Determinants of the Essential Elements and Vitamins Intake and Status during Pregnancy: A Descriptive Study in Polish Mother and Child Cohort
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Assessment of Essential Elements and Vitamins Intake Based on Questionnaire Data
2.3. Determination of Microelements and Vitamins in Biological Samples
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Factors Associated with Adequate Intake of Essential Elements and Vitamins during Pregnancy
3.3. Factors Associated with Microelements and Vitamins Concentration in Blood Collected during Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fall, C. Maternal nutrition: Effects on health in the next generation. Indian J. Med. Res. 2009, 130, 593–599. [Google Scholar] [PubMed]
- King, J.C. Maternal obesity, metabolism, and pregnancy outcomes. Annu. Rev. Nutr. 2006, 26, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Plows, F.J.; Stanley, L.J.; Baker, N.P.; Reynolds, M.C.; Vickers, H.M. The Pathophysiology of Gestational 491 Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Available online: https://www.who.int/publications/i/item/9789241549912 (accessed on 20 December 2020).
- World Health Organization (WHO). WHO Antenatal Care Recommendations for a Positive Pregnancy Experience Nutritional Interventions Update: Multiple Micronutrient Supplements during Pregnancy. Available online: https://www.who.int/publications/i/item/9789240007789 (accessed on 20 January 2021).
- Heindel, J.J.; Balbus, J.; Birnbaum, L.; Brune-Drisse, M.N.; Grandjean, P.; Gray, K.; Landrigan, P.J.; Sly, P.D.; Suk, W.; Cory Slechta, D.; et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.M.; Tielemans, M.J.; van den Broek, M.; Jaddoe, V.W.V.; Franco, O.H.; Kiefte-de Jong, J.C. Maternal dietary patterns during pregnancy and offspring cardiometabolic health at age 6 years: The generation R study. Clin. Nutr. 2017, 36, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Rifas-Shiman, S.L.; Georgiou, V.; Joung, K.E.; Koinaki, S.; Chalkiadaki, G.; Margioris, A.; Sarri, K.; Vassilaki, M.; Vafeiadi, M.; et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr. Obes. 2017, 12 (Suppl. 1), 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef]
- Chen, L.W.; Aris, I.M.; Bernard, J.Y.; Tint, M.T.; Chia, A.; Colega, M.; Gluckman, P.D.; Shek, L.P.; Saw, S.M.; Chong, Y.S.; et al. Associations of Maternal Dietary Patterns during Pregnancy with Offspring Adiposity from Birth Until 54 Months of Age. Nutrients 2016, 9, 2. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedón, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma; wheeze; and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef]
- Borge, T.C.; Aase, H.; Brantsæter, A.L.; Biele, G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: A systematic review and meta-analysis. BMJ Open 2017, 24, e016777. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 15, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 4127. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Antenatal Care Recommendations for a Positive Pregnancy Experience Nutritional Interventions Update: Vitamin D Supplements during Pregnancy. Available online: https://apps.who.int/iris/handle/10665/333562 (accessed on 20 January 2021).
