Effect of High Fat and Fructo-Oligosaccharide Consumption on Immunoglobulin A in Saliva and Salivary Glands in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics Statement
2.3. Diets
2.4. Sampling
2.5. Saliva Collection
2.6. Measurement of Immunoglobulin A (IgA) Concentration
2.7. Measurement of Serum Immunoglobulin G (IgG) Concentration
2.8. Measurement of Tyrosine Hydroxylase (TH) Concentration in Submandibular Glands (SMG)
2.9. pH in Cecal-Digesta Measurement
2.10. Gene Expression Analysis (Quantitative Real-Time Polymerase Chain Reaction (RT qPCR)) of Polymeric Immunoglobulin Receptor (pIgR) in the Submandibular Gland (SMG)
2.11. Submandibular Gland (SMG) and Liver Tissue Preparation for Hematoxylin and Eosin Staining
2.12. Statistical Analysis
3. Results
3.1. Rat Weight Gain and Energy, Diet, and Fat Intake
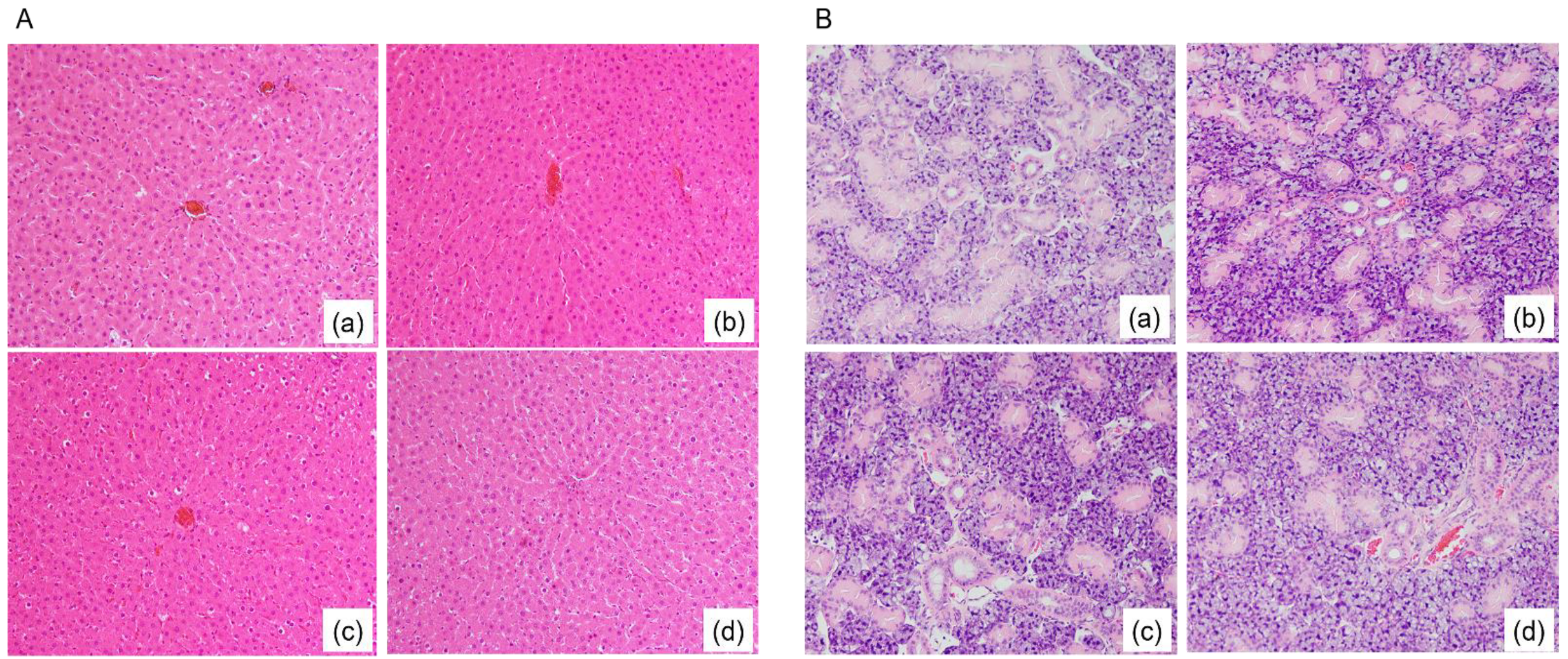
3.2. Fatty Degeneration in Rat Liver Tissue and Submandibular Gland (SMG) Tissue
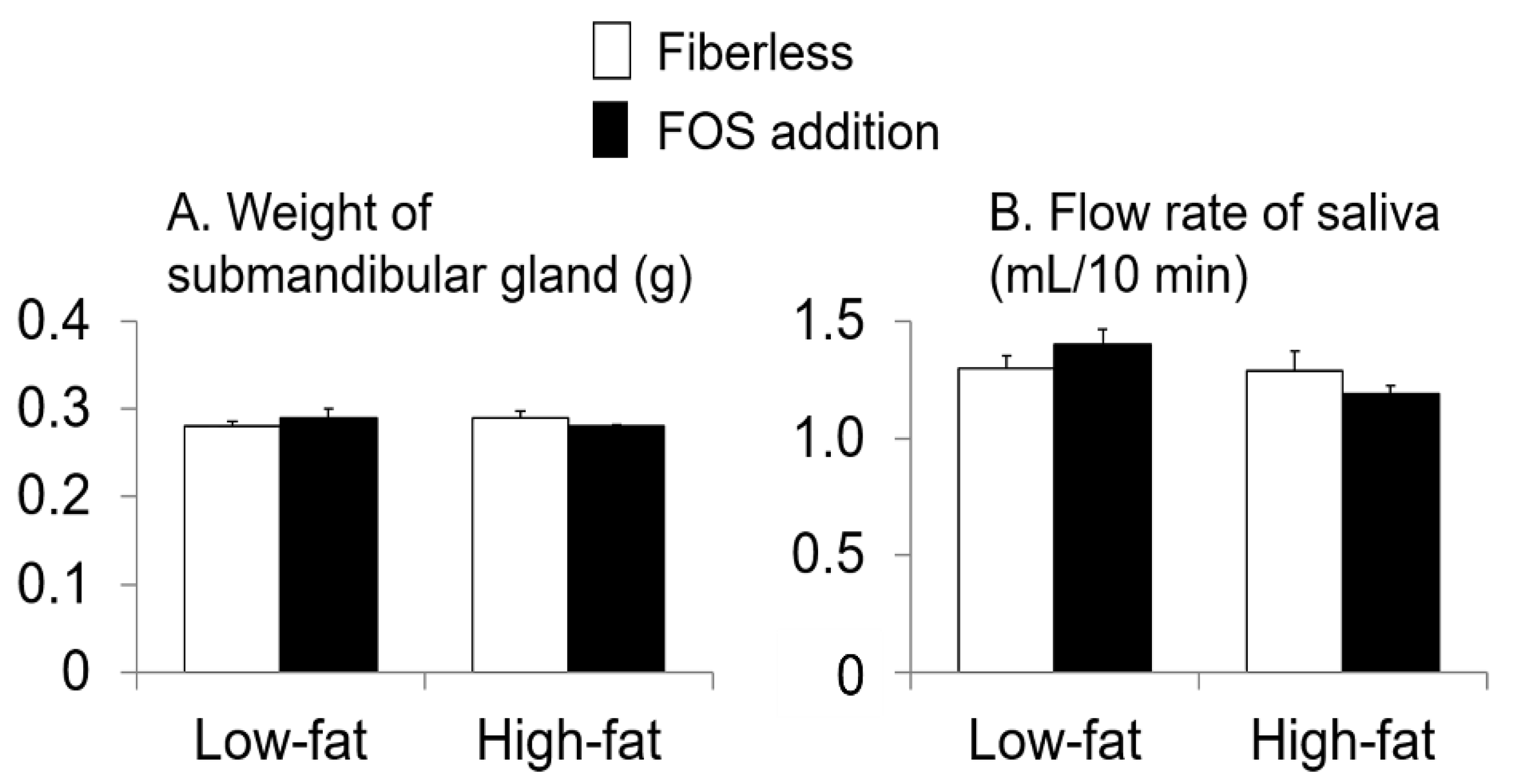
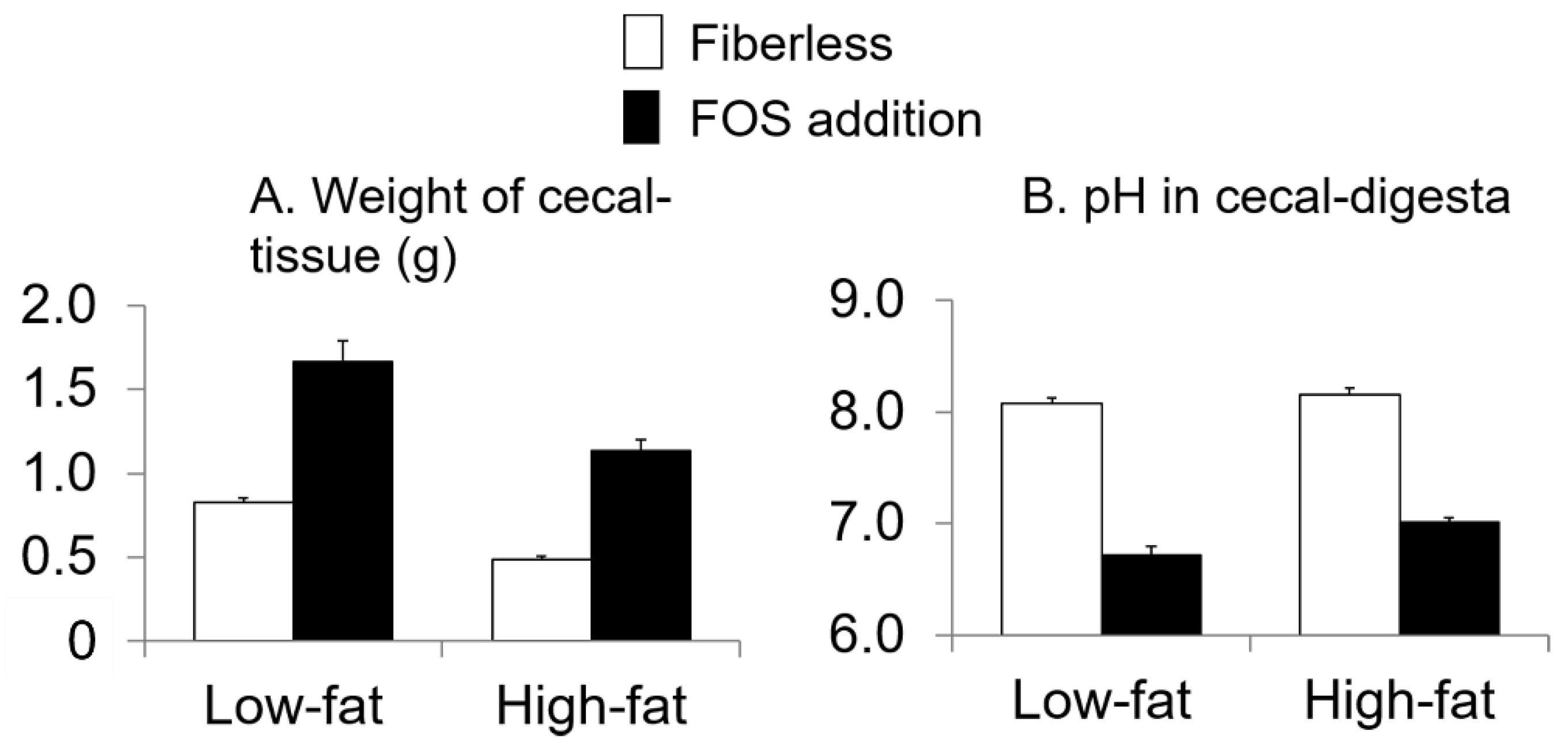
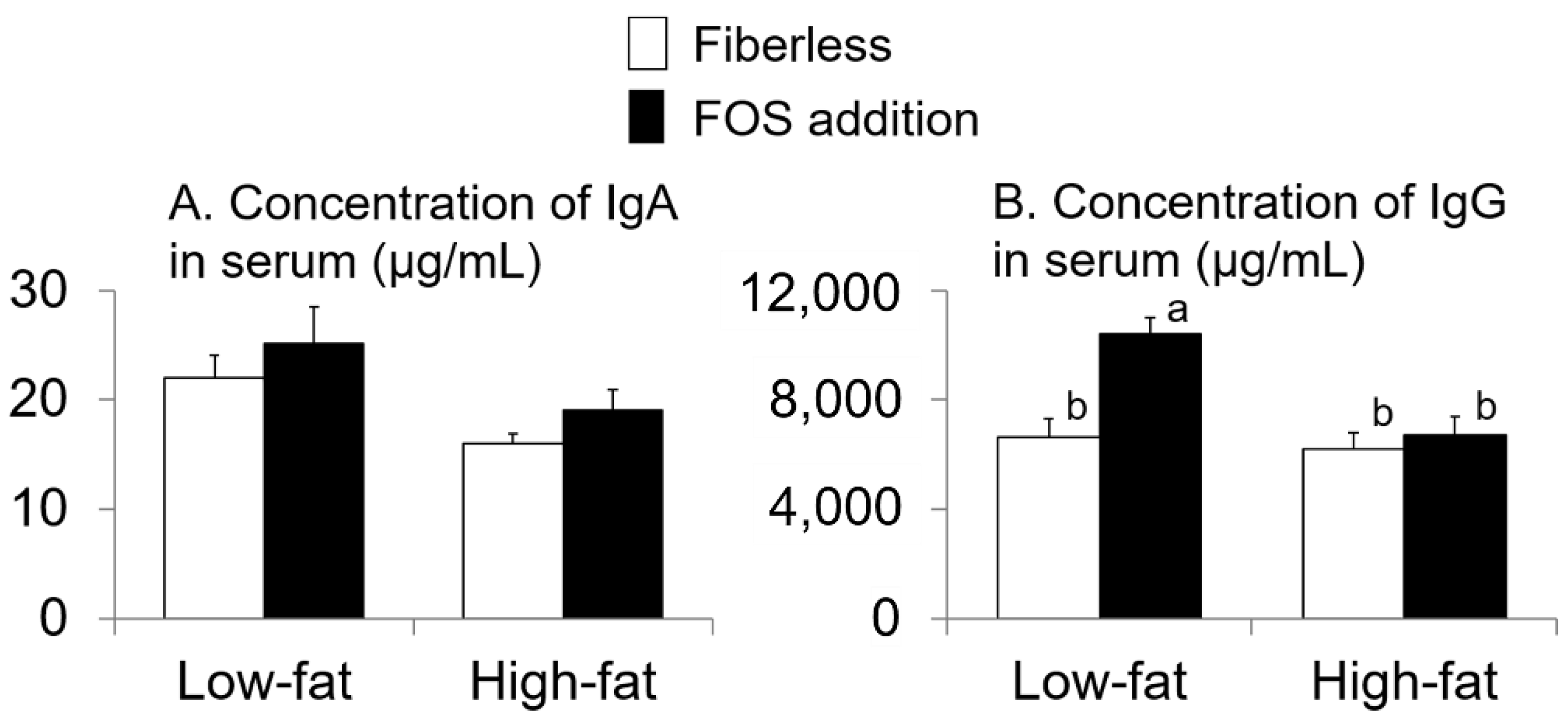
3.3. Effect of Fat Intake and Fructo-Oligosaccharide (FOS) Supplementation
3.4. Relationship with IgA Flow Rate of Saliva (IgA FR-saliva)
3.5. Relationship with Polymeric Immunoglobulin Receptor (pIgR) Expression Level in Submandibular Gland (SMG)
3.6. Relationship with Fat Intake per Day
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaffen, S.L.; Moutsopoulos, N.M. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci. Immunol. 2020, 5, eaau4594. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, S.M.; Edmisson, J.S.; Jimenez-Flores, E. Human neutrophils and oral microbiota: A constant tug-of-war between a harmonious and a discordant coexistence. Immunol. Rev. 2016, 273, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.W.; Ruhl, S. Glycan recognition at the saliva—Oral microbiome interface. Cell Immunol. 