Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Pathophysiology of Cardiovascular Disease and HDL
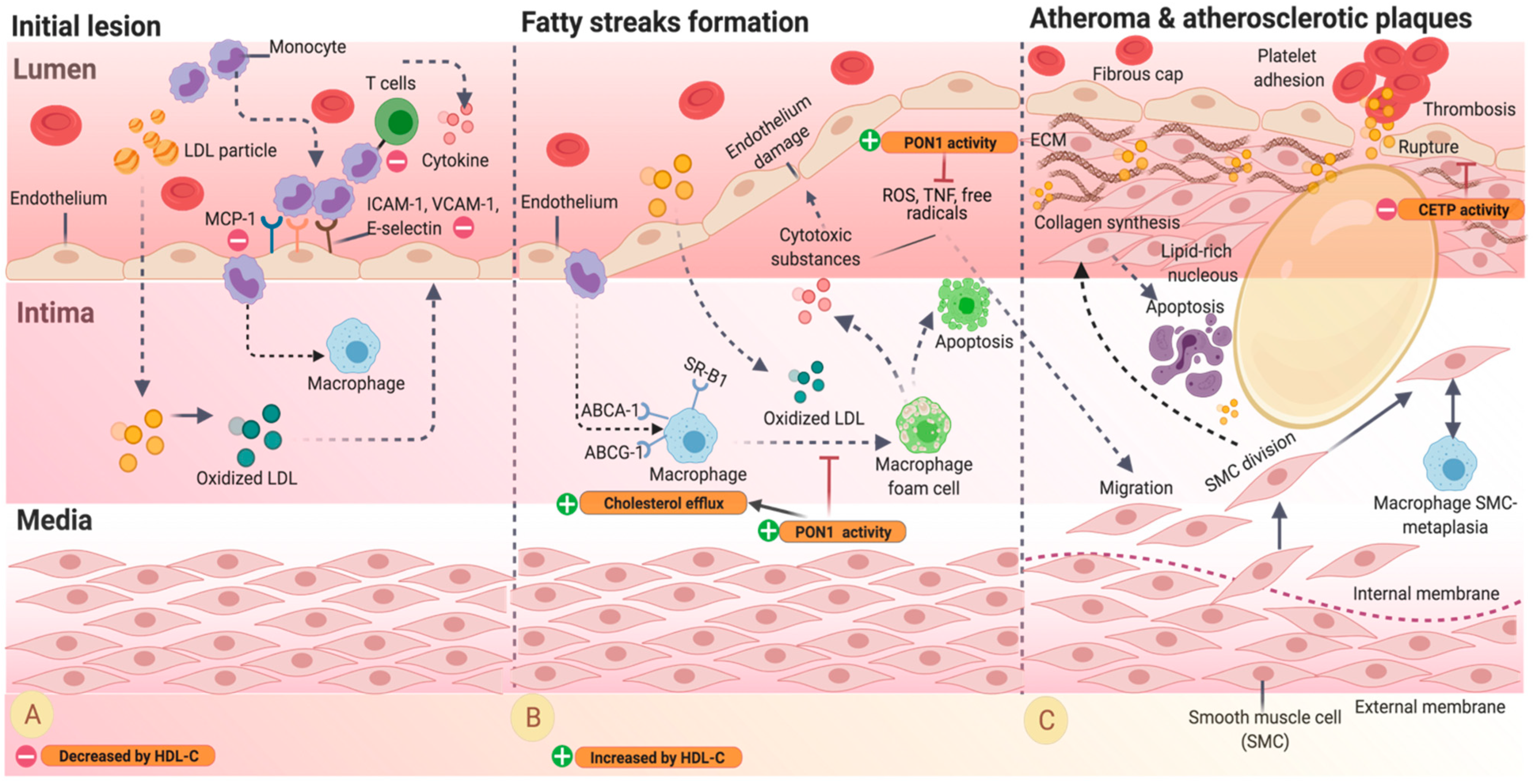
4. HDL-C Physiology and Pathophysiology
4.1. Factors That Impact the Function of HDL
4.2. Effect of Inflammation on HDL
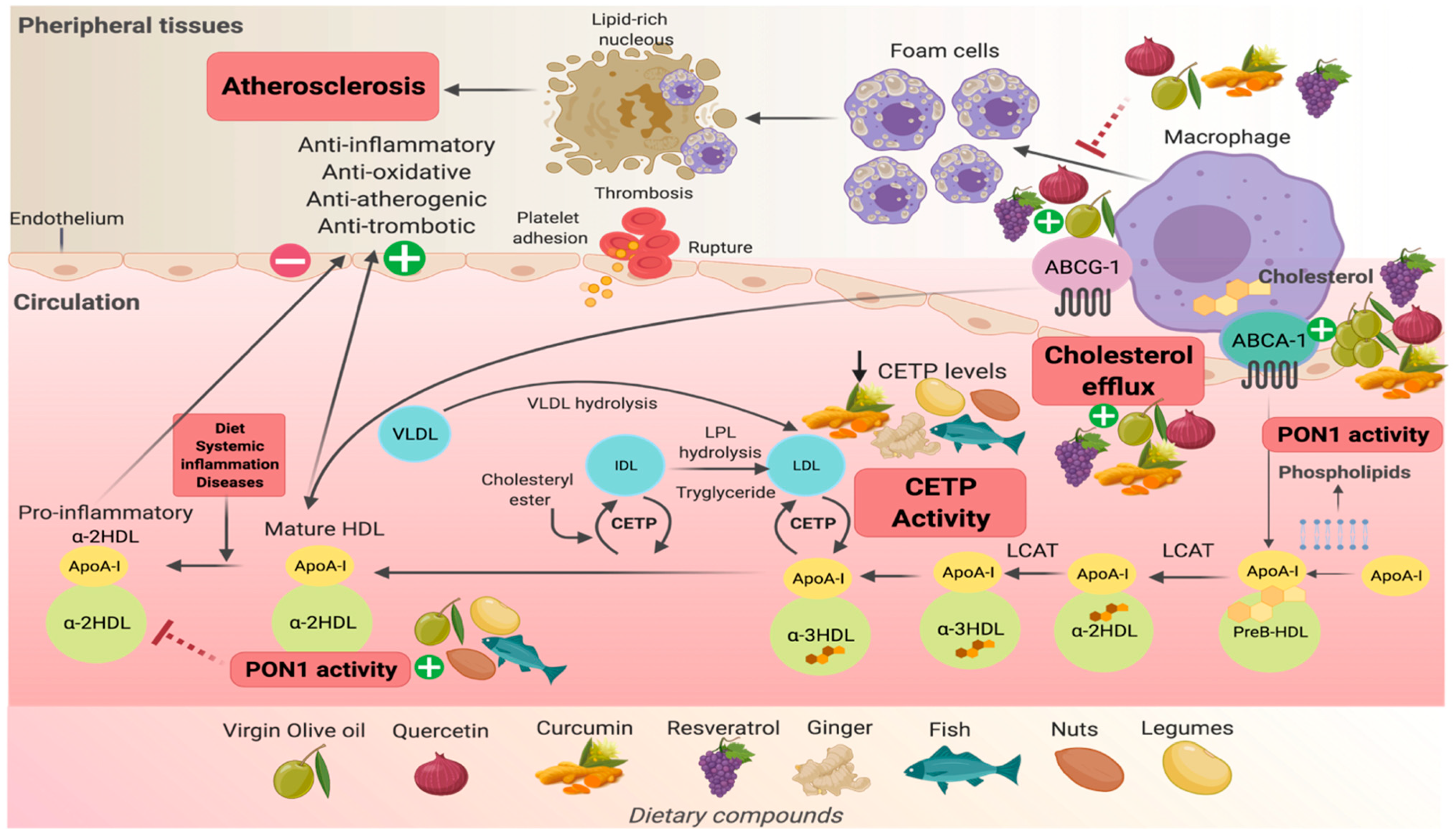
5. Dietary Compounds and Their Effects on the Modulation of HDL-C Functionality
5.1. Cholesterol Efflux Capacity
5.1.1. Extra Virgin Olive Oil
5.1.2. Resveratrol
5.1.3. Nuts
5.1.4. Legumes and Fish
5.1.5. Fruits
5.1.6. Green Tea, Cocoa, and Red Wine
5.1.7. Curcumin
5.1.8. Quercetin
5.2. Activity of Cholesteryl Ester Transfer Protein (CETP)
5.2.1. Extra Virgin Olive Oil
5.2.2. Legumes and Fresh Fish
5.2.3. Curcumin and Ginger
5.3. Antioxidant Capacity: PON1 Activity/Expression
5.3.1. Olive Oil
5.3.2. Fruits, Vegetables, and Resveratrol
5.3.3. Nuts, Fish, and Legumes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA-1 | ATP-binding cassette transporter A member 1 |
| ABCG-1 | ATP-binding cassette transporter G member 1 |
| ABCG-4 | ATP-binding cassette transporter G member 4 |
| ACC/AHA | American College of Cardiology/American Heart Association |
| ApoA-I | Apolipoprotein A-I |
| Caco-2 | Human colon carcinoma cell line |
| CE | Cholesterol efflux |
| CEC | Cholesterol efflux capacity |
| CETP | Cholesteryl ester transfer protein |
| CMCNa | Carboxymethyl cellulose sodium |
| CVD | Cardiovascular diseases |
| DHA | Docosahexaenoic acid |
| EC | Epicatechin |
| ECG | Epicatechin-gallate |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin-gallate |
| EPA | Eicosapentaenoic acid |
| EVOO | Extra virgin olive oil |
| Fu5AH | Macrophages and rat hepatoma cell |
| FVOO | Functional virgin olive oil |
| H-FVOO | High- functional olive oil |
| HAEC | Human aortic endothelial cells |
| HDL-C | High-density lipoprotein cholesterol |
| HMDM | Human monocyte-derived macrophage |