- Zimmer, M.; Sieroszewski, P.; Oszukowski, P.; Huras, H.; Fuchs, T.; Pawlosek, A. Polish Society of Gynecologists and Obstetricians recommendations on supplementation during pregnancy. Ginekol. Pol. 2020, 91, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.; Croker, H.; Barr, S.; Briley, A.; Poston, L.; Wardle, J.; on behalf of the UPBEAT Trial. Psychological predictors of dietary intentions in pregnancy. J. Hum. Nutr. Diet. 2012, 25, 345–353. [Google Scholar] [CrossRef]
- Doyle, I.M.; Borrmann, B.; Grosser, A.; Razum, O.; Spallek, J. Determinants of dietary patterns and diet quality during pregnancy: A systematic review with narrative synthesis. Public Health Nutr. 2017, 20, 1009–1028. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Hanke, W.; Gromadzińska, J.; Ligocka, D.; Gulczyńska, E.; Sobala, W.; Wąsowicz, W. Polish mother and child cohort study-defining the problem; the aim of the study and methodological assumption. Int. J. Occup. Med. Environ. Health 2009, 22, 383–391. [Google Scholar] [CrossRef]
- Polańska, K.; Hanke, W.; Jurewicz, J.; Sobala, W.; Madsen, C.; Nafstad, P.; Magnus, P. Polish mother and child cohort study (REPRO_PL)-methodology of follow-up of the children. Int. J. Occup. Med. Environ. Health 2011, 24, 391–398. [Google Scholar] [CrossRef]
- Polańska, K.; Hanke, W.; Król, A.; Potocka, A.; Waszkowska, M.; Jacukowicz, A.; Gromadzińska, J.; Wąsowicz, W.; Jerzyńska, J.; Stelmach, W.; et al. Polish Mother and Child Cohort Study (REPRO_PL)—Methodology of the follow-up of the children at the age of 7. Int. J. Occup. Med. Environ. Health 2016, 29, 883–893. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hu, F.B.; Chavarro, J.; Rosner, B.; Mozaffarian, D.; Zhang, C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch. Intern. Med. 2012, 12, 1566–1572. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, C.; Gelaye, B.; Enquobahrie, D.A.; Frederick, I.O.; Williams, M.A. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care 2011, 34, 1564–1569. [Google Scholar] [CrossRef]
- Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J.; Colditz, G.A.; Litin, L.B.; Willett, W.C. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 1992, 135, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Klipstein-Grobusch, K.; den Breeijen, J.H.; Goldbohm, R.A.; Geleijnse, J.M.; Hofman, A.; Grobbee, D.E.; Witteman, J.C. Dietary assessment in the elderly: Validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1998, 52, 588–596. [Google Scholar] [CrossRef]
- Wesołowska, E.; Jankowska, A.; Trafalska, E.; Kałużny, P.; Grzesiak, M.; Dominowska, J.; Hanke, W.; Calamandrei, G.; Polańska, K. Sociodemographic, Lifestyle, Environmental and Pregnancy-Related Determinants of Dietary Patterns during Pregnancy. Int. J. Environ. Res. Public Health 2019, 16, 754. [Google Scholar] [CrossRef] [PubMed]
- Aubert, A.M.; Forhan, A.; de Lauzon-Guillain, B.; Chen, L.W.; Polanska, K.; Hanke, W.; Jankowska, A.; Mensink-Bout, S.M.; Duijts, L.; Suderman, M.; et al. Deriving the Dietary Approaches to Stop Hypertension (DASH) Score in Women from Seven Pregnancy Cohorts from the European ALPHABET Consortium. Nutrients 2019, 11, 2706. [Google Scholar] [CrossRef]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu i Wartości Odżywczej Żywności; Publisher PZWL: Warsaw, Poland, 2017. [Google Scholar]
- Jarosz, M. Nutrition Standards for Polish Population; National Food and Nutrition Institute: Warsaw, Poland, 2017; Available online: https://ncez.pl (accessed on 20 December 2020).
- Polanska, K.; Hanke, W.; Krol, A.; Gromadzinska, J.; Kuras, R.; Janasik, B.; Wasowicz, W.; Mirabella, F.; Chiarotti, F.; Calamandrei, G. Micronutrients during pregnancy and child psychomotor development: Opposite effects of Zinc and Selenium. Environ. Res. 2017, 158, 583–589. [Google Scholar] [CrossRef]
- Gromadzinska, J.; Polanska, K.; Kozlowska, L.; Mikolajewska, K.; Stelmach, I.; Jerzyńska, J.; Stelmach, W.; Grzesiak, M.; Hanke, W.; Wasowicz, W. Vitamins A and E during Pregnancy and Allergy Symptoms in an Early Childhood-Lack of Association with Tobacco Smoke Exposure. Int. J. Environ. Res. Public Health 2018, 15, 1245. [Google Scholar] [CrossRef]
- Agarwal, R.P.; Henkon, R.L. A simple method for simultaneous estimation of zinc and copper in erythrocytes. Biol. Trace. Elem. Res. 1985, 7, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Neve, J.; Molle, L. Direct determination of selenium in human serum by graphite furnace atomic absorption spectroscopy. Improvements due to oxygen ashing in graphite tube and Zeeman effect background correction. Acta Pharmacol. Toxicol. 1986, 59, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Neve, J.; Chamart, S.; Molle, L. Optimization of direct procedure for the determination of selenium in plasma and erythrocytes using Zeeman effect atomic absorption spectroscopy. Trace Elem. Anal. Chem. Med. Biol. 1987, 4, 349–358. [Google Scholar]
- Grzelinska, Z.; Gromadzinska, J.; Swiercz, R.; Wasowicz, W. Plasma concentration of vitamin E, vitamin Aand b-carotene in healthy men. Pol. J. Environ. Study 2007, 16, 209–213. [Google Scholar]
- Stragierowicz, J.; Mikołajewska, K.; Zawadzka-Stolarz, M.; Polanska, K.; Ligocka, D. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed. Res. Int. 2013, 2013, 386784. [Google Scholar]
- R Core Team 2018. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 20 January 2021).