2018, 333, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugimoto, N.; Islam, R.; Hossain, M.E.; Sumiyoshi, E.; Katakura, M.; Shido, O. Salivary immunoglobulin A secretion and polymeric Ig receptor expression in the submandibular glands are enhanced in heat-acclimated rats. Int. J. Mol. Sci. 2020, 21, 815. [Google Scholar] [CrossRef]
- Mikami, Y.; Iwase, T.; Komiyama, Y.; Matsumoto, N.; Oki, H.; Komiyama, K. Secretory leukocyte protease inhibitor inhibits expression of polymeric immunoglobulin receptor via the NF-κB signaling pathway. Mol. Immunol. 2015, 67, 568–574. [Google Scholar] [CrossRef]
- Moreira, A.; Arsati, F.; de Oliveira Lima-Arsati, Y.B.; de Freitas, C.G.; de Araújo, V.C. Salivary immunoglobulin a responses in professional top-level futsal players. J. Strength Cond. Res. 2011, 25, 1932–1936. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Falsey, A.R. Parainfluenza virus infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554. [Google Scholar] [CrossRef]
- Ghilotti, F.; Pesonen, A.S.; Raposo, S.E.; Winell, H.; Nyrén, O.; Trolle Lagerros, Y.; Plymoth, A. Physical activity, sleep and risk of respiratory infections: A Swedish cohort study. PLoS ONE 2018, 13, e0190270. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H.; Segawa, A.; Garrett, J.R.; Ebersole, L. Constitutive secretion of immunoglobulin A and other proteins into lumina of unstimulated submandibular glands in anaesthetized rats. Exp. Physiol. 2003, 88, 7–12. [Google Scholar] [CrossRef]