| HPCOO | High polyphenol compound olive oil |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IDL-C | Intermediate-density lipoprotein cholesterol |
| IL-1 | Interleukin 1 |
| IL-8 | Interleukin 8 |
| L-FVOO | Low-functional olive oil |
| LCAT | Lecithin cholesterol acyltransferase |
| LDL-C | Low-density lipoprotein cholesterol |
| LPCOO | Low polyphenol compound olive oil |
| LPS | Lipopolysaccharides |
| LXR | Liver X receptor |
| LXR-α | Liver X receptor alfa |
| M-CSF | Macrophage colony-stimulating factor |
| M-FVOO | Medium-functional olive oil |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MD | Mediterranean diet |
| MPO | Myeloperoxidase |
| MUFAs | Monounsaturated fatty acids |
| NFk- β | Factor kappa β |
| NO | Nitric oxide |
| OO-PC | Olive Oil-Phenolic compounds |
| ox-LDL | Oxidized LDL |
| PAF-AH | Platelet-activating factor-acetylhydrolase |
| PBMC | Human peripheral blood mononuclear cell |
| PC | Phenolic compounds |
| PON1 | Paraoxonase 1 |
| PPARs | Peroxisome proliferation-activated receptors |
| PREDIMED | PREvención con DIeta MEDiterránea |
| PUFAs | Polyunsaturated fatty acids |
| RCT | Reverse cholesterol transport |
| ROS | Reactive oxygen species |
| SAA | Serum amyloid A |
| SEC + THY | Secoiridoid and thyme extracts |
| SFA | Saturated fatty acids |
| SMC | Smooth muscle cells |
| sPLA2-IIA | Secretory phospholipase A2 |
| SR-B1 | Scavenger receptor class B type 1 |
| TGE | Total ginger extract |
| THY | Thyme phenol content extract |
| TLR | Toll-like receptors |
| TMD | Traditional Mediterranean diet |
| TNF | Tumor necrosis factor |
| TPH-1 | Human acute monocyte leukemia cells line |
| VCAM-1 | Vascular adhesion molecule-1 |
| VD | Vegetarian diet |
| VLDL-C | Very low-density lipoprotein cholesterol |
| VOHF | Virgin olive oil and HDL functionality |
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, E139–E596. [Google Scholar] [CrossRef]
- GBD Compare. Institute for Health Metrics and Evaluation (IHME). Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 4 January 2021).
