Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Quality Assessment
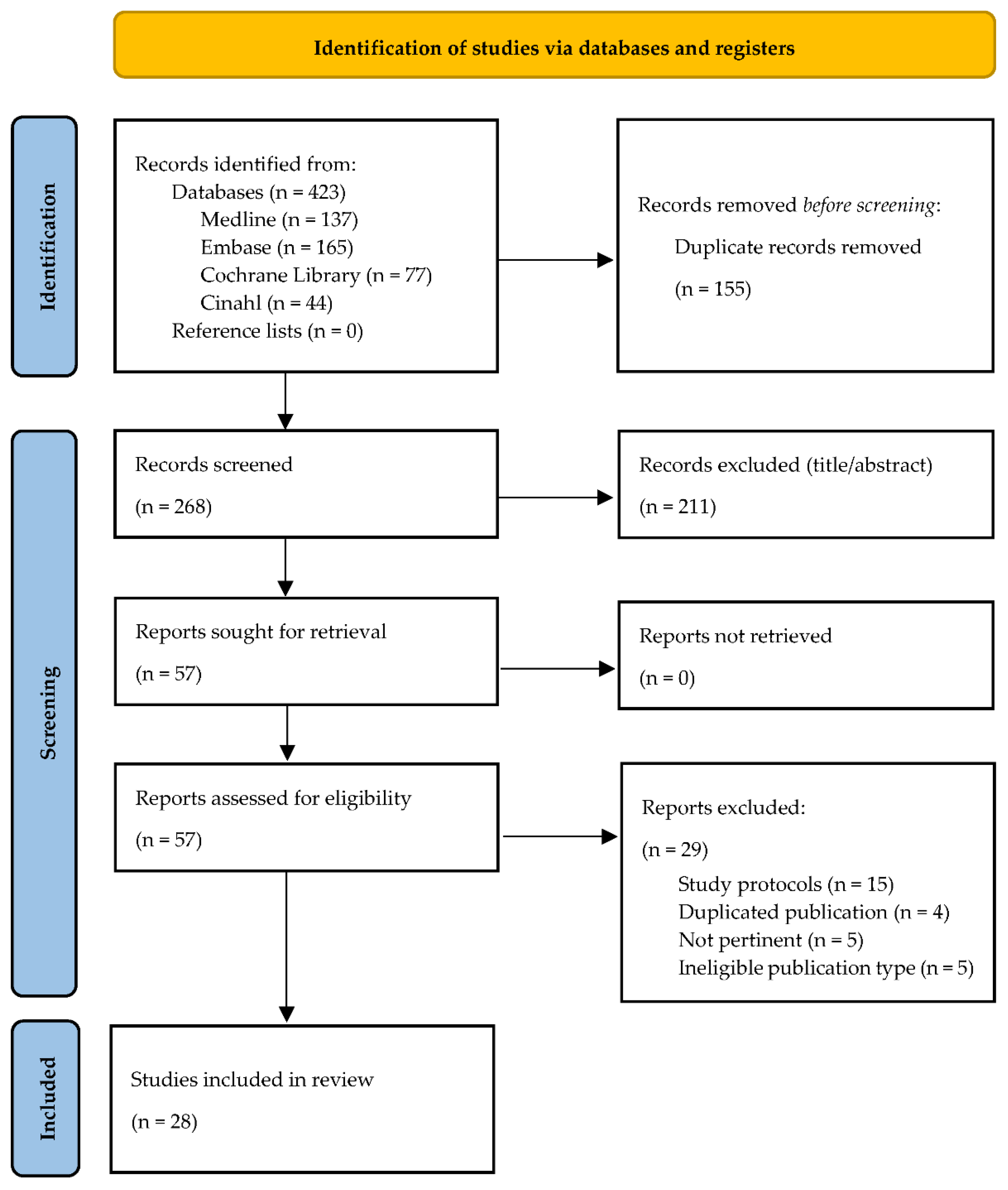
3. Results
3.1. Paper Selection and Categorization
3.2. Studies Heterogeneity
3.3. BC Topical Applications in Uro-Gynecology Setting
3.4. BC as Dietary Supplement in the Sporting Population
3.5. BC as Dietary Supplement in Pediatrics and Preterm Infants
3.6. BC as Dietary Supplement in Healthy Older Adults
3.7. BC Administration in Critically Ill Patients
3.8. Abstracts on BC Clinical Applications
3.9. Systematic Reviews on BC Clinical Applications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagwe, S.; Tharappel, L.J.P.; Kaur, G.; Buttar, H.S. Bovine Colostrum: An Emerging Nutraceutical. J. Complement Integr. Med. 2015, 12, 175–185. [Google Scholar] [CrossRef]
- Parrish, D.B.; Wise, G.H.; Hughes, J.S. Properties of the Colostrum of the Dairy Cow. I Tocopherol Levels in the Colostrum and in the Early Milk1. J. Dairy Sci. 1947, 30, 849–860. [Google Scholar] [CrossRef]
- Parrish, D.B.; Wise, G.H.; Hughes, J.S.; Atkeson, F.W. Properties of the Colostrum of the Dairy Cow. II. Effect of Prepartal Rations upon the Nitrogenous Constituents1. J. Dairy Sci. 1948, 31, 889–895. [Google Scholar] [CrossRef]
- Parrish, D.B.; Wise, G.H.; Hughes, J.S. Properties of the Colostrum of the Dairy Cow. IV. Effect of Form of Vitamin A and of Tocopherol Supplements on Concentrations of Vitamin A and Carotenoids1. J. Dairy Sci. 1949, 32, 458–464. [Google Scholar] [CrossRef]
- Moody, E.G.; Wise, G.H.; Parrish, D.B.; Atkeson, F.W. Properties of the Colostrum of the Dairy Cow. VI. Creaming and Rate of Flow1. J. Dairy Sci. 1951, 34, 106–115. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and Properties of Bovine Colostrum: A Review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Carrillo, A.E.; Koutedakis, Y.; Flouris, A.D. Exercise and Exposure to Heat Following Bovine Colostrum Supplementation: A Review of Gastrointestinal and Immune Function. Cell Mol. Biol. 2013, 59, 84–88. [Google Scholar]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune Components of Bovine Colostrum and Milk. J Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Lönnerdal, B. Bioactive Proteins in Breast Milk. J. Paediatr. Child Health 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Filipescu, I.E.; Leonardi, L.; Menchetti, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Piro, F.; Quattrone, A.; Barbato, O.; Brecchia, G. Preventive Effects of Bovine Colostrum Supplementation in TNBS-Induced Colitis in Mice. PLoS ONE 2018, 13, e0202929. [Google Scholar] [CrossRef]
- Dice Nail, C. The Immunoprotective Properties of Bovine Colostrum: A Review. Nutr. Perspect. J. Counc. Nutr. 2016, 39, 23–30. [Google Scholar]
- Gopal, P.K.; Gill, H.S. Oligosaccharides and Glycoconjugates in Bovine Milk and Colostrum. Br. J. Nutr. 2000, 84 (Suppl. 1), S69–S74. [Google Scholar] [CrossRef] [Green Version]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential Benefits of Colostrum in Gastrointestinal Diseases. Front. Biosci. 2016, 8, 331–351. [Google Scholar] [CrossRef] [Green Version]
- Marnila, P.; Korohnen, H. Colostrum. Encyclopedia of Dairy Sciences; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Tsioulpas, A.; Grandison, A.S.; Lewis, M.J. Changes in Physical Properties of Bovine Milk from the Colostrum Period to Early Lactation. J. Dairy Sci. 2007, 90, 5012–5017. [Google Scholar] [CrossRef]
