Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males
Abstract
1. Introduction
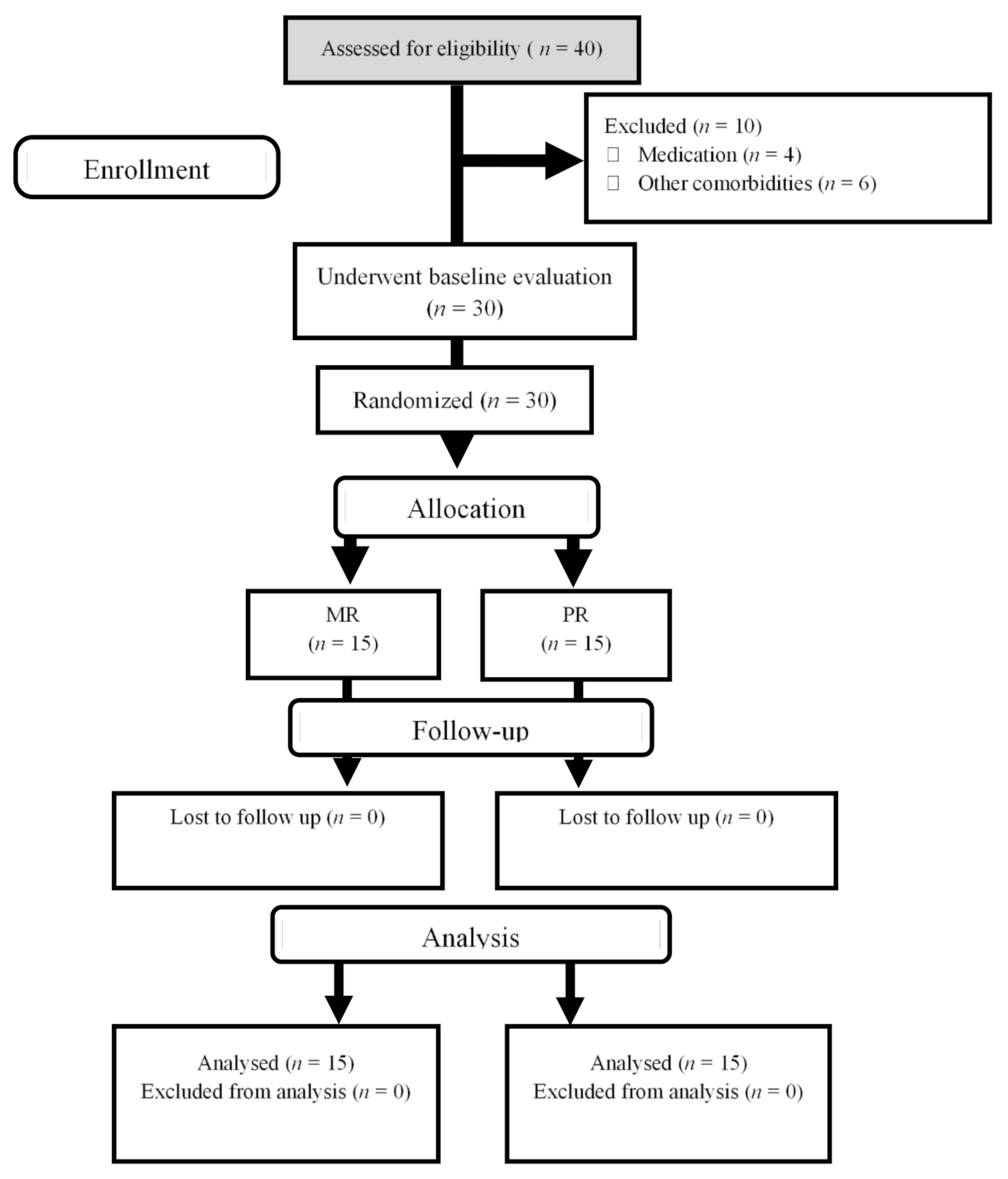
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Resistance Training Program
2.4. Nutritional Intervention
2.5. Body Composition
2.6. Blood
2.7. Strength
2.8. Power
2.9. Diet
2.10. Statistical Analysis
3. Results
3.1. Compliance, Adverse Effects, and Diet
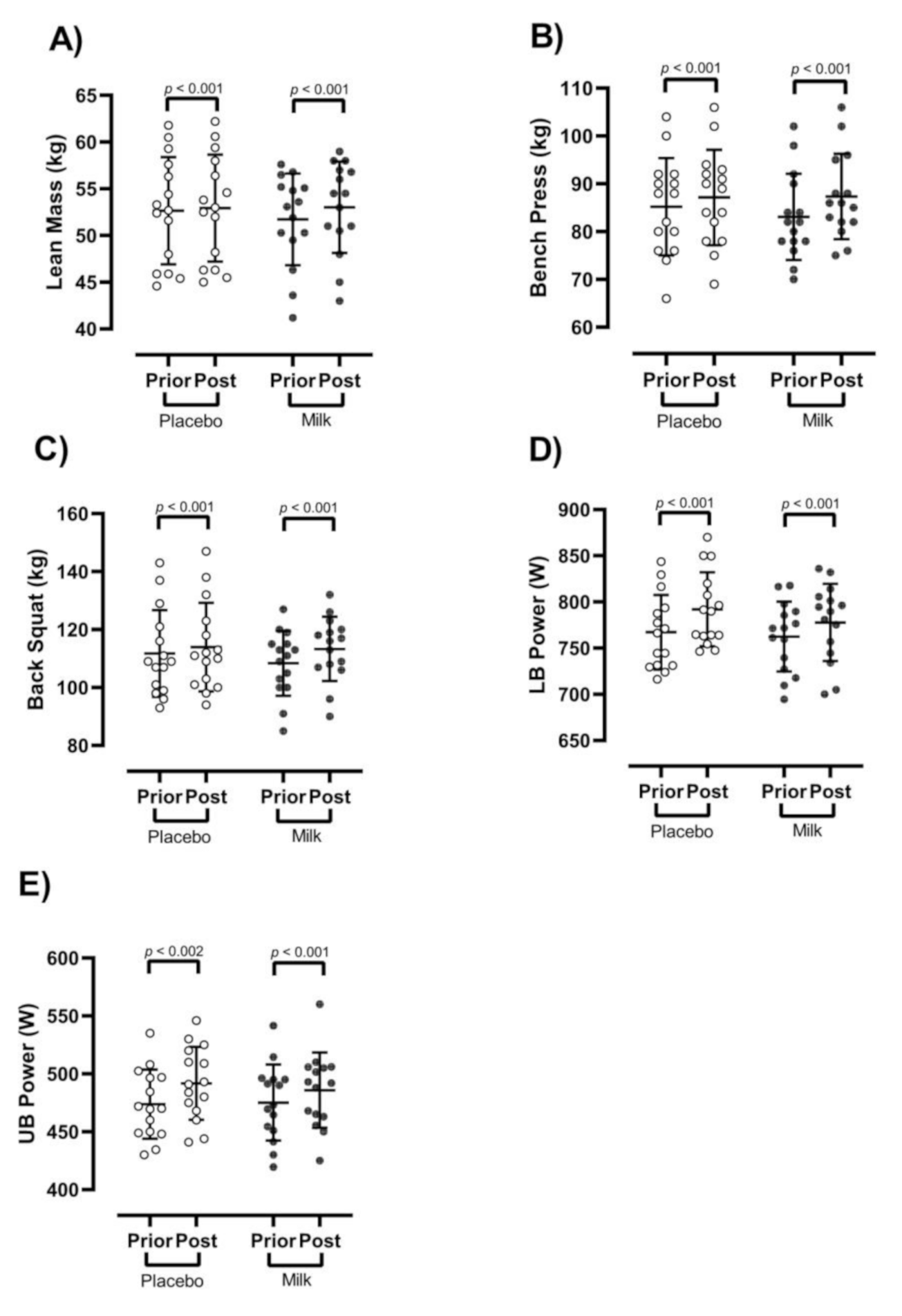
3.2. Body Composition
3.3. Strength and Power
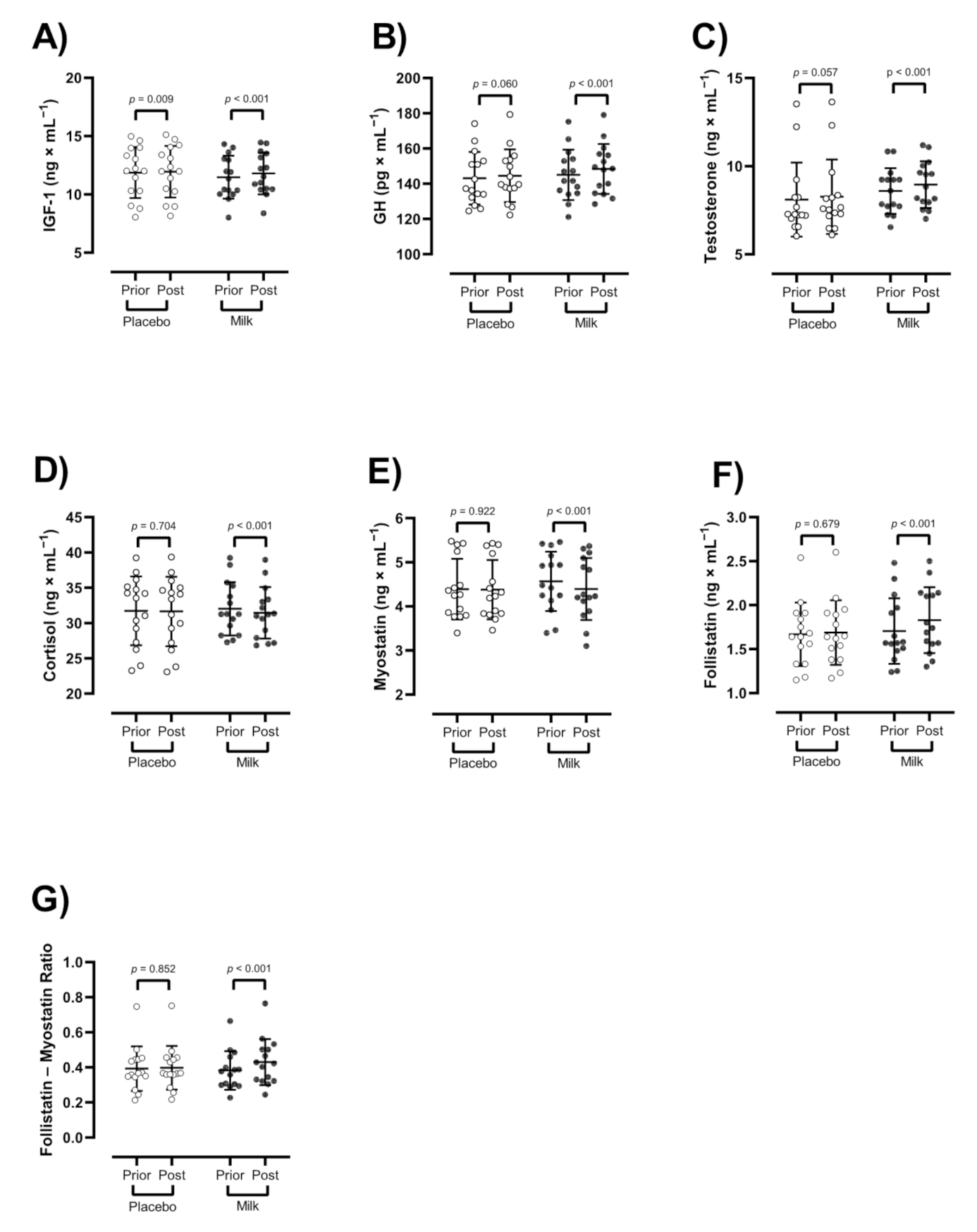
3.4. Hormones and Myokines
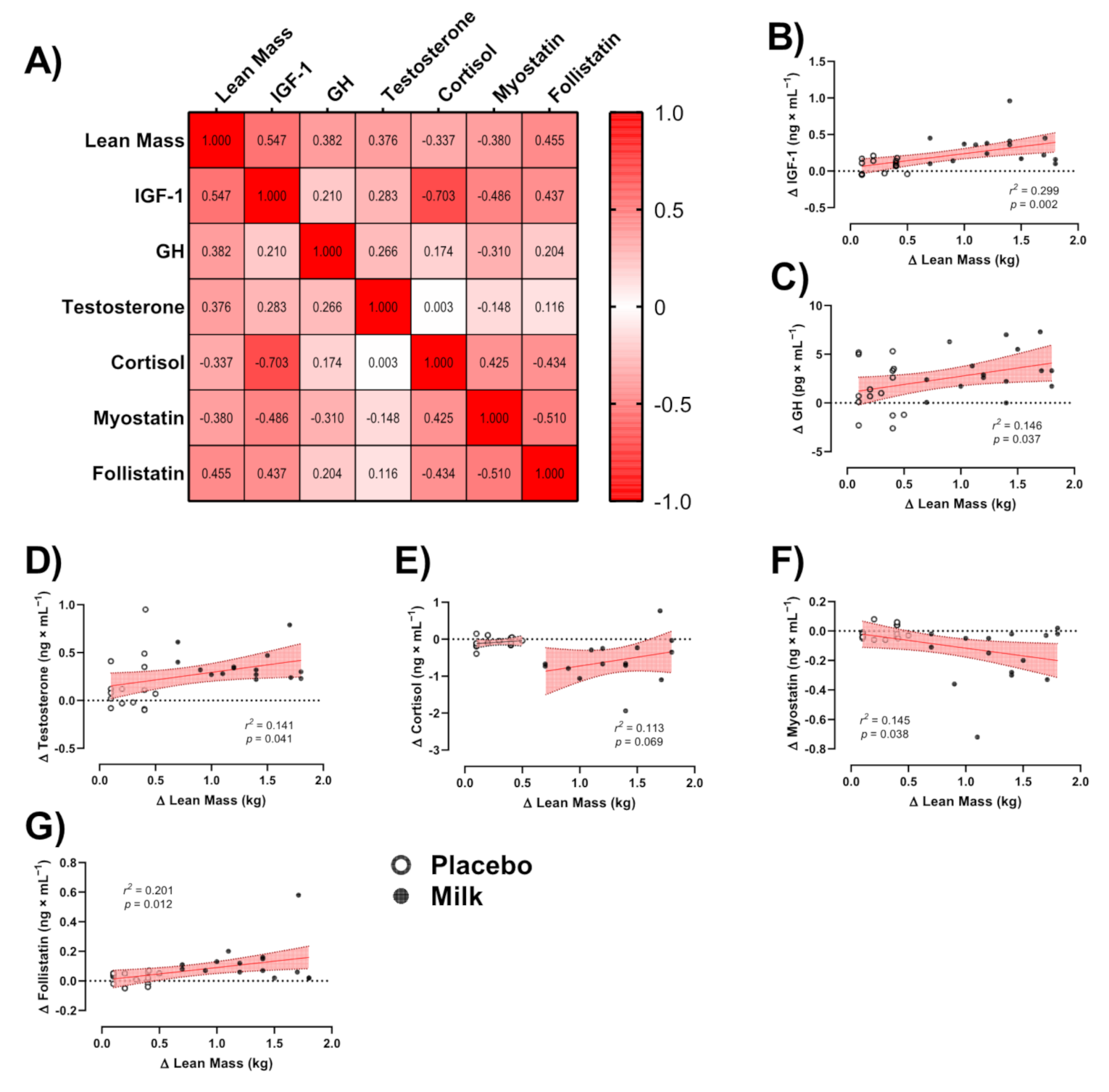
3.5. Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burd, N.A.; Tang, J.E.; Moore, D.R.; Philips, S.M. Exercise training and protein metabolism: Influences of contraction, protein intake, and sex-based differences. J. Appl. Physiol. 2009, 106, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk-and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Snijders, T.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Verdijk, L.B.; van Loon, L.J.C. The impact of pre-sleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: An update. Front. Nutr. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Beelen, M.; Koopman, R.; Gijsen, A.P.; Vandereyt, H.; Kies, A.K.; Kuipers, H.; Saris, W.H.; van Loon, L.J. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E70–E77. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Aarsland, A.A.; Sanford, A.P.; Wolfe, R.R. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E71–E76. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; McCargar, L.; Jelen, P.; Bell, G.J. Dose response of whey protein isolate in addition to a typical mixed meal on blood amino acids and hormonal concentrations. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 188–195. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef]