- LatinComm, S.A. Manual para la Reducción del Riesgo Cardiovascular: Basado en las Guías del National Cholesterol Educaction Program Adult Treatment Panel III (NCEP-ATPIII); LatinComm: Buenos Aires, Argentina, 2011; pp. 2–28. [Google Scholar]
- Rye, K.A.; Ong, K.L. HDL Function as a Predictor of Coronary Heart Disease Events: Time to Re-Assess the HDL Hypothesis? Lancet Diabetes Endocrinol. 2015, 3, 488–489. [Google Scholar] [CrossRef]
- Mahmooda, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the Epidemiology of Cardiovascular Diseases: A Historical Perspective. Lancet 2014, 383, 1933–1945. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport: Basic Mechanisms and Their Roles in Vascular Health and Disease. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhao, X.; Zhou, Q.; Zhang, Z. High-Density Lipoprotein Cholesterol Efflux Capacity Is Inversely Associated with Cardiovascular Risk: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.D.; Fryar, C.D.; Nguyen, D.T. Total and High-Density Lipoprotein Cholesterol in Adults: United States, 2015–2018. NCHS Data Brief. 2020, 363, 1–8. [Google Scholar]
- Halcox, J.P.; Banegas, J.R.; Roy, C.; Dallongeville, J.; De Backer, G.; Guallar, E.; Perk, J.; Hajage, D.; Henriksson, K.M.; Borghi, C. Prevalence and Treatment of Atherogenic Dyslipidemia in the Primary Prevention of Cardiovascular Disease in Europe: EURIKA, a Cross-Sectional Observational Study. BMC Cardiovasc. Disord. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, Q.; Wang, L.; Huang, Z.; Zhou, M.; Li, Y.; Zhao, Z.; Zhang, Y.; Wang, L. Prevalence of Dyslipidemia and Achievement of Low-Density Lipoprotein Cholesterol Targets in Chinese Adults: A Nationally Representative Survey of 163,641 Adults. Int. J. Cardiol. 2018, 260, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Bigna, J.J.; Nansseu, J.R.; Nyaga, U.F.; Balti, E.V.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Prevalence of Dyslipidaemia among Adults in Africa: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2018, 6, e998–e1007. [Google Scholar] [CrossRef]
- Vizmanos, B.; Betancourt-Nuñez, A.; Márquez-Sandoval, F.; González-Zapata, L.I.; Monsalve-Álvarez, J.; Bressan, J.; De Carvalho Vidigal, F.; Figueredo, R.; López, L.B.; Babio, N.; et al. Metabolic Syndrome among Young Health Professionals in the Multicenter Latin America Metabolic Syndrome Study. Metab. Syndr. Relat. Disord. 2020, 18, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-Density Lipoprotein (HDL) Functionality and Its Relevance to Atherosclerotic Cardiovascular Disease. Drugs Context 2018, 7, 1–9. [Google Scholar] [CrossRef]
- O’Neill, F.; Riwanto, M.; Charakida, M.; Colin, S.; Manz, J.; McLoughlin, E.; Khan, T.; Klein, N.; Kay, C.W.M.; Patel, K.; et al. Structural and Functional Changes in HDL with Low Grade and Chronic Inflammation. Int. J. Cardiol. 2015, 188, 111–116. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Translation of High-Density Lipoprotein Function into Clinical Practice: Current Prospects and Future Challenges. Circulation 2013, 128, 1256–1267. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Hu, J.; Xi, D.; Zhao, J.; Luo, T.; Liu, J.; Lu, H.; Li, M.; Xiong, H.; Guo, Z. High-Density Lipoprotein and Inflammation and Its Significance to Atherosclerosis. Am. J. Med. Sci. 2016, 352, 408–415. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Functionally Defective High-Density Lipoprotein: A New Therapeutic Target at the Crossroads of Dyslipidemia, Inflammation, and Atherosclerosis. Pharmacol. Rev. 2006, 58, 342–374. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Reddy, S.T.; Navab, M.; Fogelman, A.M. Understanding Changes in High Density Lipoproteins during the Acute Phase Response. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1687–1688. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Thorpe, S.R.; Fu, M.X.; Harper, C.M.; Yoo, J.; Kim, S.M.; Wong, H.; Peters, A.L. Glycation Impairs High-Density Lipoprotein Function. Diabetologia 2000, 43, 312–320. [Google Scholar] [CrossRef]
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between Ethnicity and Obesity with High-Density Lipoprotein (HDL) Function and Subclass Distribution. Lipids Health Dis. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lundman, P.; Harmer, J.A.; Cutri, B.; Griffiths, K.A.; Rye, K.A.; Barter, P.J.; Celermajer, D.S. Consumption of Saturated Fat Impairs the Anti-Inflammatory Properties of High-Density Lipoproteins and Endothelial Function. J. Am. Coll. Cardiol. 2006, 48, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Olivar, L.C.; Ramos, E.; Chávez-Castillo, M.; Rojas, J.; Bermúdez, V. Dysfunctional High-Density Lipoprotein: An Innovative Target for Proteomics and Lipidomics. Cholesterol 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Blazek, A.; Rutsky, J.; Osei, K.; Maiseyeu, A.; Rajagopalan, S. Exercise-Mediated Changes in High-Density Lipoprotein: Impact on Form and Function. Am. Heart J. 2013, 166, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; Mccartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the Amount and Intensity of Exercise on Plasma Lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-Medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Bardagjy, A.S.; Steinberg, F.M. Relationship between HDL Functional Characteristics and Cardiovascular Health and Potential Impact of Dietary Patterns: A Narrative Review. Nutrients 2019, 11, 1231. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W. Effects of Diet on High-Density Lipoprotein Cholesterol. Curr. Atheroscler. Rep. 2011, 13, 453–460. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, 596–646. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Raygor, V.; Khera, A. New Recommendations and Revised Concepts in Recent Guidelines on the Management of Dyslipidemias to Prevent Cardiovascular Disease: The 2018 ACC/AHA and 2019 ESC/EAS Guidelines. Curr. Cardiol. Rep. 2020, 22. [Google Scholar] [CrossRef]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The Interplay between Lipids and Immune Cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Bergheanu, S.C.; Bodde, M.C.; Jukema, J.W. Pathophysiology and Treatment of Atherosclerosis: Current View and Future Perspective on Lipoprotein Modification Treatment. Neth. Heart J. 2017, 25, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Olvera-López, E. Cardiovascular Disease. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535419/ (accessed on 20 May 2020).