- Barrington, G.M.; Besser, T.E.; Davis, W.C.; Gay, C.C.; Reeves, J.J.; McFadden, T.B. Expression of Immunoglobulin G1 Receptors by Bovine Mammary Epithelial Cells and Mammary Leukocytes. J. Dairy Sci. 1997, 80, 86–93. [Google Scholar] [CrossRef]
- Butler, J.E. The Occurrence of Immunoglobulin Fragments, Two Types of Lactoferrin and a Lactoferrin-IgG2 Complex in Bovine Colostral and Milk Whey. Biochim. Biophys. Acta Protein Struct. 1973, 295, 341–351. [Google Scholar] [CrossRef]
- Pakkanen, R.; Aalto, J. Growth Factors and Antimicrobial Factors of Bovine Colostrum. Int. Dairy J. 1997, 7, 285–297. [Google Scholar] [CrossRef]
- Apodaca, G.; Katz, L.A.; Mostov, K.E. Receptor-Mediated Transcytosis of IgA in MDCK Cells Is via Apical Recycling Endosomes. J. Cell Biol. 1994, 125, 67–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phalipon, A.; Cardona, A.; Kraehenbuhl, J.-P.; Edelman, L.; Sansonetti, P.J.; Corthésy, B. Secretory Component: A New Role in Secretory IgA-Mediated Immune Exclusion In Vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Rathe, M.; Müller, K.; Sangild, P.T.; Husby, S. Clinical Applications of Bovine Colostrum Therapy: A Systematic Review. Nutr. Rev. 2014, 72, 237–254. [Google Scholar] [CrossRef]
- van Hooijdonk, A.C.M.; Kussendrager, K.D.; Steijns, J.M. In Vivo Antimicrobial and Antiviral Activity of Components in Bovine Milk and Colostrum Involved in Non-Specific Defence. Br. J. Nutr. 2000, 84, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Sarker, S.A.; Casswall, T.H.; Mahalanabis, D.; Alam, N.H.; Albert, M.J.; Brüssow, H.; Fuchs, G.J.; Hammerström, L. Successful Treatment of Rotavirus Diarrhea in Children with Immunoglobulin from Immunized Bovine Colostrum. Pediatric Infect. Dis. J. 1998, 17, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Doillon, C.J.; Lehance, F.; Bordeleau, L.J.; Laplante-Campbell, M.P.; Drouin, R. Modulatory Effect of a Complex Fraction Derived from Colostrum on Fibroblast Contractibility and Consequences on Repair Tissue. Int. Wound J. 2011, 8, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Aalto, J.U.; Jalkanen, M.T.; Jalonen, H.G.; Kanttinen, A.P.; Laato, M.K.; Pakkanen, R.A. Method for the Improvement of Wound Healing and Compositions Therefore. WIPO (PCT) WO1995000155 A1, 5 January 1995. [Google Scholar]
- Główka, N.; Woźniewicz, M. Potential Use of Colostrum Bovinum Supplementation in Athletes—A Review. Acta Sci. Pol. Technol. Aliment. 2019, 18, 115–123. [Google Scholar] [CrossRef]
- Gingerich, D.A.; McPhillips, C.A. Analytical Approach to Determination of Safety of Milk Ingredients from Hyperimmunized Cows. Regul. Toxicol. Pharmacol. 2005, 41, 102–112. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Armijo-Olivo, S.; Stiles, C.R.; Hagen, N.A.; Biondo, P.D.; Cummings, G.G. Assessment of Study Quality for Systematic Reviews: A Comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological Research. J. Eval. Clin. Pract. 2012, 18, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358. [Google Scholar] [CrossRef] [Green Version]
- OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 13 May 2021).