- Josse, A.R.; Phillips, S.M. Impact of milk consumption and resistance training on body composition of female athletes. Med. Sport Sci. 2012, 59, 94–103. [Google Scholar]
- Granic, A.; Hurst, C.; Dismore, L.; Davies, L.; Stevenson, E.; Sayer, A.A.; Aspray, T. Milk and resistance exercise intervention to improve muscle function in community-dwelling older adults at risk of sarcopenia (MIlkMAN): Protocol for a pilot study. BMJ Open 2019, 9, e031048. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef]
- Rankin, J.W.; Goldman, L.P.; Puglisi, M.J.; Nickols-Richardson, S.M.; Earthman, C.P.; Gwazdauskas, F.C. Effect of post-exercise supplement consumption on adaptations to resistance training. J. Am. Coll. Nutr. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 1–25. [Google Scholar] [CrossRef]
- Solomon, A.M.; Bouloux, P.M. Modifying muscle mass—the endocrine perspective. J. Endocrinol. 2006, 191, 349–360. [Google Scholar] [CrossRef]
- Spillane, M.; Schwarz, N.; Willoughby, D.S. Heavy resistance training and peri-exercise ingestion of a multi-ingredient ergogenic nutritional supplement in males: effects on body composition, muscle performance and markers of muscle protein synthesis. J. Sports Sci. Med. 2014, 13, 894–903. [Google Scholar]
- Haff, G.G.; Triplett, N.T. Essentials of strength training and conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Bagheri, R.; Rashidlamir, A.; Motevalli, M.S.; Elliott, B.T.; Mehrabani, J.; Wong, A. Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. Eur. J. Appl. Physiol. 2019, 119, 1921–1931. [Google Scholar] [CrossRef]
- Murach, K.A.; Bagley, J.R. Skeletal muscle hypertrophy with concurrent exercise training: contrary evidence for an interference effect. Sports Med. 2016, 46, 1029–1039. [Google Scholar] [CrossRef]
- Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Lane, J.R.; Gray, J.L.; Partl, J.M.; Hayes, D.W.; Wilson, G.J.; Hollmer, C.A.; Minivich, J.R.; et al. The Effects of Beef, Chicken, or Whey Protein After Workout on Body Composition and Muscle Performance. J. Strength Cond. Res. 2018, 32, 2233–2242. [Google Scholar] [CrossRef]
- Colantonio, E.; Barros, R.V.; Kiss, M.A.P.D.M. Oxygen uptake during Wingate tests for arms and legs in swimmers and water polo players. Rev. Bras. Med. Esporte 2003, 9, 141–144. [Google Scholar] [CrossRef][Green Version]
- Elliot, T.A.; Cree, M.G.; Sanford, A.P.; Wolfe, R.R.; Tipton, K.D. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med. Sci. Sports Exerc. 2006, 38, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Res, P.T.; Groen, B.; Pennings, B.; Beelen, M.; Wallis, G.A.; Gijsen, A.P.; Senden, J.M.; van Loon, L.J. Protein ingestion before sleep improves postexercise overnight recovery. Med. Sci. Sports Exerc. 2012, 44, 1560–1569. [Google Scholar] [CrossRef]
- Snijders, T.; Res, P.T.; Smeets, J.S.; van Vliet, S.; van Kranenburg, J.; Maase, K.; Kies, A.K.; Verdijk, L.B.; van Loon, L.J. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J. Nutr. 2015, 145, 1178–1184. [Google Scholar] [CrossRef]
- Elliott, B.; Renshaw, D.; Getting, S.; Mackenzie, R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012, 205, 324–340. [Google Scholar] [CrossRef]
- Tortoriello, D.V.; Sidis, Y.; Holtzman, D.A.; Holmes, W.E.; Schneyer, A.L. Human follistatin-related protein: A structural homologue of follistatin with nuclear localization. Endocrinology 2001, 142, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Salek, L.; Nikfal, E.; Izadi, M.; Tufano, J.J.; Elliott, B.T.; Brughelli, M. Comparable endocrine and neuromuscular adaptations to variable vs. constant gravity-dependent resistance training among young women. J. Transl. Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Roth, S.M.; Martel, G.F.; Ferrell, R.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Myostatin gene expression is reduced in humans with heavy-resistance strength training: A brief communication. Exp. Biol. Med. 2003, 228, 706–709. [Google Scholar] [CrossRef]
- Walker, K.S.; Kambadur, R.; Sharma, M.; Smith, H.K. Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med. Sci. Sports Exerc. 2004, 36, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Dalloul, H.; Hegyesi, H.; Apor, P.; Csende, Z.; Racz, L.; Vaczi, M.; Tihanyi, J. Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur. J. Appl. Physiol. 2007, 101, 427–436. [Google Scholar] [CrossRef]
- Saremi, A.; Gharakhanloo, R.; Sharghi, S.; Gharaati, M.R.; Larijani, B.; Omidfar, K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol. Cell. Endocrinol. 2010, 317, 25–30. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Attarzadeh Hosseini, S.R. Effect of resistance training with blood flow restriction on follistatin to myostatin ratio, body composition and anaerobic power of trained-volleyball players. Med. Lab. J. 2018, 12, 28–33. [Google Scholar]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef]
- Brahm, H.; Piehl-Aulin, K.; Saltin, B.; Ljunghall, S. Net fluxes over working thigh of hormones, growth factors and biomarkers of bone metabolism during short lasting dynamic exercise. Calcif. Tissue Int. 1997, 60, 175–180. [Google Scholar] [CrossRef]
- Goldspink, G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology 2005, 20, 232–238. [Google Scholar] [CrossRef]