- World Health Organization (WHO). Healthy Diet. Fact Sheet No. 394. Geneva, Updated 30 August 2018. Available online: https://www.who.int/publications/m/item/healthy-diet-factsheet394 (accessed on 21 January 2021).
- Oliver, E.M.; Grimshaw, K.E.; Schoemaker, A.A.; Keil, T.; McBride, D.; Sprikkelman, A.B.; Ragnarsdottir, H.S.; Trendelenburg, V.; Emmanouil, E.; Reche, M.; et al. Dietary habits and supplement use in relation to national pregnancy recommendations: Data from the EuroPrevall birth cohort. Matern. Child Health J. 2014, 18, 2408–2425. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., 3rd; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open. 2019, 2, e195967. [Google Scholar] [CrossRef]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef]
- Knapik, A.; Kocot, K.; Witek, A.; Jankowski, M.; Wróblewska-Czech, A.; Kowalska, M.; Zejda, J.E.; Brożek, G. Dietary supplementation usage by pregnant women in Silesia—Population based study. Ginekol. Pol. 2018, 89, 506–512. [Google Scholar] [CrossRef]
- Arkkola, T.; Uusitalo, U.; Pietikainen, M.; Metsala, J.; Kronberg-Kippila, C.; Erkkola, M.; Ovaskainen, M.L. Dietary intake and use of dietary supplements in relation to demographic variables among pregnant Finnish women. Br. J. Nutr. 2006, 96, 913–920. [Google Scholar] [CrossRef]
- Kocyłowski, R.; Lewicka, I.; Grzesiak, M.; Gaj, Z.; Sobańska, A.; Poznaniak, J.; von Kaisenberg, C.; Suliburska, J. Assessment of dietary intake and mineral status in pregnant women. Arch. Gynecol. Obstet. 2018, 297, 1433–1440. [Google Scholar] [CrossRef]
- Bojar, I.; Owoc, A.; Humeniuk, E.; Wierzba, W.; Fronczak, A. Inappropriate consumption of vitamins and minerals by pregnant women in Poland. Ann. Agric. Environ. Med. 2012, 19, 263–266. [Google Scholar]
- Institute of Mother and Child. Dietary Recommendations for Pregnant Women. Available online: https://imid.med.pl/images/poradnik-zywienia-dla-kobiet-w-ciazy.pdf (accessed on 21 January 2021).
- Food and Nutrition Institute. Available online: https://ncez.pl/ciaza-i-macierzynstwo/plodnosc-i-ciaza (accessed on 21 January 2021).