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M. Atherosclerosis: Process, Indicators, Risk Factors and New Hope. Int. J. Med. 2014, 5, 927–946. [Google Scholar]
- Mahan, L.K.; Escott-Stump, S.; Raymond, J. Krause Dietoterapia, 14th ed.; Elsevier: Barcelona, Spain, 2017. [Google Scholar]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Ramji, D.P. Nutraceutical Therapies for Atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Lian, Z.; Di, Y.; Zhang, L.; Shahid Riaz Rajoka, M.; Zhang, Y.; Kong, J.; Jiang, C.; Shi, J. Dietary Compounds Have Potential in Controlling Atherosclerosis by Modulating Macrophage Cholesterol Metabolism and Inflammation via MiRNA. NPJ Sci. Food 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Shao, B.; Tang, C.; Sinha, A.; Mayer, P.S.; Davenport, G.D.; Brot, N.; Oda, M.N.; Zhao, X.-Q.; Heineck, J.W. Humans with Atherosclerosis Have Impaired ABCA1 Cholesterol Efflux and Enchanced HDL Oxidation by Myeloperoxidase. Circ. Res. 2014, 23, 1733–1742. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano-Alberico, L.; Norata-Giuseppe, D. Biologial Consequences of Dysfuncional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef]
- Iqbal, F.; Baker, W.S.; Khan, M.I.; Thukuntla, S.; McKinney, K.H.; Abate, N.; Tuvdendorj, D. Current and Future Therapies for Addressing the Effects of Inflammation on HDL Cholesterol Metabolism. Br. J. Pharmacol. 2017, 174, 3986–4006. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A. Anti-Oxidant Properties of High-Density Lipoprotein and Atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2010, 37, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, R.; Mu, H.; Wang, X.; Yao, Q.; Chen, C. Reverse Cholesterol Transport and Cholesterol Efflux in Atherosclerosis. QJM Mon. J. Assoc. Physicians 2005, 98, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL Functionality in Reverse Cholesterol Transport—Challenges in Translating Data Emerging from Mouse Models to Human Disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 566–583. [Google Scholar] [CrossRef]
- Joy, T.; Hegele, R.A. Is Raising HDL a Futile Strategy for Atheroprotection? Nat. Rev. Drug Discov. 2008, 7, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Yokoyama, S. ABCA1 and Biogenesis of HDL. J. Atheroscler. Thromb. 2006, 13, 1–15. [Google Scholar] [CrossRef]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and Antithrombotic Actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, O.; Shih, D.M.; Aviram, M. Paraoxonase 1 (PON1) Attenuates Macrophage Oxidative Status: Studies in PON1 Transfected Cells and in PON1 Transgenic Mice. Atherosclerosis 2005, 181, 9–18. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef]
- Ahmed, Z.; Ravandi, A.; Maguire, G.F.; Emili, A.; Draganov, D.; La Du, B.N.; Kuksis, A.; Connelly, P.W. Apolipoprotein A-I Promotes the Formation of Phosphatidylcholine Core Aldehydes That Are Hydrolyzed by Paraoxonase (PON-1) during High Density Lipoprotein Oxidation with a Peroxvnitrite Donor. J. Biol. Chem. 2001, 276, 24473–24481. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.W.; Rye, K.A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. Ability of Reconstituted High Density Lipoproteins to Inhibit Cytokine- Induced Expression of Vascular Cell Adhesion Molecule-1 in Human Umbilical Vein Endothelial Cells. J. Lipid Res. 1999, 40, 345–353. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Ortiz-Rodríguez, M.A.; Medina-Briseño, L.; Carreon-Torres, E.; Izquierdo-Vega, J.A.; Sharma, A.; Cancino-Díaz, J.C.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules 2018, 23, 2730. [Google Scholar] [CrossRef] [PubMed]
- Hyka, N.; Dayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K.; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I Inhibits the Production of Interleukin-1β and Tumor Necrosis Factor-α by Blocking Contact-Mediated Activation of Monocytes by T Lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef]
- Chyu, K.Y.; Shah, P.K. HDL/ApoA-1 Infusion and ApoA-1 Gene Therapy in Atherosclerosis. Front. Pharmacol. 2015, 6, 187. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Tellis, C.C.; Lekakis, J.; Rizos, I.; Varounis, C.; Charalampopoulos, A.; Zolindaki, M.; Dagres, N.; Anastasiou-Nana, M.; Tselepis, A.D. Lipoprotein-Associated Phospholipase A2 Bound on High-Density Lipoprotein Is Associated with Lower Risk for Cardiac Death in Stable Coronary Artery Disease Patients: A 3-Year Follow-Up. J. Am. Coll. Cardiol. 2012, 60, 2053–2060. [Google Scholar] [CrossRef]
- Huang, F.; Wang, K.; Shen, J. Lipoprotein-Associated Phospholipase A2: The Story Continues. Med. Res. Rev. 2020, 40, 79–134. [Google Scholar] [CrossRef]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.; Davidson, J.W.S. High Density Lipoproteins: From Biological Understanding to Clinical Exploitation; Eckardstein, A., von Kardassis, D., Eds.; Springer: Paris, France, 2015; Volume 224, ISBN 9783319096650. [Google Scholar]
- Murphy, A.J. High Density Lipoprotein: Assembly, Structure, Cargo, and Functions. ISRN Physiol. 2013, 2013, 186365. [Google Scholar] [CrossRef]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The Role and Function of HDL in Patients with Diabetes Mellitus and the Related Cardiovascular Risk. Lipids Health Dis. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Khera, A.V.; Rader, D.J. Future Therapeutic Directions in Reverse Cholesterol Transport. Curr. Atheroscler. Rep. 2010, 12, 73–81. [Google Scholar] [CrossRef]
- Ng, D.S.; Wong, N.C.W.; Hegele, R.A. HDL-Is It Too Big to Fail? Nat. Rev. Endocrinol. 2013, 9, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, L.; Carson, J.A.S.; Appel, L.; Burke, L.E.; Economos, C.; Karmally, W. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e534. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Espín, J.; Nilsson, O.; Bernfur, K.; Del Giudice, R.; Lagerstedt, J.O. Site-Specific Glycations of Apolipoprotein A-I Lead to Differentiated Functional Effects on Lipid-Binding and on Glucose Metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2822–2834. [Google Scholar] [CrossRef] [PubMed]