- Jiang, Q.; Lou, K.; Hou, L.; Lu, Y.; Sun, L.; Tan, S.C.; Low, T.Y.; Kord-Varkaneh, H.; Pang, S. The effect of resistance training on serum insulin-like growth factor 1 (IGF-1): A systematic review and meta-analysis. Complement. Ther. Med. 2020, 2020, 102360. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Wang, C.H.; Pan, C.Y.; Chen, F.C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, Y.J.; Lee, S.Y.; Jeong, D.W.; Lee, J.G.; Yi, Y.H.; Cho, Y.H.; Choi, E.J.; Kim, H.J. Interactive effects of an isocaloric high-protein diet and resistance exercise on body composition, ghrelin, and metabolic and hormonal parameters in untrained young men: A randomized clinical trial. J. Diabetes Investig. 2014, 5, 242–247. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Marchitelli, L.; Gordon, S.E.; Harman, E.; Dziados, J.E.; Mello, R.; Frykman, P.; McCurry, D.; Fleck, S.J. Hormonal and growth factor responses to heavy resistance exercise protocols. J. Appl. Physiol. 1990, 69, 1442–1450. [Google Scholar] [CrossRef]
- Ahtiainen, J.P.; Pakarinen, A.; Alen, M.; Kraemer, W.J.; Häkkinen, K. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur. J. Appl. Physiol. 2003, 89, 555–563. [Google Scholar] [CrossRef]
- Josse, A.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Body composition and strength changes in women with milk and resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1122–1130. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010, 4, 142–148. [Google Scholar] [CrossRef]
- Cheng, G.; Hilbig, A.; Drossard, C.; Alexy, U.; Kersting, M. Relative validity of a 3 d estimated food record in German toddlers. Public Health Nutr. 2013, 16, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, S.; Lindroos, A.K.; Wirfalt, E.; Leanderson, P.; Landberg, R.; Ericson, U.; Larsson, I.; Lemming Warensjo, E.; Bergstrom, G.; Hedblad, B.; et