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu Rev Nutr. 2019, 39, 121–146. [Google Scholar] [CrossRef]
- Park, K.; Rimm, E.; Siscovick, D.; Spiegelman, D.; Morris, J.S.; Mozaffarian, D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr. Res. Pract. 2011, 5, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Livock, M.; Anderson, P.J.; Lewis, S.; Bowden, S.; Muggli, E.; Halliday, J. Maternal micronutrient consumption periconceptionally and during pregnancy: A prospective cohort study. Public Health Nutr. 2017, 20, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Zilkens, R.R.; Croft, K.; Mori, T.; Burke, V.; Beilin, L.J.; Puddey, I.B. A reduction in alcohol consumption is associated with reduced plasma F2-isoprostanes and urinary 20-HETE excretion in men. Free Radic. Biol. Med. 2007, 42, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Quattrocchi, T.; Mappa, I.; Spagnuolo, T.; Licameli, A.; Chiaradia, G.; De Luca, C. Folic acid use in planned pregnancy: An Italian survey. Matern. Child Health J. 2013, 17, 661–666. [Google Scholar] [CrossRef]
- Nasr Hage, C.; Jalloul, M.; Sabbah, M.; Adib, S.M. Awareness and intake of folic acid for the prevention of neural tube defects among Lebanese women of childbearing age. Matern. Child Health J. 2012, 16, 258–265. [Google Scholar] [CrossRef]
- Roth, C.; Bjørke-Monsen, A.L.; Reichborn-Kjennerud, T.; Nilsen, R.M.; Smith, G.D.; Stoltenberg, C.; Surén, P.; Susser, E.; Ueland, P.M.; Vollset, S.E.; et al. Use of folic acid supplements in early pregnancy in relation to maternal plasma levels in week 18 of pregnancy. Mol. Nutr. Food Res. 2013, 57, 653–660. [Google Scholar] [CrossRef]
- McNally, S.; Bourke, A. Periconceptional folic acid supplementation in a nationally representative sample of mothers. Ir. Med. J. 2012, 105, 236–238. [Google Scholar]
- Forster, D.A.; Wills, G.; Denning, A.; Bolger, M. The use of folic acid and other vitamins before and during pregnancy in a group of women in Melbourne, Australia. Midwifery 2009, 25, 134–146. [Google Scholar] [CrossRef]