- Nobecourt, E.; Davies, M.J.; Brown, B.E.; Curtiss, L.K.; Bonnet, D.J.; Charlton, F.; Januszewski, A.S.; Jenkins, A.J.; Barter, P.J.; Rye, K.A. The Impact of Glycation on Apolipoprotein A-I Structure and Its Ability to Activate Lecithin:Cholesterol Acyltransferase. Diabetologia 2007, 50, 643–653. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Dyslipidemia and Inflammation: An Evolutionary Conserved Mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- De la Llera Moya, M.; McGillicuddy, F.C.; Hinkle, C.C.; Byrne, M.; Joshi, M.R.; Nguyen, V.; Tabita-Martinez, J.; Wolfe, M.L.; Badellino, K.; Pruscino, L.; et al. Inflammation Modulates Human HDL Composition and Function in Vivo. Atherosclerosis 2012, 222, 390–394. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Montecucco, F.; Favari, E.; Norata, G.; Ronda, N. Impact of Systemic Inflammation and Autoinmmune Diseases on ApoA-I and HDL Plasma Levels and Functions. Handb. Exp. Pharmacol. 2015, 224, 455–482. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. J. Am. Med. Assoc. 2004, 292, 1440–1446. [Google Scholar] [CrossRef]
- Sun, L.; Li, E.; Wang, F.; Wang, T.; Qin, Z.; Niu, S.; Qiu, C. Quercetin Increases Macrophage Cholesterol Efflux to Inhibit Foam Cell Formation through Activating PPARγ-ABCA1 Pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 10854–10860. [Google Scholar]
- Cui, Y.; Hou, P.; Li, F.; Liu, Q.; Qin, S.; Zhou, G.; Xu, X.; Si, Y.; Guo, S. Quercetin Improves Macrophage Reverse Cholesterol Transport in Apolipoprotein E-Deficient Mice Fed a High-Fat Diet. Lipids Health Dis. 2017, 16, 3–9. [Google Scholar] [CrossRef]
- Helal, O.; Berrougui, H.; Loued, S.; Khalil, A. Extra-Virgin Olive Oil Consumption Improves the Capacity of HDL to Mediate Cholesterol Efflux and Increases ABCA1 and ABCG1 Expression in Human Macrophages. Br. J. Nutr. 2013, 109, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Hai, O.; Littlefield, M.J.; Carsons, S.; Reiss, A.B. Resveratrol Mediates Anti-Atherogenic Effects on Cholesterol Flux in Human Macrophages and Endothelium via PPARγ and Adenosine. Eur. J. Pharmacol. 2013, 698, 299–309. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, J.; Fan, Z.; Li, J. Curcumin Increases Cholesterol Efflux via Hemeoxygenase-1-Mediated ABCA1 and SR-BI Expression in Macrophages. Mol. Med. Rep. 2018, 17, 6138–6143. [Google Scholar] [CrossRef] [PubMed]
- Elseweidy, M.M.; Younis, N.N.; Elswefy, S.E.; Abdallah, F.R.; El-Dahmy, S.I.; Elnagar, G.; Kassem, H.M. Atheroprotective Potentials of Curcuminoids against Ginger Extract in Hypercholesterolaemic Rabbits. Nat. Prod. Res. 2015, 29, 961–965. [Google Scholar] [CrossRef]
- Hernáez, Á.; Sanllorente, A.; Castañer, O.; Martínez-González, M.Á.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Alonso-Gómez, Á.M. Increased Consumption of Virgin Olive Oil, Nuts, Legumes, Whole Grains, and Fish Promotes HDL Functions in Humans. Mol. Nutr. Food Res. 2019, 63, 1800847. [Google Scholar] [CrossRef] [PubMed]
- Chowaniec, Z.; Skoczyńska, A. Plasma Lipid Transfer Proteins: The Role of PLTP and CETP in Atherogenesis. Adv. Clin. Exp. Med. 2018, 27, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castillejo, S.; Rubio, L.; Hernáez, Á.; Catalán, Ú.; Pedret, A.; Valls, R.-M.; Mosele, J.I.; Covas, M.I.; Reamley, A.T.; Castañer, O.; et al. Determinants of HDL Cholesterol Efflux Capacity after Virgin Olive Oil Ingestion- Interrelationships with Fluidity of HDL Monolayer. Mol. Nutr. Food Res. 2017, 61, 1700445. [Google Scholar] [CrossRef]
- Farràs, M.; Fernández-Castillejo, S.; Rubió, L.; Arranz, S.; Catalán, Ú.; Subirana, I.; Romero, M.P.; Castañer, O.; Pedret, A.; Blanchart, G.; et al. Phenol-Enriched Olive Oils Improve HDL Antioxidant Content in Hypercholesterolemic Subjects. A Randomized, Double-Blind, Cross-over, Controlled Trial. J. Nutr. Biochem. 2018, 51, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castillejo, S.; García-Heredia, A.I.; Solà, R.; Camps, J.; López de la Hazas, M.C.; Farràs, M.; Pedret, A.; Catalán, Ú.; Rubió, L.; Motilva, M.J.; et al. Phenol-Enriched Olive Oils Modify Paraoxonase-Related Variables: A Randomized, Crossover, Controlled Trial. Mol. Nutr. Food Res. 2017, 61, 1–39. [Google Scholar] [CrossRef]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute Consumption of Walnuts and Walnut Components Differentially Affect Postprandial Lipemia, Endothelial Function, Oxidative Stress, and Cholesterol Efflux in Humans with Mild Hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Kris-Etherton, P.M.; Petersen, K.S. Replacing Saturated Fats with Unsaturated Fats from Walnuts or Vegetable Oils Lowers Atherogenic Lipoprotein Classes Without Increasing Lipoprotein(A). J. Nutr. 2020, 150, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Ritcher, C.K.; Skulas-Ray, A.C.; Fleming, J.A.; Link, C.J.; Mukherjea, R.; Krul, E.S. Effects of Isoflavone-Containing Soya Protein on Ex Vivo Cholesterol Efflux, Vascular Function and Blood Markers of CVD Risk in Adults with Moderately Elevated Blood Pressure: A Dose −response Randomised Controlled Trial. Br. J. Nutr. 2017, 117, 1403–1413. [Google Scholar] [CrossRef]
- Manninen, S.; Lankinen, M.; Erkkilä, A.; Nguyen, S.D.; Ruuth, M.; de Mello, V.; Öörni, K.; Schwab, U. The Effect of Intakes of Fish and Camelina Sativa Oil on Atherogenic and Anti-Atherogenic Functions of LDL and HDL Particles: A Randomized Controlled Trial. Atherosclerosis 2019, 281, 56–61. [Google Scholar] [CrossRef]
- Yang, Z.H.; Amar, M.; Sorokin, A.V.; Troendle, J.; Courville, A.B.; Sampson, M.; Playford, M.P.; Yang, S.; Stagliano, M.; Ling, C.; et al. Supplementation with Saury Oil, a Fish Oil High in Omega-11 Monounsaturated Fatty Acids, Improves Plasma Lipids in Healthy Subjects. J. Clin. Lipidol. 2020, 14, 53–65.e2. [Google Scholar] [CrossRef]
