Effect of Improvement in Sarcopenia on Functional and Discharge Outcomes in Stroke Rehabilitation Patients
Abstract
:1. Introduction
2. Materials and Methods
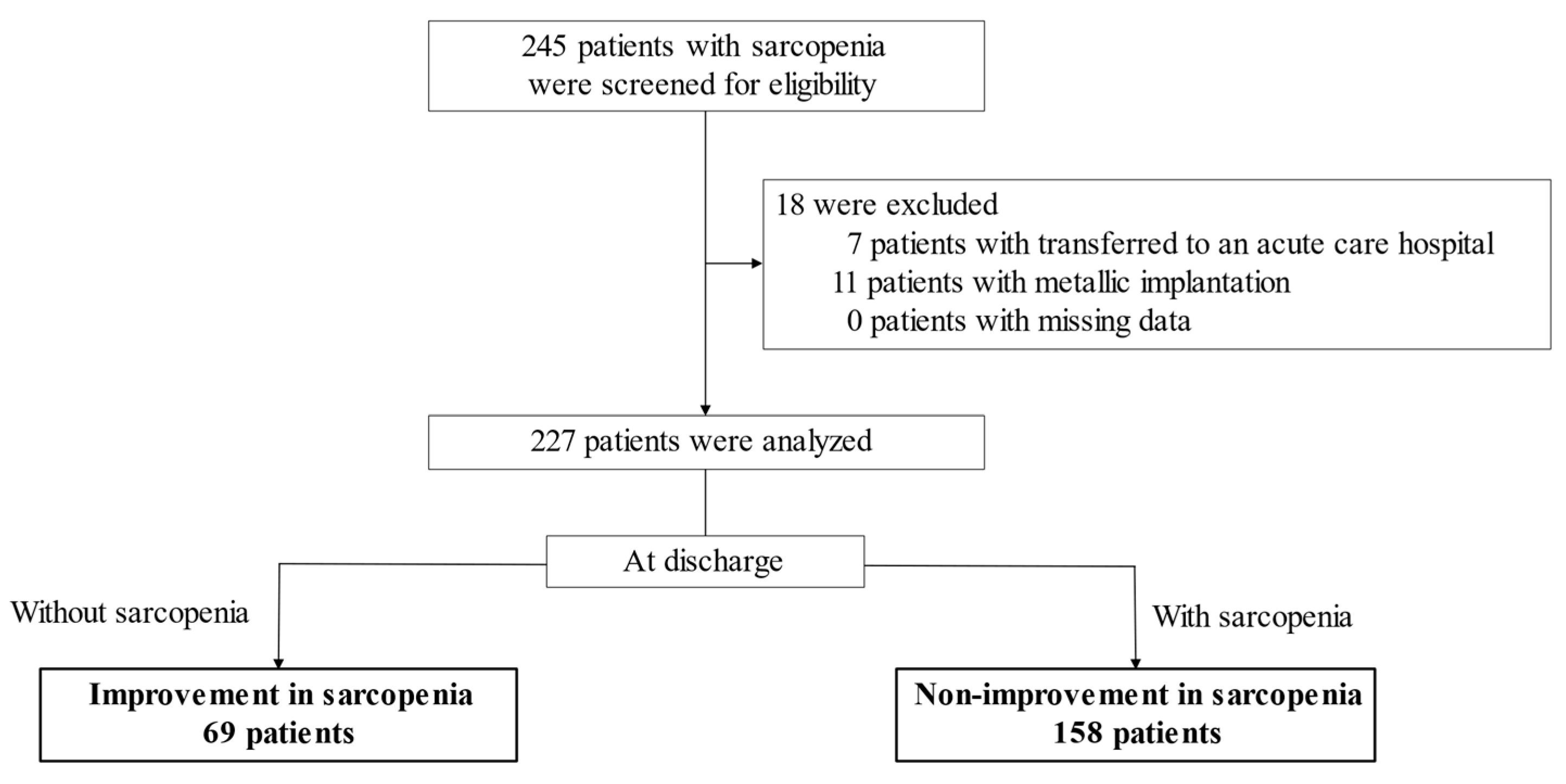
2.1. Study Participants and Design
2.2. Ethical Consideration
2.3. Outcome Measures
2.4. Data Collection
2.5. Definition of Sarcopenia
2.6. Sample Size Calculation
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bischoff-Ferrari, H.A.; Orav, J.E.; Kanis, J.A.; Rizzoli, R.; Schlögl, M.; Staehelin, H.B.; Willett, W.C.; Dawson-Hughes, B. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos. Int. 2015, 26, 2793–2802. [Google Scholar] [CrossRef]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The longitudinal aging study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Beaudart, C.; Biver, E.; Reginster, J.Y.; Rizzoli, R.; Rolland, Y.; Bautmans, I.; Petermans, J.; Gillain, S.; Buckinx, F.; Dardenne, N.; et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for sarcopenia. J. Cachexia Sarcopenia Muscle 2017, 8, 238–244. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, J.; Geerlings, M.A.J.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef]
- Scherbakov, N.; Sandek, A.; Doehner, W. Stroke-related sarcopenia: Specific characteristics. J. Am. Med. Dir. Assoc. 2015, 16, 272–276. [Google Scholar] [CrossRef]
- Hunnicutt, J.L.; Gregory, C.M. Skeletal muscle changes following stroke: A systematic review and comparison to healthy individuals. Top Stroke Rehabil. 2017, 24, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, T.; Liu, Y. New understanding of the pathogenesis and treatment of stroke-related sarcopenia. Biomed. Pharmacother. 2020, 131, 110721. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Ivey, F.M.; Serra, M.C.; Hartstein, J.; Hafer-Macko, C.E. Sarcopenia and physical function in middle-aged and older stroke survivors. Arch. Phys. Med. Rehabil. 2017, 98, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, Y.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Systemic inflammation in the recovery stage of stroke: Its association with sarcopenia and poor functional rehabilitation outcomes. Prog. Rehabil. Med. 2018, 3, 20180011. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, T.; Nishioka, S.; Taguchi, S.; Yamanouchi, A. Sarcopenia as a predictor of activities of daily living capability in stroke patients undergoing rehabilitation. Geriatr. Gerontol. Int. 2019, 19, 1124–1128. [Google Scholar] [CrossRef]
- King, D.; Wittenberg, R.; Patel, A.; Quayyum, Z.; Berdunov, V.; Knapp, M. The future incidence, prevalence and costs of stroke in the UK. Age Ageing 2020, 49, 277–282. [Google Scholar] [CrossRef]