- Nappi, R.E.; Benedetto, C.; Campolo, F.; Martella, S.; Tosti, C.; Cianci, A.; Caruso, S.; Guaschino, S.; Grimaldi, E.; Bagolan, M.; et al. Efficacy, Tolerability and Safety of a New Medical Device, Monurelle Biogel® Vaginal Gel, in the Treatment of Vaginal Dryness: A Randomized Clinical Trial in Women of Reproductive Age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 82–88. [Google Scholar] [CrossRef]
- Schiavi, M.C.; Di Tucci, C.; Colagiovanni, V.; Faiano, P.; Giannini, A.; D’Oria, O.; Prata, G.; Perniola, G.; Monti, M.; Zullo, M.A.; et al. A Medical Device Containing Purified Bovine Colostrum (Monurelle Biogel) in the Treatment of Vulvovaginal Atrophy in Postmenopausal Women: Retrospective Analysis of Urinary Symptoms, Sexual Function, and Quality of Life. Low Urin. Tract Symptoms 2019, 11, O11–O15. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Liverani, C.A.; Bianco, V.; Penna, C.; Guarnieri, T.; Comparetto, C.; Monti, E.; Valente, I.; Pieralli, A.L.; Fiaschi, C.; et al. Spontaneous Regression of Low-Grade Cervical Intraepithelial Lesions Is Positively Improved by Topical Bovine Colostrum Preparations (GINEDIE®). A Multicentre, Observational, Italian Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 728–733. [Google Scholar]
- Jones, A.W.; Cameron, S.J.S.; Thatcher, R.; Beecroft, M.S.; Mur, L.A.J.; Davison, G. Effects of Bovine Colostrum Supplementation on Upper Respiratory Illness in Active Males. Brain. Behav. Immun. 2014, 39, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Thatcher, R.; March, D.S.; Davison, G. Influence of 4 Weeks of Bovine Colostrum Supplementation on Neutrophil and Mucosal Immune Responses to Prolonged Cycling. Scand. J. Med. Sci. Sports 2015, 25, 788–796. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.W.; March, D.S.; Thatcher, R.; Diment, B.; Walsh, N.P.; Davison, G. The Effects of Bovine Colostrum Supplementation on in Vivo Immunity Following Prolonged Exercise: A Randomised Controlled Trial. Eur. J. Nutr. 2019, 58, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Davison, G.; Jones, A.W.; Marchbank, T.; Playford, R.J. Oral Bovine Colostrum Supplementation Does Not Increase Circulating Insulin-like Growth Factor-1 Concentration in Healthy Adults: Results from Short- and Long-Term Administration Studies. Eur. J. Nutr. 2020, 59, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, Y.; Mikellidi, A.; Aresti, C.; Persia, E.; Sotiropoulos, A.; Panagiotakos, D.B.; Antonopoulou, S.; Nomikos, T. A Low-Dose, 6-Week Bovine Colostrum Supplementation Maintains Performance and Attenuates Inflammatory Indices Following a Loughborough Intermittent Shuttle Test in Soccer Players. Eur. J. Nutr. 2018, 57, 1181–1195. [Google Scholar] [CrossRef] [Green Version]
- Kotsis, Y.; Methenitis, S.; Mikellidi, A.; Aresti, C.; Persia, E.; Antonopoulou, S.; Nomikos, T. Changes of Rate of Torque Development in Soccer Players after a Loughborough Intermittent Shuttle Test: Effect of Bovine Colostrum Supplementation. Isokinet. Exerc. Sci. 2020, 28, 59–72. [Google Scholar] [CrossRef]
- March, D.S.; Jones, A.W.; Thatcher, R.; Davison, G. The Effect of Bovine Colostrum Supplementation on Intestinal Injury and Circulating Intestinal Bacterial DNA Following Exercise in the Heat. Eur. J. Nutr. 2019, 58, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, S.A.; Cheung, S.S.; Hurst, R.D.; Cotter, J.D. Cognitive Function and Blood-Brain Barrier Permeability during Exercise in the Heat: Effect of Fitness and Bovine Colostrum Supplementation. J. Therm. Biol. 2013, 38, 374–383. [Google Scholar] [CrossRef]
- Morrison, S.A.; Cheung, S.S.; Cotter, J.D. Bovine Colostrum, Training Status, and Gastrointestinal Permeability during Exercise in the Heat: A Placebo-Controlled Double-Blind Study. Appl. Physiol. Nutr. Metab. 2014, 39, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. A Pilot Study: Bovine Colostrum Supplementation and Hormonal and Autonomic Responses to Competitive Cycling. J. Sports Med. Phys. Fit. 2013, 53, 490–501. [Google Scholar]
- Aunsholt, L.; Jeppesen, P.B.; Lund, P.; Sangild, P.T.; Ifaoui, I.B.R.; Qvist, N.; Husby, S. Bovine Colostrum to Children with Short Bowel Syndrome: A Randomized, Double-Blind, Crossover Pilot Study. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 99–106. [Google Scholar] [CrossRef]
- Balachandran, B.; Dutta, S.; Singh, R.; Prasad, R.; Kumar, P. Bovine Colostrum in Prevention of Necrotizing Enterocolitis and Sepsis in Very Low Birth Weight Neonates: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. J. Trop. Pediatr. 2017, 63, 10–17. [Google Scholar] [CrossRef]
- Barakat, S.H.; Meheissen, M.A.; Omar, O.M.; Elbana, D.A. Bovine Colostrum in the Treatment of Acute Diarrhea in Children: A Double-Blinded Randomized Controlled Trial. J. Trop. Pediatr. 2020, 66, 46–55. [Google Scholar] [CrossRef]