- Nicod, N.; Chiva-Blanch, G.; Giordano, E.; Dávalos, A.; Parker, R.S.; Visioli, F. Green Tea, Cocoa, and Red Wine Polyphenols Moderately Modulate Intestinal Inflammation and Do Not Increase High-Density Lipoprotein (HDL) Production. J. Agric. Food Chem. 2014, 62, 2228–2232. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Garcia, C.; Norris, G.H.; Lemos, B.S.; DiMarco, D.M.; Fernandez, M.L. Effects of Freeze-Dried Grape Powder on High-Density Lipoprotein Function in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Study. Metab. Syndr. Relat. Disord. 2018, 16, 464–469. [Google Scholar] [CrossRef]
- Marín-Echeverri, C.; Blesso, C.N.; Fernández, M.L.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Nuñez-Rangel, V.; Aristizábal, J.C.; Barona-Acevedo, J. Effect of Agraz (Vaccinium Meridionale Swartz) on High-Density Lipoprotein Function and Inflammation in Women with Metabolic Syndrome. Antioxidants 2018, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Michaličková, D.; Belović, M.; Ilić, N.; Kotur-Stevuljević, J.; Slanař, O.; Šobajić, S. Comparison of Polyphenol-Enriched Tomato Juice and Standard Tomato Juice for Cardiovascular Benefits in Subjects with Stage 1 Hypertension: A Randomized Controlled Study. Plant. Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef]
- Lazavi, F.; Mirmiran, P.; Sohrab, G.; Nikpayam, O.; Angoorani, P.; Hedayati, M. The Barberry Juice Effects on Metabolic Factors and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized Clinical Trial. Complement. Ther. Clin. Pract. 2018, 31, 170–174. [Google Scholar] [CrossRef]
- Balsan, G.; Pellanda, L.C.; Sausen, G.; Galarraga, T.; Zaffari, D.; Pontin, B.; Portal, V.L. Effect of Yerba Mate and Green Tea on Paraoxonase and Leptin Levels in Patients Affected by Overweight or Obesity and Dyslipidemia: A Randomized Clinical Trial. Nutr. J. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.P.J.; Mensink, R.P.; Smolders, L.; Bakeroot, V.; Plat, J. Theobromine Does Not Affect Fasting and Postprandial HDL Cholesterol Efflux Capacity, While It Decreases Fasting MiR-92a Levels in Humans. Mol. Nutr. Food Res. 2018, 62, 1800027. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, M.; Abdollahi, S.; Salehi-Abargouei, A.; Clark, C.C.T.; Karimi-Nazari, E.; Fallahzadeh, H.; Rahmanian, M.; Mozaffari-Khosravi, H. The Effect of Resveratrol Supplementation on Serum Levels of Asymmetric De-Methyl-Arginine and Paraoxonase 1 Activity in Patients with Type 2 Diabetes: A Randomized, Double-Blind Controlled Trial. Phyther. Res. 2020, 34, 2023–2031. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Catalán, Ú.; López de las Hazas, M.C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.J.; Solà, R. Protective Effect of Hydroxytyrosol and Its Predominant Plasmatic Human Metabolites against Endothelial Dysfunction in Human Aortic Endothelial Cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef]
- Derosa, G.; Catena, G.; Raddino, R.; Gaudio, G.; Maggi, A.; D’Angelo, A.; Maffioli, P. Effects on Oral Fat Load of a Nutraceutical Combination of Fermented Red Rice, Sterol Esters and Stanols, Curcumin, and Olive Polyphenols: A Randomized, Placebo Controlled Trial. Phytomedicine 2018, 42, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol Blunts the Positive Effects of Exercise Training on Cardiovascular Health in Aged Men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef] [PubMed]
- Anastasius, M.; Kockx, M.; Jessup, W.; Sullivan, D.; Rye, K.A.; Kritharides, L. Cholesterol Efflux Capacity: An Introduction for Clinicians. Am. Heart J. 2016, 180, 54–63. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Origins and Hallmarks of Macrophages: Development, Homeostasis, and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; de Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; de Guimarães, R.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Hunter, P.M.; Hegele, R.A. Functional Foods and Dietary Supplement for the Management of Dyslipidemia. Nat. Rev. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The Effects of Resveratrol on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral Resveratrol Supplementation Improves Metabolic Syndrome Features in Obese Patients Submitted to a Lifestyle-Changing Program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef] [PubMed]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stødkilde-Jørgensen, H.; Jessen, N.; Jørgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No Beneficial Effects of Resveratrol on the Metabolic Syndrome: A Randomized Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Bitok, E.; Sabaté, J. Nuts and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 33–37. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional Quality of Legumes, and Their Role in Cardiometabolic Risk Prevention: A Review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Blesso, C.N.; Banach, M.; Pirro, M.; Majeed, M.; Sahebkar, A. Effects of Curcumin on HDL Functionality. Pharmacol. Res. 2017, 119, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Quercitin. Dict. Gems Gemol. 2009, 16, 172–195. [Google Scholar] [CrossRef]
| Author, Year | Dietary Compounds | Dose/Time | Study Design n | Main Results on Efflux Capacity on HDL-C |
|---|---|---|---|---|
| Hernáez, 2017 [27] | TMD enriched with EVOO TMD enriched with nuts (walnuts, hazelnuts, and almonds) | 1 L/week 30 g/d (15 g walnuts, 7.5 g hazelnuts, 7.5 g almonds) 1 year | A randomized controlled trial subsample PREDIMED study 296 subjects (TMD-EVOO; n = 100 and TMD-Nuts; n = 100, low-fat control diet; n = 96). | ↑ CEC TMD-EVOO interventions relative to baseline (0.01 ± 0.007; p = 0.018) ↑ CEC TMD-Nuts interventions relative to baseline (0.02 ± 0.09; p = 0.013) |
| Hernáez, 2019 [81] | EVOO Whole grains | 10 g/d (one spoonful) 25 g/d 1 year | A randomized controlled trial subsample PREDIMED study 296 older adults’ high cardiovascular risk (50–80 years) | ↑ 0.7 % CEC (0.08–1.2; p = 0.026) with EVOO ↑0.6% CEC (0.1–1.1; p = 0.017) with whole grains. |