- Yang, H.; Duan, Z. The local defender and functional mediator: Gut microbiome. Digestion 2018, 97, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.D.; Chen, F.Q.; Wu, T.X.; Tang, H.G.; Zhao, Z.Y. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B 2009, 10, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takahashi, T.; To, M.; Nakagawa, Y.; Hayashi, T.; Shimizu, T.; Kamata, Y.; Saruta, J.; Tsukinoki, K. The salivary IgA flow rate is increased by high concentrations of short-chain fatty acids in the cecum of rats ingesting fructooligosaccharides. Nutrients 2016, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Morozumi, T.; Takahashi, T.; Saruta, J.; To, M.; Sakaguchi, W.; Shimizu, T.; Kubota, N.; Tsukinoki, K. Faster short-chain fatty acid absorption from the cecum following polydextrose ingestion increases the salivary immunoglobulin A flow rate in rats. Nutrients 2020, 12, 1745. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Sumida, H.; Suzuki, M. Effects of chemical sympathectomy on lipoprotein lipase activities in peripheral tissues of rats fed high fat diets consisting of different fats. J. Nutr. Sci. Vitaminol. 1995, 41, 377–386. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, P.M.; Gavião, M.B.; Carpenter, G.H. Altered autophagy and sympathetic innervation in salivary glands from high-fat diet mice. Arch. Oral. Biol. 2017, 75, 107–113. [Google Scholar] [CrossRef]
- Birch, J.; Petty, R.; Hooper, L.; Bauld, L.; Rosenberg, G.; Vohra, J. Clustering of behavioural risk factors for health in UK adults in 2016: A cross-sectional survey. J. Public Health 2019, 41, e226–e236. [Google Scholar] [CrossRef]
- Nemati, M.; Zardooz, H.; Rostamkhani, F.; Abadi, A.; Foroughi, F. High-fat diet effects on metabolic responses to chronic stress. Arch. Physiol. Biochem. 2017, 123, 182–191. [Google Scholar] [CrossRef]