al. Carotenoids and alkylresorcinols as objective biomarkers of diet quality when assessing the validity of a web-based food record tool and a food frequency questionnaire in a middle-aged population. BMC Nutr. 2016, 2, 53. [Google Scholar] [CrossRef]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L.; Graves, J.E.; Mahar, M.T. Reliability and validity of bioelectrical impedance in determining body composition. J. Appl. Physiol. 1988, 64, 529–534. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Nickerson, B.S.; Wilborn, C.D.; Urbina, S.L.; Hayward, S.B.; Krieger, J.; Aragon, A.A.; Tinsley, G.M. Comparison of multifrequency bioelectrical impedance vs. dual-energy X-ray absorptiometry for assessing body composition changes after participation in a 10-week resistance training program. J. Strength Cond. Res. 2020, 34, 678–688. [Google Scholar] [CrossRef] [PubMed]
| Descriptive | Milk | Placebo | p-Value |
|---|---|---|---|
| Age (years) | 26 (1) | 27 (1) | 0.495 |
| Stature (cm) | 178.6 (1.1) | 177.1 (1.5) | 0.429 |
| Body mass (kg) | 77.1 (1.3) | 78.4 (1.5) | 0.511 |
| BMI (kg × m−2) | 24.2 (0.5) | 25.1 (0.8) | 0.312 |
| Lean Mass (kg) | 51.7 (1.2) | 52.6 (1.4) | 0.638 |
| Fat mas (kg) | 16.4 (0.3) | 16.8 (0.3) | 0.328 |
| Bench press (kg) | 83 (2.3) | 85.2 (2.6) | 0.549 |
| Squat (kg) | 108.4 (2.8) | 111.7 (3.8) | 0.496 |
| Lower body power (watt) | 767.1 (10.4) | 762.4 (9.7) | 0.741 |
| Upper body power (watt) | 473.7 (7.6) | 475.1 (8.4) | 0.905 |
| IGF-1 (ng × mL−1) | 11.4 (0.4) | 11.8 (0.5) | 0.587 |
| GH (pg × mL−1) | 145 (3.6) | 143.1 (3.8) | 0.721 |
| Testosterone (ng × mL−1) | 8.5 (0.3) | 8.1 (0.5) | 0.450 |
| Cortisol (ng × mL−1) | 32 (0.9) | 31.7 (1.2) | 0.852 |
| Myostatin (ng × mL−1) | 4.5 (0.1) | 4.3 (0.1) | 0.484 |