| Fernández-Castillejo, 2017 [83] | VOO (80 ppm) FVOO enriched with its own PC (500 ppm) FVOOT with its own PC plus thyme (500 ppm) | 25 mL/day 3 weeks | Crossover, double-blind, controlled trial from the VOHF 33 hypercholesterolemic subjects | ↑ CEC post-intervention vs. pre-intervention values (4.1% ± 1.4; p = 0.042). ↑ HDL ApoA-I concentration (0.6 ± 0.1; p = 0.014). Independent of VOO type. CEC was related to concentration in HDL of ApoA-I (p = 0.004). |
| Farràs, 2017 [84] | VOO (80 ppm) FVOO enriched with its own PC (500 ppm) FVOOT with its own PC plus thyme (500 ppm) | 25 mL/day raw OO (between meals) 3 weeks 2 weeks wash-out periods | Randomized, double-blind, crossover, controlled trial from the VOHF 33 hypercholesterolemic subjects | FVOOT versus FVOO intervention ↑ CEC (1.3% ± 3.9 and 1.2% ± 3.8, respectively; p = 0.019) FVOOT versus VOO ↓ (−0.03% + 5.4) FVOOT post-intervention versus baseline. ↑ CEC (29.7% ± 5.6 vs. 28.3% ± 6.7; p = 0.086) |
| Tindall, 2019 [87] | Walnuts Vegetable oils | WD WFMD ORAD | Randomized, crossover, controlled-feeding study 34 individuals at risk of cardiovascular disease (aged 30–65 years) | ~ CEC mediated for ABCA1 (p = 0.1) or global efflux in all diets (WD 3.5% ± 0.2, WFMD 3.5% ± 0.2, ORAD 3.8% ± 0.2; p = 0.1). ↓ global efflux after the WFMD compared with WD and ORAD (p = 0.01). |
| Manninen, 2019 [89] | Fish Camelina sativa oil | 20 mL of CSO * 4 meals/week of lean Fish 1 g EPA + DHA per day of fatty fish Control * * CSO and control allow one fish per week 12 weeks | Randomized controlled trial 79 impaired glucose metabolism subjects (40–75 years) | ~ CEC of HDL (p = 0.123) had no significant effect after 12 weeks of fatty fish ingestion. |
| Yang, 2019 [90] | Fish LCMUFA, omega-3 FA, MUFA | 12 g saury oil, control oil (sardine + olive oil) | Randomized, doble blind, crossover trial 30 healthy normolipidemic subjects [>18 years, (34.8 ± 12.5)] 8 weeks | ↑ 6.2% HDL-C levels, ↑ 8% CEC |
| Richter, 2017 [88] | Soya protein (isoflavone) | 0, 25 and 50 g/day soya protein 8 weeks | Randomized, placebo-controlled, three period crossover study 20 adults with moderately elevated blood pressure (35–60 years) | ~ CEC No significant effects in CE ex vivo. ↓ 12.7 % ABCA1-specific efflux (p = 0.02) from baseline following supplementation with the control Change not significant compared with ABCA1 efflux by 50 g/day of soya protein (3.1%; p = 0.4). |
| Millar, 2018 [92] | Grape | 60 g/day of freeze-dried grape powder (GRAPE, 195 mg polyphenols) 60 g/day of placebo powder (without polyphenols) 4 weeks 3 weeks washout | Randomized, double-blind, crossover placebo-controlled study 20 adults with MS (aged 32–70 years) | ~ CEC after interventions with grape and placebo (15.1% ± 5.0 and 14.4 ± 5.5; respectively). Grape not affect HDL CEC compared with placebo (0.7 ± 4.2; p = 0.47) |
| Marín-Echeverri, 2018 [93] | Agraz (fruit) | 200 mL freeze-dried agraz reconstituted/day Placebo (similar beverage without any polyphenols) 12 weeks | Double-blind crossover study 40 women with MS | ~ CEC (0.5% ± 2.9; p = 0.324) after comparing the end of both intervention periods (placebo versus agraz) |
| Talbot, 2018 [97] | Cocoa (theobromine) | 20 mL drink (500 mg of theobromine) 20 mL placebo drink per day 4 weeks | Randomized, double-blind, controlled, crossover study 44 overweight and obese subjects (aged 45–70 years) | Not affect fasting CEC after theobromine intervention (+0.4% point; −2.81, 3.57; p = 0.81). ~ CEC after theobromine on fasting and postprandial CEC (97.5% ± 9.2 to 99.1 ± 11.7). |
| Nicod, 2014 [91] | Polyphenols (red wine, cocoa, or green tea) | 50 μM total polyphenols (gallic acid equivalents) 24 h | In vitro study Caco-2 monolayer model | No change of cholesterol efflux, via SR-B1 (cholesterol is taken up by SR-B1) |
| Voloshyna, 2013 [78] | Resveratrol | 10, 25 μM 4 h (CEC of ApoA-1) 6 h (CEC to HDL) | In vitro study TPH-1 monocytes and macrophages, HAEC, PBMC, HMDM 18 h | ↑ ABCA1 message (10 μM) in TPH1 and HAEC vs. control (168.2 ± 13.3; 141.3 ± 15.4%; p < 0.001) ↑ ABCG1 expression in TPH-1 (169.9 ± 15.1%; p < 0.001) ↑ LXRα mRNA (10 μM) in TPH-1 and HAEC vs. control (148.9 ± 13.3% vs. 125.8 ± 10.3%; p < 0.05) ↑ 4.6% CEC to ApoA-1 in TPH-1 (20 μM/mL, 4 h) vs. 3.8% control (p < 0.05). ↑ 136.2 (±8.5%; p < 0.001) PPARγ expression vs. control |
| Sun, 2015 [75] | Quercetin | 0, 25,50, 100, 200 μM 0, 4, 8, 16, 24, 32 h | In vitro study TPH-1 derived foam cells | 200 uM, 32 h ↑ ApoA-I dependent CEC after 200 μM, 32 h vs. without treatment (>30% vs. 10%; p < 0.001) ↑ PPARγ expression and activation (p < 0.001) in 200 μM, 32 h. |
| Cui, 2017 [76] | Quercetin | Quercetin 12.5 mg/Kg/d in 0.5% CMCNa 2.5, 5.0, 10.0 μM 8 weeks | In vivo study Experimental animal model (apoE-deficient mice fed a high-fat diet) 24 mice CMCNa group (n = 12), quercetin group (n = 12). | ↑ 31.8% CEC from macrophages in the quercetin-treated mice vs. controls (p < 0.01) ↑22% HDL in quercetin group (p < 0.01) ↑ CEC in a concentration-depend manner 5.0 and 10.0 μM (p < 0.01) |
| Zhong, 2017 [79] | Curcumin | 10, 20, 40 μM 12 h | In vitro study Murine macrophage RAW264.7 cell line and monocyte TPH-1 cell line | ↑ CEC in macrophage in a dose-dependent manner (10, 20, 40 μM) vs. untreated group (p < 0.05). ↑ ABCA1 and SRB1 expression and protein level (10, 20, 40 μM) vs. control group (p < 0.05). ~ SRB1 expression. |
| Author, Year | Bioactive Compounds | Dose/Time | Study Design n | Main Results on CETP Activity |
|---|---|---|---|---|
| Hernáez, 2017 [27] | TMD- EVOO TMD- Nuts | 1 L/week 1 year | Randomized controlled trial subsample PREDIMED study 296 subjects (TMD-VOO; n = 100 and TMD-Nuts; n = 100, low-fat control diet; n = 96). | ↓ CETP activity after TMD-EVOO intervention to baseline (−0.039; p = 0.008). |