- Pouchieu, C.; Lévy, R.; Faure, C.; Andreeva, V.A.; Galan, P.; Hercberg, S.; Touvier, M. Socioeconomic, lifestyle and dietary factors associated with dietary supplement use during pregnancy. PLoS ONE 2013, 13, e70733. [Google Scholar] [CrossRef]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.-Y.; Lee, S.-Y. A Prospective Study of Serum Trace Elements in Healthy Korean Pregnant Women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef]
- Liang, C.M.; Wu, X.Y.; Huang, K.; Yan, S.Q.; Li, Z.J.; Xia, X.; Pan, W.J.; Sheng, J.; Tao, Y.R.; Xiang, H.Y.; et al. Trace element profiles in pregnant women’s sera and umbilical cord sera and influencing factors: Repeated measurements. Chemosphere 2019, 218, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.; Mujkić, A.; Todorović, G.; Tulchinsky, T. Is level of knowledge, attitude and use of folic acid among pregnant women in Croatia a call for public health action? Period. Biol. 2009, 111, 329–335. [Google Scholar]
- Northstone, K.; Emmett, P.; Rogers, I. Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. Eur. J. Clin. Nutr. 2008, 62, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Arkkola, T.; Uusitalo, U.; Kronberg-Kippilä, C.; Männistö, S.; Virtanen, M.; Kenward, M.G.; Veijola, R.; Knip, M.; Ovaskainen, M.L.; Virtanen, S.M. Seven distinct dietary patterns identified among pregnant Finnish women—Associations with nutrient intake and sociodemographic factors. Public Health Nutr. 2008, 11, 176–182. [Google Scholar] [CrossRef]
- Völgyi, E.; Carroll, K.N.; Hare, M.E.; Ringwald-Smith, K.; Piyathilake, C.; Yoo, W.; Tylavsky, F.A. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 2013, 5, 1511–1530. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.B.; Freitas Vilela, A.A.; de Oliveira, A.S.; Cabral, M.; de Souza, R.A.; Kac, G.; Sichieri, R. Sociodemographic characteristics determine dietary pattern adherence during pregnancy. Public Health Nutr. 2016, 19, 1245–1251. [Google Scholar] [CrossRef]