| Follistatin (ng × mL−1) | 1.7 (0.1) | 1.6 (0.1) | 0.786 |
| Follistatin–Myostatin ratio (ng × mL−1) | 0.3 (0.02) | 0.4 (0.03) | 0.812 |
| Weeks 1–2–3 | Weeks 4–5–6 | Rest | ||
|---|---|---|---|---|
| Day 1 and 3 | Bench press | 12, 10, 8 | 8, 6, 4 | 1.5–2 min |
| Lateral pull | 12, 10, 8 | 8, 6, 4 | 1.5–2 min | |
| Shoulder press | 12, 10, 8 | 8, 6, 4 | 1.5–2 min | |
| Seated rows | 12, 10, 8 | 8, 6, 4 | 1.5–2 min | |
| Shoulder shrugs | 3 × 12 | 3 × 8 | 30–60 sec | |
| Chest Flies | 3 × 12 | 3 × 8 | 30–60 sec | |
| Bicep curl | 3 × 12 | 3 × 8 | 30–60 sec | |
| Triceps Press down | 3 × 12 | 3 × 8 | 30–60 sec | |
| Abdominal curls | 125 weighted reps | 125 weighted reps | none | |
| Day 2 and 4 | Leg press | 12, 10, 8 | 8, 6, 4 | 1.5–2.5 min |
| Back squats | 12, 10, 8 | 8, 6, 4 | 1.5–2.5 min | |
| Step-ups | 12, 10, 8 | 8, 6, 4 | 30–60 sec | |
| Leg curls | 12, 10, 8 | 8, 6, 4 | 30–60 sec | |
| Back extension | 3 × 12 | 3 × 8 | 30–60 sec | |
| Calf raises | 3 × 12 | 3 × 8 | 30–60 sec | |
| Abdominal curls | 200 unweighted reps | 200 unweighted reps | none | |
| Variables | Group | Pre-Training | Post-Training | p-Value |
|---|---|---|---|---|
| Energy (kcal/day) | Milk | 2623.8 ± 107.4 | 2909.2 ± 109.2 *, # | <0.001 |
| Placebo | 2618.6 ± 104.9 | 2653.3 ± 109.1 | ||
| Absolute Protein (g/day) | Milk | 114.4 ± 7.7 | 178.2 ± 13 *, # | <0.001 |
| Placebo | 112.8 ± 11.7 | 114.2 ± 11.3 | ||
| Relative Protein (g/kg/day) | Milk | 1.5 ± 0.1 | 2.3 ± 0.2 | <0.001 |
| Placebo | 1.4 ± 0.1 | 1.4 ± 0.2 | ||
| Absolute Fat (g/day) | Milk | 95.2 ± 5.4 | 97.5 ± 4.4 | 0.285 |
| Placebo | 94 ± 6.2 | 95.4 ± 4.6 | ||
| Relative Fat (g/kg/day) | Milk | 1.2 ± 0.1 | 1.2 ± 0.08 | 0.323 |
| Placebo | 1.2 ± 0.1 | 1.2 ± 0.08 | ||
| Absolute Carbohydrate (g/day) | Milk | 327.1 ± 17 | 329.6 ± 15.4 | 0.385 |