- Bedê, T.P.; de Jesus, V.; Rosse de Souza, V.; Mattoso, V.; Abreu, J.P.; Dias, J.F.; Teodoro, A.J.; de Azeredo, V.B. Effect of grape juice, red wine and resveratrol solution on antioxidant, anti-inflammatory, hepatic function and lipid profile in rats feds with high-fat diet. Nat. Prod. Res. 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Singh, R.B.; Demeester, F.; Wilczynska, A. The Tsim Tsoum approaches for prevention of cardiovascular disease. Cardiol. Res. Pract. 2010, 2010, 824938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, T.; Zhou, J.; Zhu, J.; Zhang, S.; Zhang, N.; Zhao, Y.; Ding, C.; Shi, X.; Yao, J. Carnosic acid protects non-alcoholic fatty liver-induced dopaminergic neuron injury in rats. Metab. Brain Dis. 2017, 32, 483–491. [Google Scholar] [CrossRef]
- Woods, M.N.; Gorbach, S.L.; Longcope, C.; Goldin, B.R.; Dwyer, J.T.; Morrill-LaBrode, A. Low-fat, high-fiber diet and serum estrone sulfate in premenopausal women. Am. J. Clin. Nutr. 1989, 49, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Tada, N.; Maruyama, C.; Koba, S.; Tanaka, H.; Birou, S.; Teramoto, T.; Sasaki, J. Japanese dietary lifestyle and cardiovascular disease. J. Atheroscler Thromb. 2011, 18, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Nankova, B.B.; Parab, S.; La Gamma, E.F. Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res. 2006, 1107, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; South, T.; Han, M.; Chen, J.; Wang, R.; Huang, X.F. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009, 1268, 181–189. [Google Scholar] [CrossRef]
- Shimomura, Y.; Tamura, T.; Suzuki, M. Less body fat accumulation in rats fed a safflower oil diet than in rats fed a beef tallow diet. J. Nutr. 1990, 120, 1291–1296. [Google Scholar] [CrossRef]