- Polanska, K.; Krol, A.; Sobala, W.; Gromadzinska, J.; Brodzka, R.; Calamandrei, G.; Chiarotti, F.; Wasowicz, W.; Hanke, W. Selenium status during pregnancy and child psychomotor development-Polish Mother and Child Cohort study. Pediatr. Res. 2016, 79, 863–869. [Google Scholar] [CrossRef]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Fu, H.; Li, L.; Ren, T. Different levels of prenatal zinc and selenium had different effects on neonatal neurobehavioral development. Neurotoxicology 2013, 37, 35–39. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Pathak, P.; Kapil, U.; Kapoor, S.K.; Saxena, R.; Kumar, A.; Gupta, N.; Dwivedi, S.N.; Singh, R.; Singh, P. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J. Pediatr. 2004, 71, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Li, X.; Xiang, W.; Jiang, X. The Relationship between Serum Copper and Overweight/Obesity: A Meta-analysis. Biol. Trace Elem. Res. 2020, 194, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.N.; Wolf, R.M.; Ralle, M.; Dev, S.; Pierson, H.; Askin, F.; Steele, K.E.; Magnuson, T.H.; Schweitzer, M.A.; et al. Obesity is associated with copper elevation in serum and tissues. Metallomics 2019, 11, 1363–1371. [Google Scholar] [CrossRef]
- Chen, H.; Qian, N.; Yan, L.; Jiang, H. Role of serum vitamin A and E in pregnancy. Exp. Ther. Med. 2018, 16, 5185–5189. [Google Scholar] [CrossRef]
- Ramage, S.M.; McCargar, L.J.; Berglund, C.; Harber, V.; Bell, R.C.; APrON Study Team. Assessment of Pre-Pregnancy Dietary Intake with a Food Frequency Questionnaire in Alberta Women. Nutrients 2015, 7, 6155–6166. [Google Scholar] [CrossRef]
- Trijsburg, L.; de Vries, J.H.; Boshuizen, H.C.; Hulshof, P.J.; Hollman, P.C.; van’t Veer, P.; Geelen, A. Comparison of duplicate portion and 24 h recall as reference methods for validating a FFQ using urinary markers as the estimate of true intake. Br. J. Nutr. 2015, 114, 1304–1312. [Google Scholar] [CrossRef]
 Below EAR,
Below EAR,  Meeting EAR. Note: EAR (Estimated Average Requirement) for essential elements and vitamins during pregnancy are presented in Supplementary Materials (Table S1).
Meeting EAR. Note: EAR (Estimated Average Requirement) for essential elements and vitamins during pregnancy are presented in Supplementary Materials (Table S1).
 Below EAR,
Below EAR,  Meeting EAR. Note: EAR (Estimated Average Requirement) for essential elements and vitamins during pregnancy are presented in Supplementary Materials (Table S1).