- Juhl, S.M.; Ye, X.; Zhou, P.; Li, Y.; Iyore, E.O.; Zhang, L.; Jiang, P.; van Goudoever, J.B.; Greisen, G.; Sangild, P.T. Bovine Colostrum for Preterm Infants in the First Days of Life: A Randomized Controlled Pilot Trial. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 471–478. [Google Scholar] [CrossRef]
- Patıroğlu, T.; Kondolot, M. The Effect of Bovine Colostrum on Viral Upper Respiratory Tract Infections in Children with Immunoglobulin A Deficiency. Clin. Respir. J. 2013, 7, 21–26. [Google Scholar] [CrossRef]
- Rathe, M.; De Pietri, S.; Wehner, P.S.; Frandsen, T.L.; Grell, K.; Schmiegelow, K.; Sangild, P.T.; Husby, S.; Müller, K. Bovine Colostrum Against Chemotherapy-Induced Gastrointestinal Toxicity in Children with Acute Lymphoblastic Leukemia: A Randomized, Double-Blind, Placebo-Controlled Trial. JPEN J. Parenter. Enteral. Nutr. 2020, 44, 337–347. [Google Scholar] [CrossRef]
- Saad, K.; Abo-Elela, M.G.M.; El-Baseer, K.A.A.; Ahmed, A.E.; Ahmad, F.-A.; Tawfeek, M.S.K.; El-Houfey, A.A.; Aboul Khair, M.D.; Abdel-Salam, A.M.; Abo-Elgheit, A.; et al. Effects of Bovine Colostrum on Recurrent Respiratory Tract Infections and Diarrhea in Children. Medicine 2016, 95, e4560. [Google Scholar] [CrossRef]
- Duff, W.R.D.; Chilibeck, P.D.; Rooke, J.J.; Kaviani, M.; Krentz, J.R.; Haines, D.M. The Effect of Bovine Colostrum Supplementation in Older Adults during Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, G.; Ardehali, S.H.; Baghestani, A.-R.; Vahdat Shariatpanahi, Z. Effects of Early Enteral Bovine Colostrum Supplementation on Intestinal Permeability in Critically Ill Patients: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2019, 60, 106–111. [Google Scholar] [CrossRef]
- Caysido, E.C.; Caysido, E.C. Effect of bovine colostrum on the absolute neutrophil count of pediatric patients with acute lymphocytic leukemia undergoing chemotherapy—A double blind randomized placebo controlled study. In Proceedings of the 2nd World Congress on Pediatrics and Clinical Pediatrics, Edinburgh, Scotland, 12–13 June 2019. [Google Scholar]
- Barakat, S.H.M.; Omar, O.; Meheissen, M. Bovine Colostrum in the Treatment of Acute Diarrhea in Egyptian Children: A Randomised Double-Blinded, Placebo-Controlled Trial. Pediatrics 2019, 144, 235. [Google Scholar] [CrossRef]
- Donowitz, J.; Alam, M.; Kabir, M.; Ferdous, T.; Zerin, A.; Nayak, U.; Haque, R.; Petri, W.A. PTM202, A Bovine Colostrum Based Nutritional Supplement, Decreases the Enteric Inflammation of Environmental Enteric Dysfunciton in Bangladeshi Infants. Am. J. Trop. Med. Hygiene 2019, 101, 194. [Google Scholar]
- Oloroso-Chavez, K.; Andaya, P.; Wong, C. OR082 Bovine Colostrum Supplementation in Respiratory Allergies According to Sensitization: Subgroup Analysis of Randomized Controlled Trial. Ann. Allergy Asthma Immunol. 2017, 119, S11–S12. [Google Scholar] [CrossRef]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal Fatty Acid-Binding Protein and Gut Permeability Responses to Exercise. Eur. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.R.; Wallace, L.E.; Hartmann, B.; Holst, J.J.; Demchyshyn, L.; Toney, K.; Sigalet, D.L. Nutrient-Stimulated GLP-2 Release and Crypt Cell Proliferation in Experimental Short Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G431–G438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, M.; Kellow, N.J.; Dordevic, A.L.; Evans, S.; Caissutti, J.; McCaffrey, T.A. Health Benefits of Whey or Colostrum Supplementation in Adults ≥ 35 Years; a Systematic Review. Nutrients 2020, 12, 299. [Google Scholar] [CrossRef] [Green Version]
| Evidence Level (Treatment Benefits) | Level 1 * |
| Systematic review of randomized trials or n-of-1 trials | |
| Level 2 * | |
| Randomized trial or observational study with dramatic effect | |
| Level 3 * | |
| Non-randomized controlled cohort/follow-up study ** | |
| Level 4 * | |
| Case-series, case control studies, or historically controlled studies ** | |
| Level 5 * | |
| Mechanism-based reasoning |
| Authors | Study Design | Population Number Groups Gender Mean Age | Intervention Matrix | TG Size Dosage Frequency Duration | Control Matrix | CG Size Dosage Frequency Duration | Endpoints Data Collection Tools | Adverse Events | Results | OCEBM | EPHPP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nappi, R. E., et al. (2016) [33] | RCT | Women with vaginal dryness. n = 95; >18 ys | Monurelle Biogel® | TG (n = 48) 5 mL, 1–2 time per day during intermenstrual period (23 days) | No-treatment, nonactive lubricants on demand were allowed | CG (n = 47) | PE: Vaginal discomfort (VRS) SE: (1) Symptoms (VRS); (2) Vaginal health (VHI mean sum score); (3) Sexual function (FSFI); (4) Sexual distress (FSDS-R). | No severe or serious AEs Mild AEs in 16.7% CG: AEs in 8.5% | ↓ Vaginal discomfort ↓ Vaginal symptoms ↑ Vaginal health Sexual function improved ↓ Sexual distress | Level 1 | 1 |
| Schiavi, M. C., et al. (2019) [34] | Retrospective | Postmenopausal women with VVA. n = 172 mean age 60.8 ys | Monurelle Biogel® | 5 mL once daily for 12 weeks | No CG | No CG | PE: Vaginal health (VHI); SE: (1) Sexual function (FSFI); (2) Sexual distress (FSDS); (3) Urinary symptoms (4) Urogenital distress (UDI-6); (5) Overactive bladder symptoms (OAB-Q) (6) QoL (HRQL) | No significant AEs | ↑ Vaginal health Sexual function improved ↓ Sexual distress ↓ Urinary symptoms ↓ Urogenital distress ↓ OAB symptoms ↑ QoL | Level 3 | 3 |