- Matsuo, T.; Suzuki, M. Brain beta-adrenergic receptor binding in rats with obesity induced by a beef tallow diet. Metabolism 1997, 46, 18–22. [Google Scholar] [CrossRef]
- Vissers, L.E.T.; Rijksen, J.; Boer, J.M.A.; Verschuren, W.M.M.; van der Schouw, Y.T.; Sluijs, I. Fatty acids from dairy and meat and their association with risk of coronary heart disease. Eur. J. Nutr. 2019, 58, 2639–2647. [Google Scholar] [CrossRef]
- Carpenter, G.H.; Garrett, J.R.; Hartley, R.H.; Proctor, G.B. The influence of nerves on the secretion of immunoglobulin A into submandibular saliva in rats. J. Physiol. 1998, 512, 567–573. [Google Scholar] [CrossRef]
- Yamamoto, Y.; To, M.; Hayashi, T.; Shimizu, T.; Kamata, Y.; Saruta, J.; Takahashi, T.; Tsukinoki, K. Intake of indigestible carbohydrates influences IgA response and polymeric Ig receptor expression in the rat submandibular gland. Br. J. Nutr. 2015, 113, 1895–1902. [Google Scholar] [CrossRef]
- Bellahcene, M.; O’Dowd, J.F.; Wargent, E.T.; Zaibi, M.S.; Hislop, D.C.; Ngala, R.A.; Smith, D.M.; Cawthorne, A.; Stocker, C.J.; Arch, J.R. Male mice lacking the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br. J. Nutr. 2013, 109, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H.; Anderson, L.C.; Garrett, J.R. Nerve-evoked secretion of immunoglobulin A in relation to other proteins by parotid glands in anaesthetized rat. Exp. Physiol. 2000, 85, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H.; Proctor, G.B.; Ebersole, L.E.; Garrett, J.R. Secretion of IgA by rat parotid and submandibular cells in response to autonomimetic stimulation in vitro. Int. Immunopharmacol. 