Meeting EAR. Note: EAR (Estimated Average Requirement) for essential elements and vitamins during pregnancy are presented in Supplementary Materials (Table S1).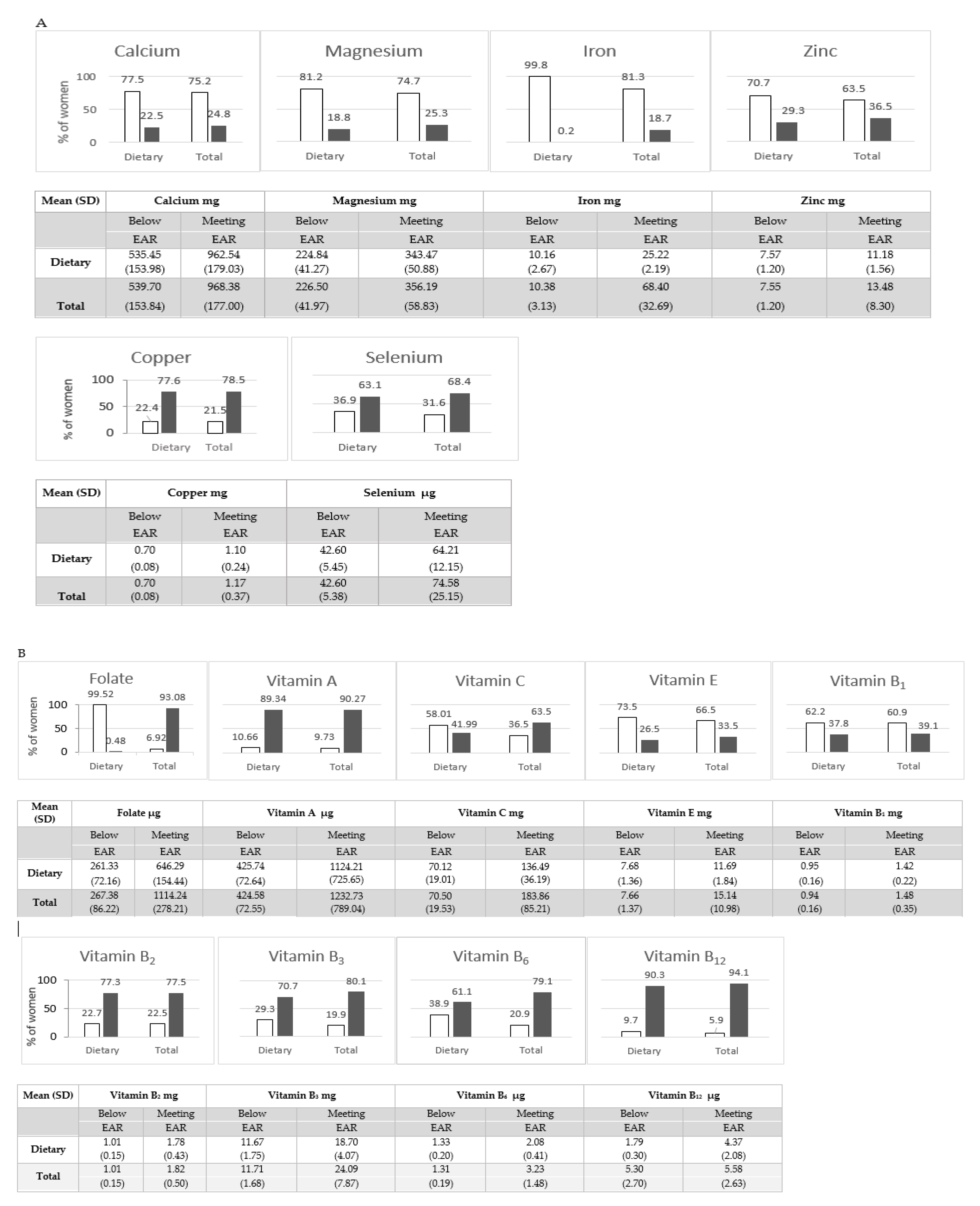
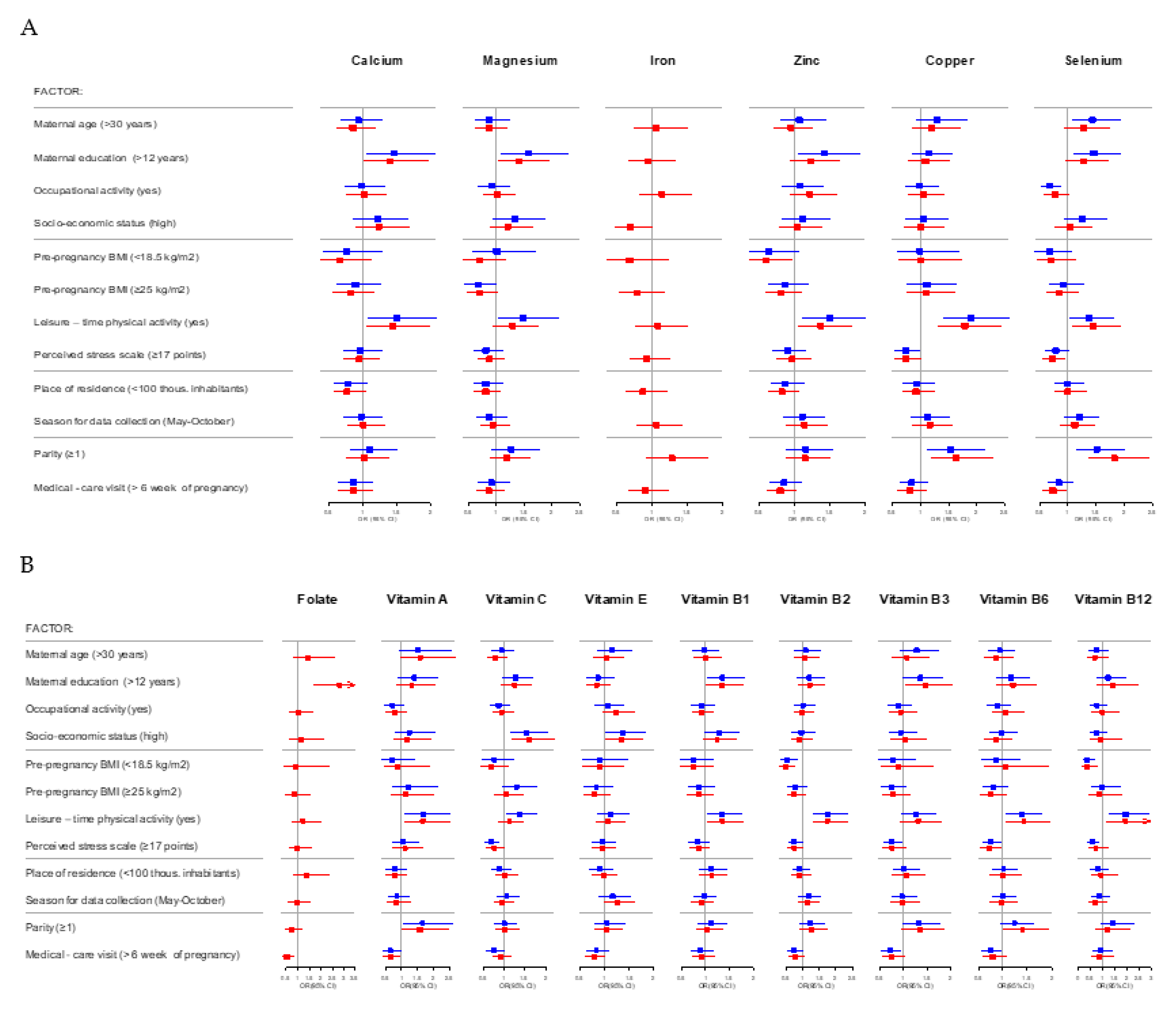
| Determinant | Zinc (mg/L) | Copper (mg/L) | Selenium (µg/L) | Vitamin A (mg/L) | Vitamin E (mg/L) |
|---|---|---|---|---|---|
| β (95% CI) | |||||
| Maternal age | |||||
| >30 | 0.04 (−0.03, 0.10) | −0.08 (−0.22, 0.07) | 2.56 (−0.15, 5.27) | 0.01 (−0.06, 0.08) | 1.51 (0.62, 2.39) * |
| Maternal education (years) | |||||
| >12 | −0.05 (−0.12, 0.02) | 0.04 (−0.11, 0.19) | −0.98 (−3.76, 1.80) | 0.01 (−0.06, 0.09) | −0.23 (−1.14, 0.68) |
| Occupational activity between the 8th and 12th week of pregnancy | |||||
| Yes | −0.06 (−0.12, 0.01) | −0.09 (−0.23, 0.04) | 0.22 (−2.30, 2.73) | 0.09 (−0.03, 0.16) | −0.32 (−1.12, 0.47) |
| Socio-economic status (SES) | |||||
| High | 0.03 (−0.04, 0.11) | −0.04 (−0.19, 0.12) | 3.07 (0.21, 5.94) ^ | 0.03 (−0.04, 0.09) | −0.35 (−1.22, 0.52) |
| Pre-pregnancy BMI (kg/m2) | |||||
| <18.5 | 0.01 (−0.10, 0.12) | -0.11 (−0.35, 0.14) | −0.89 (−5.46, 3.68) | −0.08 (−0.21, 0.05) | −1.41 (−3.00, 0.18) |
| ≥25 | 0.07 (−0.00, 0.15) | 0.19 (0.03, 0.35) ^ | 1.98 (−1.04, 5.00) | 0.05 (−0.03, 0.13) | −0.28 (−1.25, 0.70) |
| Cotinine level | |||||
| ≥10 ng/mL | 0.05 (−0.05, 0.15) | 0.05 (−0.17, 0.27) | −2.29 (−6.35, 1.77) | 0.03 (−0.08, 0.13) | 0.49 (−0.82, 1.80) |
| Alcohol consumption | |||||
| Yes | 0.01 (−0.09, 0.11) | −0.02 (−0.24, 0.20) | −2.30 (−6.33, 1.73) | 0.08 (−0.03, 0.19) | 1.28 (−0.10, 2.66) |
| Leisure–time physical activity (LTPA) | |||||
| Yes | 0.05 (−0.01, 0.12) | 0.04 (−0.10, 0.18) | 2.45 (−0.14, 5.04) | 0.03 (−0.03, 0.10) | −0.24 (−1.06, 0.59) |
| Perceived Stress Scale (range: 0–38 points)(PSS) | |||||
| ≥17 points | 0.07 (0.00, 0.13) ^ | −0.05 (−0.18, 0.08) | −0.40 (−2.85, 2.05) | −0.03 (−0.09, 0.03) | 0.22 (−0.56, 0.99) |
| Place of residence (thousands of inhabitants) | |||||
| <100 | −0.06 (−0.13, 0.01) | 0.09 (−0.06, 0.24) | 0.29 (−2.51, 3.09) | −0.09 (−0.16, 0.02) | 0.21 (−0.63, 1.06) |