- Motyer, R.; Asadi, H.; Thornton, J.; Nicholson, P.; Kok, H.K. Current evidence for endovascular therapy in stroke and remaining uncertainties. J. Intern. Med. 2018, 283, 2–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia, and a lower proportion of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Aragane, H.; Suzuki, N.; Yoshimura, Y.; Fujiwara, D.; Mori, T.; Kanehisa, Y.; Iida, Y.; Higashi, K.; Yoshimura-Yokoi, Y.; et al. Clinical practice guidelines for rehabilitation nutrition in cerebrovascular disease, hip fracture, cancer, and acute illness: 2020 update. Clin. Nutr. ESPEN 2021, 43, 90–103. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Moriwaki, M.; Wakabayashi, H.; Sakata, K.; Domen, K. The effect of branched chain amino acids-enriched nutritional supplements on Activities of Daily Living and muscle mass in inpatients with gait impairments: A randomized controlled trial. J. Nutr. Health Aging 2019, 23, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Miyai, I.; Sonoda, S.; Nagai, S.; Takayama, Y.; Inoue, Y.; Kakehi, A.; Kurihara, M.; Ishikawa, M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural Repair 2011, 25, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, M. Screening for malnutrition: A multidisciplinary responsibility. In Development and Use of the Malnutrition Universal Screening Tool (‘MUST’) for Adults; BAPEN: Redditch, UK, 2003. [Google Scholar]
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, T.; Nishioka, S.; Taguchi, S.; Yamanouchi, A.; Nakashima, R.; Wakabayashi, H. Sarcopenic obesity and activities of daily living in stroke rehabilitation patients: A cross-sectional study. Healthcare 2020, 8, 255. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Sakai, M.; Nishimura, K.; Fujiwara, K.; Fujisaki, K.; Shimpo, M.; Akamatsu, R. Criterion validity of the visual estimation method for determining patients’ meal intake in a community hospital. Clin. Nutr. 2016, 35, 1543–1549. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Beninato, M.; Gill-Body, K.M.; Salles, S.; Stark, P.C.; Black-Schaffer, R.M.; Stein, J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 32–39. [Google Scholar] [CrossRef]
- Schwitzguébel, A.J.; Benaïm, C.; Carda, S.; Torea Filgueira, A.M.; Frischknecht, R.; Rapin, P.A. GABAergic drug use and global, cognitive, and motor functional outcomes after stroke. Ann. Phys. Rehabil. Med. 2016, 59, 320–325. [Google Scholar] [CrossRef]
- Shimizu, A.; Fujishima, I.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ohno, T.; Nomoto, A.; Kayashita, J.; Mori, N.; The Japanese Working Group on Sarcopenic Dysphagia. Nutritional management enhances the recovery of swallowing ability in older patients with sarcopenic dysphagia. Nutrients 2021, 13, 596. [Google Scholar] [CrossRef]
- Nagano, F.; Yoshimura, Y.; Bise, T.; Shimazu, S.; Shiraishi, A. Muscle mass gain is positively associated with functional recovery in patients with sarcopenia after stroke. J. Stroke Cereb. Dis. 2020, 29, 105017. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet. 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Peng, T.C.; Chen, W.L.; Wu, L.W.; Chang, Y.W.; Kao, T.W. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2695–2701. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Sui, S.X.; Hordacre, B.; Pasco, J.A. Are sarcopenia and cognitive dysfunction comorbid after stroke in the context of brain-muscle crosstalk? Biomedicines 2021, 9, 223. [Google Scholar] [CrossRef]