| Placebo | 330.2 ± 16 | 334.3 ± 14 | ||
| Relative Carbohydrate (g/kg/day) | Milk | 4.2 ± 0.3 | 4.1 ± 0.3 | 0.919 |
| Placebo | 4.2 ± 0.3 | 4.2 ± 0.3 |
| Variables | Group | Pre-Training | Post-Training | p-Value |
|---|---|---|---|---|
| Body mass (kg) | Milk | 77.1 ± 5.1 | 79.1 ± 5.3 | <0.001 |
| Placebo | 78.5 ± 5.9 | 79.3 ± 5.9 | ||
| Body mass index (kg/m2) | Milk | 24.2 ± 1.8 | 24.6 ± 1.8 | <0.001 |
| Placebo | 25.1 3.1 | 25.3 ± 3 | ||
| Fat mass (kg) | Milk | 16.4 ± 1.1 | 16.3 ± 1.1 | 0.432 |
| Placebo | 16.8 ± 1.3 | 16.8 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pourabbas, M.; Bagheri, R.; Hooshmand Moghadam, B.; Willoughby, D.S.; Candow, D.G.; Elliott, B.T.; Forbes, S.C.; Ashtary-Larky, D.; Eskandari, M.; Wong, A.; et al. Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males. Nutrients 2021, 13, 948. https://doi.org/10.3390/nu13030948
Pourabbas M, Bagheri R, Hooshmand Moghadam B, Willoughby DS, Candow DG, Elliott BT, Forbes SC, Ashtary-Larky D, Eskandari M, Wong A, et al. Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males. Nutrients. 2021; 13(3):948. https://doi.org/10.3390/nu13030948
Chicago/Turabian StylePourabbas, Maryam, Reza Bagheri, Babak Hooshmand Moghadam, Darryn S. Willoughby, Darren G. Candow, Bradley T. Elliott, Scott C. Forbes, Damoon Ashtary-Larky, Mozhgan Eskandari, Alexei Wong, and et al. 2021. "Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males" Nutrients 13, no. 3: 948. https://doi.org/10.3390/nu13030948
APA StylePourabbas, M., Bagheri, R., Hooshmand Moghadam, B., Willoughby, D. S., Candow, D. G., Elliott, B. T., Forbes, S. C., Ashtary-Larky, D., Eskandari, M., Wong, A., & Dutheil, F. (2021). Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males. Nutrients, 13(3), 948. https://doi.org/10.3390/nu13030948