| Season for data collection | |||||
| May–October | 0.01 (−0.06, 0.07) | 0.04 (−0.09, 0.17) | 2.03 (−0.39, 4.46) | −0.04 (−0.10, 0.02) | 0.70 (−0.06, 1.46) |
| Parity | |||||
| ≥1 | 0.01 (−0.05, 0.08) | 0.21 (0.07, 0.36) * | 0.18 (−2.46, 2.82) | −0.00 (−0.07, 0.06) | −0.61 (−1.45, 0.24) |
| Week of pregnancy of the 1st medical-care visit | |||||
| >6 | 0.02 (−0.05, 0.08) | −0.01 (−0.15, 0.12) | 0.58 (−1.92, 3.09) | 0.02 (−0.05, 0.08) | −0.38 (−1.18, 0.41) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowska, A.; Grzesiak, M.; Krekora, M.; Dominowska, J.; Jerzyńska, J.; Kałużny, P.; Wesołowska, E.; Szadkowska-Stańczyk, I.; Trafalska, E.; Kaleta, D.; et al. Determinants of the Essential Elements and Vitamins Intake and Status during Pregnancy: A Descriptive Study in Polish Mother and Child Cohort. Nutrients 2021, 13, 949. https://doi.org/10.3390/nu13030949
Jankowska A, Grzesiak M, Krekora M, Dominowska J, Jerzyńska J, Kałużny P, Wesołowska E, Szadkowska-Stańczyk I, Trafalska E, Kaleta D, et al. Determinants of the Essential Elements and Vitamins Intake and Status during Pregnancy: A Descriptive Study in Polish Mother and Child Cohort. Nutrients. 2021; 13(3):949. https://doi.org/10.3390/nu13030949
Chicago/Turabian StyleJankowska, Agnieszka, Mariusz Grzesiak, Michał Krekora, Jolanta Dominowska, Joanna Jerzyńska, Paweł Kałużny, Ewelina Wesołowska, Irena Szadkowska-Stańczyk, Elżbieta Trafalska, Dorota Kaleta, and et al. 2021. "Determinants of the Essential Elements and Vitamins Intake and Status during Pregnancy: A Descriptive Study in Polish Mother and Child Cohort" Nutrients 13, no. 3: 949. https://doi.org/10.3390/nu13030949
APA StyleJankowska, A., Grzesiak, M., Krekora, M., Dominowska, J., Jerzyńska, J., Kałużny, P., Wesołowska, E., Szadkowska-Stańczyk, I., Trafalska, E., Kaleta, D., Kowalska, M., Jabłońska, E., Janasik, B., Gromadzińska, J., Hanke, W., Wąsowicz, W., Calamandrei, G., & Polańska, K. (2021). Determinants of the Essential Elements and Vitamins Intake and Status during Pregnancy: A Descriptive Study in Polish Mother and Child Cohort. Nutrients, 13(3), 949. https://doi.org/10.3390/nu13030949