- Scisciola, L.; Fontanella, R.A.; Surina Cataldo, V.; Paolisso, G.; Barbieri, M. Sarcopenia and cognitive function: Role of myokines in muscle brain cross-talk. Life 2021, 11, 173. [Google Scholar] [CrossRef]
- Menant, J.C.; Weber, F.; Lo, J.; Sturnieks, D.L.; Close, J.C.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: Time to abandon the term sarcopenia? Osteoporos. Int. 2017, 28, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prospective Urban Rural Epidemiology (PURE) Study investigators. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Everink, I.H.; van Haastregt, J.C.; van Hoof, S.J.; Schols, J.M.; Kempen, G.I. Factors influencing home discharge after inpatient rehabilitation of older patients: A systematic review. BMC Geriatr. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakkarainen, T.W.; Arbabi, S.; Willis, M.M.; Davidson, G.H.; Flum, D.R. Patients discharged to skilled nursing facilities after acute care hospitalizations. Ann. Surg. 2016, 263, 280–285. [Google Scholar] [CrossRef] [Green Version]
| Overall | Improvement in Sarcopenia | Non-Improvement in Sarcopenia | p-Value | |
|---|---|---|---|---|
| Patients, n | 227 | 69 | 158 | |
| Age, years, mean (SD) | 80.5 (7.7) | 78.1 (6.8) | 81.5 (7.9) | 0.002 § |
| Female sex, n (%) | 125 (55) | 41 (59) | 84 (53) | 0.468 ‡ |
| Stroke subtype, n (%) | 0.259 ‡ | |||
| Ischemic stroke | 164 (72) | 46 (67) | 118 (75) | |
| Hemorrhagic stroke | 63 (28) | 23 (33) | 40 (25) | |
| CCI, score, median [IQR] | 1 [0, 2] | 0 [0, 2] | 2 [0, 2] | 0.017 † |
| Days between onset and admission, days, median [IQR] | 23 [19, 29] | 23 [19, 31] | 23 [19, 28] | 0.643 † |
| FIM, score, median [IQR] | ||||
| Total | 68 [44, 86.5] | 74 [56, 87] | 63 [42.25, 85.75] | 0.123 † |
| Motor domain | 45 [25.5, 60] | 50 [32, 59] | 42.5 [24, 60] | 0.314 † |
| Cognitive domain | 21 [16, 28.5] | 25 [19, 29] | 20 [15, 27.75] | 0.009 † |
| Pre-stroke care needs *, n (%) | 54 (24) | 7 (10) | 47 (30) | <0.001 ‡ |
| Lower limb motor paralysis, n (%) | 0.505 ‡ | |||
| BRS I–IV | 55 (24) | 20 (29) | 35 (22) | |
| BRSV–VI | 123 (54) | 36 (52) | 87 (55) | |
| absence | 49 (22) | 13 (19) | 36 (23) | |
| Height, cm, mean (SD) | 153.9 (8.1) | 155.1 (7.8) | 153.4 (8.2) | 0.139 § |
| Body weight, kg, mean (SD) | 49.9 (9.2) | 52.4 (8.5) | 48.7 (9.3) | 0.005 § |
| Body mass index, kg/m2, mean (SD) | 21.0 (3.2) | 21.7 (2.7) | 20.7 (3.3) | 0.019 § |
| Skeletal muscle mass index, kg/m2, mean (SD) | 5.3 (0.9) | 5.6 (0.8) | 5.1 (0.9) | <0.001 § |
| Hand-grip strength, kg, mean (SD) | 15.4 (6.3) | 17.7 (6.6) | 14.4 (5.9) | <0.001§ |
| Tube feeding, n (%) | 19 (8) | 7 (10) | 12 (8) | 0.603 ‡ |