| Stefani, C., et al. (2014) [35] | Retrospective | Women diagnosed as CIN1. n = 256; mean age 37.7 ys | Ginedie® vaginal tablets | twice/week at bedtime for 6 months. | No CG | No CG | ORR to negative histology (Cervical cytology) | NR | 75.5% ORR | Level 4 | 3 |
| Authors | Study Design | Population Number Groups Gender Mean Age | Intervention Matrix | TG size Dosage Frequency Duration | Control Matrix | CG Size Dosage Frequency Duration | Endpoints/Data Collection Tools | Adverse Events | Results | OCEBM | EPHPP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jones, A. W., et al. (2014) [36] | RCT, DB, PC | Regularly exercising males n = 53; mean age 50.5 ys Colostrum (n = 25) vs. Placebo (n = 28) | BC (Neovite® UK, London) | TG (n = 25) 20 g/day 12 weeks. | Isoenergetic/ Isomacronutrient placebo | CG (n = 28) 20 g/day 12 weeks. | PE: (1) Incidence of URI; SE: (1) URI days; (2) URI duration (episodes) (3) Immune-system parameters (4) Salivary antimicrobial proprieties (sIgA/AMPs) (5) Salivary microbiome composition | NR | ↓ URI incidence; ↓ URI days; ↓ salivary bacterial load; No significant effects on: severity and duration of URI episodes, immune-system parameters, salivary sIgA/AMPs | Level 2 | 1 |
| Davison G., et al. (2019) [39] | RCT, DB, PC, CB, CO | Recreationally active males n = 16; mean age 25.0 ys CO after a week | BC (Neovite® UK, London) during 4.5 h long moderate exercise | TG (n = 16): 40 g | Isoenergetic/ Isomacronutrient placebo during 4.5 h long moderate exercise | CG (n = 16) 40 g | IGF-1 blood levels | NR | No significant effects on IGF-1 blood levels | Level 2 | 1 |
| RCT, DB, PC | Recreationally active males n = 20; mean age 28.0 ys | BC (Neovite® UK, London) + training program | TG (n = 10) 20 g/day 4 weeks | Isoenergetic/ Isomacronutrient Placebo + training program | CG (n = 10) 20 g/day 4 weeks | IGF-1 blood levels | NR | No significant effects on IGF-1 blood levels | Level 3 | ||
| RCT, DB, PC | Recreationally active males n = 57; mean age NR Colostrum (n = NR) vs. Placebo (n = NR) n = 4 excluded from the analysis | BC (Neovite® UK, London) + training program | TG (n = 25): BC 20 g/day 12 weeks | Isoenergetic/ Isomacronutrient Placebo + training program | CG (n = 28) 20 g/day 12 weeks | IGF-1 blood levels | NR | No significant effects on IGF-1 blood levels | Level 3 | ||
| Halasa, M., et al. (2017) [40] | RCT, DB, PC | Competitive athletic males n = 16; mean age 27.5 ys | Freeze-dried whole BC obtained within 2 h of calf delivery (Genactiv®, Poznan, Poland) was packaged in pouches BC 500 mg and desiccated banana 500 mg. | TG (n = 8) 1 g/day 20 days | Identical pouches (500 mg of dehydrated whey and 500 mg of desiccated banana) were used as the placebo. | CG (n = 8) 1 g/day 20 days | Gut permeability: sugar absorption test, zonulin concentration | No AEs in the TG. Mild AEs in 50% of CG | ↓ sugar absorption ↓ zonulin concentration | Level 3 | 1 |
| Jones, A. W., et al. (2019) [38] | RCT, DB, PC | Recreationally active males n = 34; mean age NR Colostum (n = 17) vs. Placebo (n = 17) n = 3 excluded from the analysis | BC + water + training program Day 28: 2 h of 60% maximal aerobic capacity and immune system sensitisation Day 56: elicitation of immunity | TG (n = 15) 20 g/day 58 days. | isoenergetic/ isomacronutrient Placebo + training program Day 28: 2 h of 60% maximal aerobic capacity and immune system sensitisation Day 56: elicitation of immunity | CG (n = 16) 20 g/day 58 days | PE: Cell mediated response following prolonged exercise (skinfold reactivity) SE: (1) IGF-1 blood levels (2) Immune cell counts (3) Biochemical parameters | NR | ↓immune sensitivity decreasing after prolonged exercise No significant effects on in-vivo immune responsiveness, IGF-1 blood levels, Immune cell counts and other biochemical parameters | Level 2 | 1 |
| Jones, A. W., et al. (2015) [37] | RCT, DB, PC | Recreationally active males n = 20, mean age 28.0 ys | BC (Neovite® UK, London) | TG (n = 10) 20 g/day 4 weeks | isoenergetic/ isomacronutrient Placebo | CG (n = 10) 20 g/day 4 weeks | PE: (1) In-vitro blood neutrophil function: fMLP and PMA (2) Mucosal responses: sIgA and AMP SE: (1) Circulating cells count (2) Biochemical parameters | NR | Beneficial in vitro effects on receptor- dependent (fMLP-stimulated) oxidative burst responses. No in vitro effect on PMA-stimulated oxidative burst, sIgA and AMP. No effects on leukocyte trafficking and other biochemical parameters | Level 2 | 1 |