| Hernáez, 2019 [81] | Legumes Fresh fish | 25 g/d (2 servings/week) each one 1 year | Randomized controlled trial subsample PREDIMED study 296 older adults of high cardiovascular risk (50–80 years) | 25 g legumes ↓ 4.8% (p = 0.0028) CETP activity 25 g fish consumption ↓ 2.3% CEPT activity |
| Elseweidy, 2015 [80] | Curcuminoids and ginger | 50 mg/kg/d 200 mg/kg/d 6 weeks | In vivo Study Experimental animal model (rabbit model) Fed high-cholesterol diet 6 weeks 3 groups: 1.TGE (n = 6) 2. Curcuminoids (n = 6) 3. Placebo (n = 6) | ↓ hepatic CETP mRNA expression TGE, curcuminoids vs. placebo (8.7 ± 0.7; 8.4 ± 0.5 vs. 11 ± 0.5; p < 0.001); respectively. ↓ plasma CETP (199 pg/mL ± 4; 152 ± 5 vs. 315 ± 12; p < 0.001); respectively. Ginger was more effective in ↓ plasma CETP (152 pg/mL ± 5 vs. 199 ± 4; p < 0.001) than curcuminoids. |
| Author, Year | Bioactive Compounds | Dose/Time | Study Design n | Main Results on PON1 Activity/ Expression |
|---|---|---|---|---|
| Michaličková, 2019 [94] | Polyphenol-enriched tomato Juice | IG: 200 g tomato fruit juice enriched with 1 g of ethanolic extract or whole tomato fruit CG: 200 g tomato fruit juice 4 weeks | Randomized controlled single-blind study 26 subjects (aged 45–60 years) with Stage 1 Hypertension | ~ PON1 in both groups No significative changes baseline and 4 weeks after IG and CG [157 U/L (141–541)-172 U/L (157–447); 413 U/L (264–484)-405 U/L (294–514)]; p = 0.769 |
| Lazavi, 2018 [95] | Barberry juice | IG: 200 mL/d of BJ CG: no intervention 8 weeks | Randomized clinical trial 41 diabetic subjects (aged 30–75 years) | ↑56.0 mg/dL PON1 concentrations (±68.29; p = 0.015) for IG at the end of the trial. |
| Millar, 2018 [92] | Grape | 60 g/d of freeze-dried grape powder (GRAPE, 195 mg polyphenols) 60 g/d of placebo powder (without polyphenols) 4 weeks 3 weeks washout | Randomized, double-blind, crossover placebo-controlled study 20 adults with MS (aged 32–70 years) | ~ PON1 arylesterase and PON1 lactonase activities after interventions with grape and placebo (84.5 kU/L ± 17.4 and 86.3 kU/L ± 16.2) and (15.8 kU/L ± 3.2 and 15.6 kU/L ± 2.5; respectively) Grape not affect PON1 lactonase activity compared with placebo (0.2 kU/L ± 1.8; p = 0.6). |
| Tabatabaie, 2020 [98] | Resveratrol | 2 capsules (1000 mg) of resveratrol per day 2 capsules of methylcellulose (placebo) per day 8 weeks | Randomized, double-blind controlled trial 71 patients with type 2 diabetes (aged 30–60 years) | ↑ PON1 activity after supplementation with resveratrol (15.3 U/L ± 13.9; p < 0.001) and compared with placebo group (p = 0.04) Significantly after adjusting confounding variables (p < 0.001). |
| Marín-Echeverri, 2018 [93] | Agraz (fruit) | 200 mL freeze-dried agraz reconstituted/day Placebo (similar beverage without any polyphenols) 12 weeks | Double-blind crossover study 40 women with MS (aged 25–60 years). | ~ PON1 arylesterase and lactonase activities (-0.7 kU/L ± 8.8, p = 0.643; 0.2 kU/L ± 1.6, 0.862) after comparing the end of both intervention periods (placebo versus agraz); respectively. |
| Hernáez, 2017 [27] | TMD- EVOO TMD- Nuts | 1 L/week 1 year | Randomized controlled trial subsample PREDIMED Study 296 subjects (older adults) TMD-EVOO TMD-Nuts Low-fat control diet. | ~ PON1 in both groups |
| Hernáez, 2019 [81] | EVOO Nuts Legumes Fish | 1 L/week 30 g per day 25 g per day 25 g per day 2 servings/week each (one) 1 year | Randomized controlled trial PREDIMED Study 296 older adult high cardiovascular risk (aged 50–80 years) | Nuts, legumes and fish ↑ 12.2%, 11.7% and 3.9% PON1 antioxidant activity (0.13–24.2; p < 0.049; 0.44–22.8; p= 0.043; 0.40–7.45; p = 0.030); respectively. |
| Fernández-Castillejo, 2017 [85] | First Study (acute intake) FVOOT (different concentrations) Second Study (sustained intake) Olive oil PC Thyme PC | 30 mL single dose L-FVOO 250 ppm M-FVOO 500 ppm H-FVOO, 750 ppm 25 mL per day FVOO (80 ppm) + OO-PC control FVOO (550 ppm) own PC FVOOT (550 ppm) own PC (50% secoiridoid derivatives) FVOOT plus thyme (50%; flavonoids, PC, and monoterpenes) | Two randomized, crossover-controlled trial 12 healthy subjects and 33 hypercholesterolemic subjects; respectively. Single-dose and 3 weeks | ↓ PON1 protein after 2 h of 30 mL of L-FVOO and M-FVOO (5.1–6.4%; p < 0.005) ↑PON1 raw activity at 4 h time point (p < 0.05). ↓ 10.9–12.4% PON1 protein levels after VOO and FVOO (p < 0.05) ↑ 5.1% PON3 protein levels and PON1 catalytic activity (p < 0.05) Pon1 gene expression correlated with PPARγ (r = 0.966; p = 0.034). |
| Balsan, 2019 [96] | Green tea Yerba mate | 1000 mL per day of: GT YM AT (control) 8 weeks | Randomized, controlled, clinical trial 142 overweight or obesity and dyslipidemia (aged 35–60 years) | ↑9.7% PON1 serum levels after YM intervention (2625 pg/mL to 2880 pg/mL, change 255 pg/mL; p = 0.005). ~ PON1 serum levels after green tea intervention (2899 pg/mL to 2745 pg/mL, change -154 pg/mL; p = 0.154). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Castillo, K.P.; Lin, S.; Muñoz-Valle, J.F.; Vizmanos, B.; López-Quintero, A.; Márquez-Sandoval, F. Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review. Nutrients 2021, 13, 1165. https://doi.org/10.3390/nu13041165
Luna-Castillo KP, Lin S, Muñoz-Valle JF, Vizmanos B, López-Quintero A, Márquez-Sandoval F. Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review. Nutrients. 2021; 13(4):1165. https://doi.org/10.3390/nu13041165
Chicago/Turabian StyleLuna-Castillo, Karla Paulina, Sophia Lin, José Francisco Muñoz-Valle, Barbara Vizmanos, Andres López-Quintero, and Fabiola Márquez-Sandoval. 2021. "Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review" Nutrients 13, no. 4: 1165. https://doi.org/10.3390/nu13041165
APA StyleLuna-Castillo, K. P., Lin, S., Muñoz-Valle, J. F., Vizmanos, B., López-Quintero, A., & Márquez-Sandoval, F. (2021). Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review. Nutrients, 13(4), 1165. https://doi.org/10.3390/nu13041165