2004, 4, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Ke, X.; Walker, A.; Haange, S.B.; Lagkouvardos, I.; Liu, Y.; Schmitt-Kopplin, P.; von Bergen, M.; Jehmlich, N.; He, X.; Clavel, T.; et al. Symbiotic-driven improvement of metabolic activity disturbances are associated with changes in the gut microbiome of diet-induced obese mice. Mol. Metab. 2019, 22, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Zhang, C.; Xu, Z.; Shen, J.; Du, X.; Zhang, H.; Zhang, K.; Zhang, D. Experimental study of the anti-atherosclerotic effect of demethylzeylasteral. Exp. Ther. Med. 2017, 13, 2787–2792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Csernus, B.; Czeglédi, L. Physiological, antimicrobial, intestine morphological, and immunological effects of fructooligosaccharides in pigs. Arch. Anim. Breed. 2020, 63, 325–335. [Google Scholar] [CrossRef]
- Kelley, D.S.; Daudu, P.A.; Branch, L.B.; Johnson, H.L.; Taylor, P.C.; Mackey, B. Energy restriction decreases number of circulating natural killer cells and serum levels of immunoglobulins in overweight women. Eur. J. Clin. Nutr. 1994, 48, 9–18. [Google Scholar]
- Fan, W.; Xu, Y.; Liu, Y.; Zhang, Z.; Lu, L.; Ding, Z. Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Front. Physiol. 2018, 8, 1117. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, S.; Green-Johnson, J.M.; Murray, L.; Wan, W.; Nance, D.M.; Greenberg, A.H. Interleukin-2-induced enhancement of an antigen-specific IgM plaque-forming cell response is mediated by the sympathetic nervous system. J. Pharmacol. Exp. Ther. 1994, 271, 977–982. [Google Scholar] [PubMed]
- Stoof, S.P.; van der Klis, F.R.; van Rooijen, D.M.; Bogaert, D.; Trzciński, K.; Sanders, E.A.; Berbers, G.A. Salivary antibody levels in adolescents in response to a meningococcal serogroup C conjugate booster vaccination nine years after priming: Systemically induced local immunity and saliva as potential surveillance tool. Vaccine 2015, 33, 3933–3939. [Google Scholar] [CrossRef] [PubMed]