| Kotsis, Y., et al. (2019) [42] | RCT, DB, PC | Soccer players n = 22; mean age 21.1 ys | Commercial BC 378 Kcal, 67 g protein, 17 g carbohydrates and 4.7 g fat per 100 g Pre and post supplementation LIST exercise program | TG (n = 11) 3.2 g/day 6 weeks. | Commercial whey protein 369 Kcal, 90 g protein, 1 g carbohydrates and 0.5 g fat per 100 g Pre and post supplementation LIST exercise program | CG (n = 11) 3.2 g/day 6 weeks. | Post-LIST RTD reduction | NR | ↓ RTD decline in both groups without significant difference | Level 3 | 1 |
| Kotsis, Y., et al. (2018) [41] | RCT, DB, PC | Soccer players n = 22; mean age NR n = 4 excluded from the analysis | Commercial BC 378 Kcal, 67 g protein, 17 g carbohydrates and 4.7 g fat per 100 g Pre and post supplementation LIST exercise program | TG (n = 10) 3.2 g/day 6 weeks + 4 days | Commercial whey protein 369 Kcal, 90 g protein, 1 g carbohydrates and 0.5 g fat per 100 g Pre and post supplementation LIST exercise program | CG (n = 8) 3.2 g/day 6 weeks + 4 days | EIMD: MIVC, SQJ, CMJ, PMS, biochemical parameters | NR | ↑ SQJ, CRP, CK, IL-6 recovery. No significant differences on MIVC, CMJ, PMS and other outcome | Level 2 | 1 |
| March, D. S., et al. (2018) [43] | RCT, DB, PC, CO | Regularly exercising males n = 12; mean age 26 ys Colostrum (n = 12) vs. Placebo (n = 12) CO after 2 weeks of washout | BC (Neovite® UK, London) | TG (n = 12): BC 20 g/day for 14 days Exercise program 70% aerobic capacity for 1 h | CG (n = 12): Isoenergetic/Isomacronutrient placebo for 14 days Exercise program 70% aerobic capacity for 1 h | PE: Exercise-induced intestinal cell damage (I-FABP) SE: (1) Bacterial translocation (plasmatic bacterial DNA) (2) Other physical parameters | NR | ↓ I-FABP plasma concentration after exercise No effects Bacterial DNA plasmatic concentration No significant differences on other outcome | Level 2 | 1 | |
| Morrison, S. A., et al. (2014) [45] | RCT, DB, PC, CO | Trained and Untrained males n = 28 (14 trained, 14 untrained) Colostrum (n = 14) vs. Placebo (n = 14) n = 13 (6 untrained, 7 trained) lost and excluded from the analysis CO time NR | BC (Hokitika, New Zealand) protein, 58.2% m/m; fat, 1.4% m/m; lactose, 29.3% m/m; and IgG, 15.3%. Before 90 min multi-mode exercise session | TG (n = 7 trained, n = 8 untrained): BC 1.7 g/kg/day for 7 days | Corn flour placebo | CG (n = 7 trained, n = 8 untrained): corn flour placebo for 7 days before 90 min multi-mode exercise session | PE: GI permeability (Double sugar model, I-FABP) SE: (1) cytokine level and other blood parameters (2) thermal and cardiovascular measures (3) Other parameters | No AEs | ↑ I-FABP in trained group No significant differences on other outcome | Level 3 | 1 |
| Morrison, S. A., et al. (2013) [44] | RCT, DB, PC, CO | Healthy males n = 15 (7 highly-fit, 8 moderately-fit); mean age 22 ys | BC (Hokitika, New Zealand) protein, 58.2% m/m; fat, 1.4% m/m; lactose, 29.3% m/m; and IgG, 15.3%. Before 90 min multi-mode exercise session | TG (n = 7 highly fit, n = 8 moderately-fit) 1.7 g/kg/day 7 days | Corn flour placebo | CG (n = 7 highly fit, n = 8 moderately fit) 1.77 g/kg/day 7 days | PE: (1) BBB permeability (S100ß protein, cerebral oxygenation) (2) Cognitive function (Stroop test and perceptions) SE: (1) thermal and cardiovascular measures (2) Other parameters | NR | No effects on BBB, cognitive and physical performance | Level 3 | 1 |
| Shing, C. M., et al. (2013) [46] | RCT, DB, PC Pilot study | Highly-trained males n = 10; mean age NR CPC (n = 4) vs. WPC placebo (n = 6) | Intact® bovine CPC (Numico Research Australia Pty Ltd., South Australia) before and during a 5 days cycling race | TG (n = 4) 10 g/day for 8 weeks + 5 days | Whey protein concentrate (Alacen® 80’’ Fonterra Co-op Group Limited, Auckland, New Zealand) before and during a 5 days cycling race | CG (n = 6) 10 g/day for 8 weeks + 5 days | PE: hormonal (salivary hormones level), immune (salivary IgA) and autonomic (parasympathetic indices of HRV) response SE: Mood profile (POMS) | NR | ↑ testosterone concentration maintenance ↑ cortisol concentration before the race ↑ parasympathetic indices of HRV No significant differences on cortisol concentration during race, POMS and salivary IgA concentration | Level 3 | 1 |
| Authors | Study Design | Population Number Groups Gender Mean Age | Intervention Matrix | TG size Dosage Frequency Duration | Control Matrix | CG Size Dosage Frequency Duration | Endpoints/Data Collection Tools | Adverse Events | Results | OCEBM | EPHPP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aunsholt, L., et al. (2014) [47] | RCT, DB, PC, CO, PS | Children with SBS n = 9 (4 females, 5 males); median age 39 months CO after 4 weeks washout | “Fresh” BC from 15 different cows (Danish Holstein) within the first 24 h after calving | TG (n = 9) 20% of BFR | Mixed milk, cream, and whey protein | CG (n = 9) 20% of BFR | PE: Nutrients and Fluid balances SE: Anthropometric, knemometry, biological parameters | NR | No significant differences in intestinal energy and wet weight absorption, knemometry, IGF-1, IGF-BP3 levels | Level 2 | 1 |