| MUST, score, median [IQR] | 1 [0, 2] | 1 [0, 1] | 1 [0, 2] | 0.157 † |
| Energy intake, kcal/kg/day, mean (SD) | 27.5 (7.1) | 26.4 (6.5) | 28.0 (7.3) | 0.126 § |
| Protein intake, g/kg/day, mean (SD) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 0.329 § |
| Overall | Improvement in Sarcopenia | Non-Improvement in Sarcopenia | p-Value | |
|---|---|---|---|---|
| FIM, score, median [IQR] | ||||
| Total | 104 [ 76.5, 117] | 112 [91, 120] | 101 [73, 116] | 0.003 * |
| Motor domain | 77 [54, 85] | 81 [64, 87] | 73.5 [50, 84] | 0.009 * |
| Cognitive domain | 28 [21, 32] | 30 [26, 33] | 26 [20, 31] | 0.001 * |
| FIM gain, score, median [IQR] | ||||
| Total | 28 [18.5, 38.5] | 33 [26, 39] | 24.5 [17, 36] | 0.002 * |
| Motor domain | 23 [15, 33] | 28 [20, 33] | 21 [12.25, 32] | 0.002 * |
| Cognitive domain | 4 [2, 7] | 5 [2, 7] | 4 [2, 6.75] | 0.208 * |
| Length of stay, days, mean (SD) | 101.7 (43.3) | 105.7 (47.1) | 99.9 (41.5) | 0.351 ‡ |
| Discharge outcome, n (%) | 0.015 † | |||
| Home | 185 (82) | 63 (91) | 122 (77) | |
| Others | 42 (19) | 6 (8) | 36 (23) | |
| Dairy rehabilitation dose, min/day, median [IQR] | 176 [174, 178] | 176 [174, 178] | 176 [174, 178] | 0.634 * |
| Skeletal muscle mass index, kg/m2, mean (SD) | 5.5 (1.0) | 6.1 (0.9) | 5.3 (0.9) | <0.001 ‡ |
| Hand-grip strength, kg, mean (SD) | 17.5 (6.4) | 21.2 (6.6) | 15.9 (5.6) | <0.001 ‡ |
| B | 95% CI | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| FIM-total | 5.378 | 0.710 | 10.047 | 0.024 |
| FIM-motor | 3.587 | –0.194 | 7.368 | 0.063 |
| FIM-cognitive | 1.792 | 0.300 | 3.283 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsushita, T.; Nishioka, S.; Taguchi, S.; Yamanouchi, A.; Okazaki, Y.; Oishi, K.; Nakashima, R.; Fujii, T.; Tokunaga, Y.; Onizuka, S. Effect of Improvement in Sarcopenia on Functional and Discharge Outcomes in Stroke Rehabilitation Patients. Nutrients 2021, 13, 2192. https://doi.org/10.3390/nu13072192
Matsushita T, Nishioka S, Taguchi S, Yamanouchi A, Okazaki Y, Oishi K, Nakashima R, Fujii T, Tokunaga Y, Onizuka S. Effect of Improvement in Sarcopenia on Functional and Discharge Outcomes in Stroke Rehabilitation Patients. Nutrients. 2021; 13(7):2192. https://doi.org/10.3390/nu13072192
Chicago/Turabian StyleMatsushita, Tatsuya, Shinta Nishioka, Shiori Taguchi, Anna Yamanouchi, Yuka Okazaki, Kana Oishi, Ryusei Nakashima, Tatsuya Fujii, Yoshiharu Tokunaga, and Shinya Onizuka. 2021. "Effect of Improvement in Sarcopenia on Functional and Discharge Outcomes in Stroke Rehabilitation Patients" Nutrients 13, no. 7: 2192. https://doi.org/10.3390/nu13072192
APA StyleMatsushita, T., Nishioka, S., Taguchi, S., Yamanouchi, A., Okazaki, Y., Oishi, K., Nakashima, R., Fujii, T., Tokunaga, Y., & Onizuka, S. (2021). Effect of Improvement in Sarcopenia on Functional and Discharge Outcomes in Stroke Rehabilitation Patients. Nutrients, 13(7), 2192. https://doi.org/10.3390/nu13072192