| LFFL ‡ | LFFOS § | HFFL * | HFFOS # | p† | |
|---|---|---|---|---|---|
| Weight gain (g/70 days) | 178 ± 6 | 176 ± 6 | 181 ± 6 | 179 ± 6 | 0.9 |
| Diet intake (g/day) | 17.5 ± 0.1 a | 17.7 ± 0.1 a | 14.3 ± 0.0 b | 14.6 ± 0.1 b | <0.0001 |
| Energy intake (kJ/day) | 292 ± 1 | 292 ± 2 | 293 ± 1 | 294 ± 1 | 0.6 |
| Fat intake (g/day) | 1.05 ± 0.01 a | 1.06 ± 0.01 a | 3.15 ± 0.01 b | 3.18 ± 0.01 b | <0.0001 |
| FOS intake (g/day) | 0 a | 0.531 ± 0.004 b | 0 a | 0.438 ± 0.002 b | <0.0001 |
| IgA FR-Saliva per Weight of SMG | |||
|---|---|---|---|
| r * | p | n | |
| Weight of cecal-tissue | 0.66 | <0.0001 | 32 |
| TH concentration in SMG | 0.61 | 0.0003 | 32 |
| pIgR expression level in SMG | 0.61 | 0.0003 | 32 |
| Concentration of IgG in serum | 0.54 | 0.002 | 32 |
| Weight of cecal-digesta | 0.52 | 0.002 | 32 |
| Concentration of IgA in serum | 0.38 | 0.03 | 32 |
| pH in cecal-digesta | −0.59 | 0.0005 | 32 |
| IgA concentration in cecal-digesta | 0.16 | 0.4 | 32 |
| pIgR Expression Level in SMG | |||
|---|---|---|---|
| r * | p | n | |
| TH concentration in SMG | 0.71 | <0.0001 | 32 |
| Weight of cecal-tissue | 0.70 | <0.0001 | 32 |
| Weight of cecal-digesta | 0.65 | <0.0001 | 32 |
| Concentration of IgG in serum | 0.53 | 0.002 | 32 |
| Concentration of IgA in serum | 0.44 | 0.01 | 32 |
| pH in cecal-digesta | −0.70 | <0.0001 | 32 |
| IgA concentration in cecal-digesta | 0.19 | 0.3 | 32 |
| Fat Intake per Day | |||
|---|---|---|---|
| r * | p | n | |
| Concentration of IgA in serum | −0.47 | 0.008 | 32 |