| Balachandran, B., et al. (2016) [48] | RCT, DB, PC, PS | VLBW Neonates n = 86; chronological age < 96 h Colostrum (n = 43) vs. Placebo (n = 43) | BC Pedimmune® (Mumbai, India) | TG (n = 43) 1.2–2.0 g/dose + feeding 4 times a day 21 days | Equal dose placebo (not specified) | CG (n = 43) 1.2–2.0 g/die + feeding 4 times a day 21 days | PE: NECSE: sepsis, mortality and stool interleukin-6 (IL-6) levels | No AEs | No significant differences in NEC, sepsis and mortality. ↑IL-6 and radiological features of NEC in TG | Level 2 | 1 |
| Barakat et al. (2019) [49] | RCT, DB, PC | Pediatrics with acute diarrhea n = 160; aged 6 months to 2 ys | ImmuGuard®, sachets (London, England) | TG (n = 80) 3 g/sachet + standard therapy | Equal dose pla-cebo (not speci-fied) | CG (n = 80) + standard therapy | PE: Diarrhea frequency and duration, vomiting duration SE: fever duration and Vesikari scoring | NR | ↓ Diarrhea and vomit frequency and duration ↓ Vesikari Scoring after 48 h | Level 2 | 1 |
| Meinich Juhl, S., et al. (2018) [50] | RCT, OL, PS | NPI n = 40; gestational age 27–32 weeks Country stratification (China-Denmark) | Unmodified intact BC powder (ColoDan®; Gesten, Denmark) as supplement of MM, DM or IF | TG (n = 21) max 4.5 g/kg/day 10–14 days | Standard feeding with MM, DM or IF | CG (n = 19) 10–14 days | PE: Tolerability and safety SE: nutritional outcomes | 1 death for NEC not related to BC intake Late onset sepsis (2 TG, 1 CG), pulmonary dysplasia (1 TG), ROP (2 TG), metabolic acidosis (2 TG) No significant differences | ↑ protein intake in TG (China group) ↑ in TG Temporary elevation in plasmatic tyrosine levels on day 7 No significant differences on dietary intolerances and other outcomes | Level 3 | 2 |
| Patıroğlu, T. and M. Kondolot (2013) [51] | RCT, DB, PC | IgA deficient paediatrics with viral URI n = 31; median age 8.5 ys; n = 18 males and 13 females Colostrum (n = 16) vs. Placebo (n = 15) | BC sucking tablet that contains 14 mg of colostrum and 2.2 mg of lysozyme (Igazym®; Vejle, Denmark) | TG (n = 16) 3 times a day 1 week | placebo sucking tablets | CG (n = 15) 3 times a day for 1 week | PE: sIgA SE: Infection severity | No AEs 1 patient included 2 times and 1 included 3 time for different infections | No significant differences in sIgA secretion ↓infection severity score in BC after 1 week | Level 2 | 1 |
| Rathe, M., et al. (2020) [52] | RCT, DB, PC Multicentre study | Pediatrics with ALL n = 62, aged 1–18 ys n = 32 males and 30 females Colostrum (n = 30) vs. Placebo (n = 32) | Intact, spray-dried BC powder (Gesten, Denmark®). | TG (n = 30) 0.5–1 g/kg/day 4 weeks | Isocaloric placebo. whole-milk powder enriched with whey protein isolate powder | CG (n = 32) 4 weeks | PE: fever level and duration.SE: CRP levels, neutrophil count, bacteraemia or fungaemia episodes, treatment delay, mucositis severity, PROs on chemotherapy toxicity, compliance | No AEs | ↓ Peak severity of oral mucositis No significant differences on PE and other SE, low compliance reported | Level 2 | 1 |
| Saad, K., et al. (2016) [53] | Cohort Prospective Multicentric | Children with recurrent acute URI or diarrhea due to infection n = 160; aged 1–6 ys. n = 81 males and 79 females | ImmuGuard®, sachets (London, England) | TG n = 160 3 g/sachet 4 weeks. | No CG | No CG | URI or diarrhea episodes and frequency of hospitalizations Follow up period 24 weeks | Mild transient AEs reported in 12 patients: Skin rush (9), itching (1), and diarrhea (2). 6 patients discontinued the BC treatment. | ↓ Infection episodes at 2 and 6 months ↓ Hospitalizations | Level 3 | 3 |
| Authors | Study Design | Population Number Groups Gender Mean Age | Intervention Matrix | TG Size Dosage Frequency Duration | Control Matrix | CG Size Dosage Frequency Duration | Endpoints/Data Collection Tools | Adverse Events | Results | OCEBM | EPHPP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duff, W. R., et al. (2014) [54] | RCT, DB, PC | Older adults n = 40; mean age 59 ys n = 15 males and 25 females | spray-dried BC (Eterna Gold®, Saskatoon, Canada) + resistance training program | TG (n = 19) 60 g/day of BC 8 weeks | whey placebo (Cereal®, Illinois, US) + resistance training program | CG (n = 21): 38 g/day WP complex (60 g total) for 8 weeks | PE: Body composition and strength SE: Muscle thickness, serum assessment, bone resorption, cognitive function | Mild-moderate GI AEs was reported by 5 participants (2 in TG, 3 in CG) | ↑ leg press strength ↓ bone resorption No differences in body composition, muscle thickness and other outcomes | Level 2 | 1 |
| Authors | Study Design | Population Number Groups Gender Mean Age | Intervention Matrix | TG Size Dosage Frequency Duration | Control Matrix | CG Size Dosage Frequency Duration | Endpoints/Data Collection Tools | Adverse Events | Results | OCEBM | EPHPP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eslamian, G., et al. (2019) [55] | RCT, DB, PC | ICU patients n = 70; mean age 62 ys Colostrum (n = 35) vs. Placebo (n = 35) n = 8 not included in the analysis | Neovite ® (London, UK). | TG (n = 32): BC 20 g/day + enteral feeding for 10 days | ENTERA Meal®; (Tehran, Iran) | CG (n = 30): Isocaloric maltodextrin + enteral feeding for 10 days | PE: Intestinal permeability (plasmatic endotoxin and zonulin) SE: mortality, LOS, GI complications | No AEs | ↓ Plasmatic endotoxin and zonulin concentrations at day 10. ↓ Diarrhea No significant differences in other comparisons | Level 2 | 1 |