| Concentration of IgG in serum | −0.47 | 0.008 | 32 |
| TH concentration in SMG | −0.41 | 0.02 | 32 |
| Weight of cecal-tissue | −0.27 | 0.1 | 32 |
| IgA FR-saliva per weight of SMG | −0.27 | 0.1 | 32 |
| pIgR expression level in SMG | −0.23 | 0.2 | 32 |
| pH in cecal-digesta | −0.17 | 0.4 | 32 |
| Weight of cecal-digesta | −0.12 | 0.5 | 32 |
| IgA concentration in cecal-digesta | 0.11 | 0.6 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, Y.; Morozumi, T.; Takahashi, T.; Saruta, J.; Sakaguchi, W.; To, M.; Kubota, N.; Shimizu, T.; Kamata, Y.; Kawata, A.; et al. Effect of High Fat and Fructo-Oligosaccharide Consumption on Immunoglobulin A in Saliva and Salivary Glands in Rats. Nutrients 2021, 13, 1252. https://doi.org/10.3390/nu13041252
Yamamoto Y, Morozumi T, Takahashi T, Saruta J, Sakaguchi W, To M, Kubota N, Shimizu T, Kamata Y, Kawata A, et al. Effect of High Fat and Fructo-Oligosaccharide Consumption on Immunoglobulin A in Saliva and Salivary Glands in Rats. Nutrients. 2021; 13(4):1252. https://doi.org/10.3390/nu13041252
Chicago/Turabian StyleYamamoto, Yuko, Toshiya Morozumi, Toru Takahashi, Juri Saruta, Wakako Sakaguchi, Masahiro To, Nobuhisa Kubota, Tomoko Shimizu, Yohei Kamata, Akira Kawata, and et al. 2021. "Effect of High Fat and Fructo-Oligosaccharide Consumption on Immunoglobulin A in Saliva and Salivary Glands in Rats" Nutrients 13, no. 4: 1252. https://doi.org/10.3390/nu13041252
APA StyleYamamoto, Y., Morozumi, T., Takahashi, T., Saruta, J., Sakaguchi, W., To, M., Kubota, N., Shimizu, T., Kamata, Y., Kawata, A., & Tsukinoki, K. (2021). Effect of High Fat and Fructo-Oligosaccharide Consumption on Immunoglobulin A in Saliva and Salivary Glands in Rats. Nutrients, 13(4), 1252. https://doi.org/10.3390/nu13041252