| Authors | Study Design | Population Number Groups Gender Mean Age | Interventions Dosage Frequency Duration | Control Dosage Frequency Duration | Endpoints Data Collection Tools | Adverse Events | Results | OCEBM |
|---|---|---|---|---|---|---|---|---|
| Caysido et al. (2017) [56] | RCT, DB, PC | Pediatrics undergoing chemotherapy for ALL n = 21; aged 6 months to 18 ys | TG (n = NR): BC twice a day for a week from the first day of chemotherapy | CG (n = NR) Placebo twice a day for a week from the first day of chemotherapy | PE: neutropenia (CBC, ANC) | No AEs | ↑ ANC, WBC and PLT blood levels | Level 5 |
| Barakat, S., et al. (2019)b [57] | RCT, DB, PC | Pediatrics with acute diarrhea n = 160; aged 6 months to 2 ys | TG (n = 80) BC 3 g/day for 1 week | CG (n = 80) placebo for 1 week | PE: n° of patients with diarrhea after 72 h | NR | Diarrhea stopped in 65% of TG vs. 95% of CG after 72 h | Level 5 |
| Donowitz, J., et al. (2019) [58] | RCT, PC | Infants (income country) n = NR; 6 to 9 months | TG (n = NR) 7 g of PTM202 twice a day 30 days | CG (n = NR) micronutrient sprinkles twice a day 30 days | PE: EED (fecal MPO, fecal Reg 1B, serum CRP, serum sCD14, and L:M | NR | ↓ fecal MPO and reg1B No significant differences in other parameters | Level 5 |
| Oloroso-Chavez, K., et al. (2017) [59] | RCT Subgroup analysis | Pediatrics with respiratory allergies n = 38; aged 7 to 18 ys | TG (n = 19) BC 1000 mg day 3 months. | CG (n = 19) Placebo 1000 mg day 3 months | PE: Symptoms improvement (TNSS, ACT, CASI and pulmonary function test) | NR | ↓ nasal congestion (TNSS) and lung function in monosensitized subjects ↑ ACT and CASI scores in polysensitized subjects | Level 5 |
| Authors | Study Design | n of Paper Included Heterogeneity n of Participants | Bovine Colostrum Effects Measured | Evidence | Adverse Events | OCEBM | AMSTAR 2 |
|---|---|---|---|---|---|---|---|
| Rathe, M., et al. (2014) [21] | Systematic review | 49 record covering 51 studies High heterogeneity: settings, methodologies, treatment/placebos preparations and dosages, population, diseases, endpoints and outcomes 2326 participants |
|
| No serious AEs Mild/moderate AEs were reported: unpleasant taste, nausea, flatulence, diarrhea, skin rash, and unspecified abdominal discomfort. Nine studies reported an absence of side effects. | Level 1 | HIGH |
| Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | |
|---|---|---|---|---|---|---|---|
| Nappi, R. E., et al. (2016) | + | + | - | ? | + | ? | + |
| Jones, A. W., et al. (2014) | ? | ? | + | + | + | + | + |
| Davison G., et al. (2019) | ? | ? | + | + | + | ? | + |
| Halasa, M., et al. (2017) | + | + | + | + | + | ? | ? |
| Jones, A. W., et al. (2019) | + | + | + | + | + | ? | ? |
| Jones A.W., et al. (2015) | ? | ? | + | + | + | + | + |
| Kotsis, Y., et al. (2019) | + | + | + | + | ? | ? | + |
| Kotsis, Y., et al. (2018) | + | + | + | + | + | + | ? |
| March, D. S., et al. (2018) | + | + | + | + | + | + | + |
| Morrison S. A., et al. (2014) | ? | ? | + | + | - | ? | ? |
| Morrison, S. A., et al. (2013) | ? | ? | + | + | + | ? | - |
| Shing, C. M., et al. (2013) | + | ? | + | ? | + | ? | + |
| Aunsholt, L., et al. (2014) | + | ? | + | + | + | + | + |
| Balachandran, B., et al. (2016) | + | + | + | ? | + | ? | ? |
| Barakat et al. (2019) | + | + | + | + | + | + | + |
| Meinich Juhl, S., et al. (2018) | + | + | - | - | + | ? | ? |
| Patıroğlu, T. and M. Kondolot (2013) | + | ? | + | ? | + | + | + |
| Rathe, M., et al. (2020) | + | + | + | + | + | ? | + |
| Duff, W. R., et al. (2014) | + | + | + | + | + | + | + |
| Eslamian, G., et al. (2019) | + | + | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guberti, M.; Botti, S.; Capuzzo, M.T.; Nardozi, S.; Fusco, A.; Cera, A.; Dugo, L.; Piredda, M.; De Marinis, M.G. Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients 2021, 13, 2194. https://doi.org/10.3390/nu13072194
Guberti M, Botti S, Capuzzo MT, Nardozi S, Fusco A, Cera A, Dugo L, Piredda M, De Marinis MG. Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients. 2021; 13(7):2194. https://doi.org/10.3390/nu13072194
Chicago/Turabian StyleGuberti, Monica, Stefano Botti, Maria Teresa Capuzzo, Sara Nardozi, Andrea Fusco, Andrea Cera, Laura Dugo, Michela Piredda, and Maria Grazia De Marinis. 2021. "Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review" Nutrients 13, no. 7: 2194. https://doi.org/10.3390/nu13072194
APA StyleGuberti, M., Botti, S., Capuzzo, M. T., Nardozi, S., Fusco, A., Cera, A., Dugo, L., Piredda, M., & De Marinis, M. G. (2021). Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients, 13(7), 2194. https://doi.org/10.3390/nu13072194







