Plant-Derived Trans-β-Caryophyllene Boosts Glucose Metabolism and ATP Synthesis in Skeletal Muscle Cells through Cannabinoid Type 2 Receptor Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. CB2 Receptor (CB2R) Immunofluorescence Staining
2.4. Glucose Uptake Measurements
2.5. Glycolysis Enzyme Activity
2.6. Mitochondria Extraction
2.7. Pyruvate Dehydrogenase (PDH) and Tricarboxylic Acid (TCA) Enzymes Activity
2.8. Electron Transport Chain (ETC) and Mitochondrial ATP
2.9. Statistical Analysis
3. Results
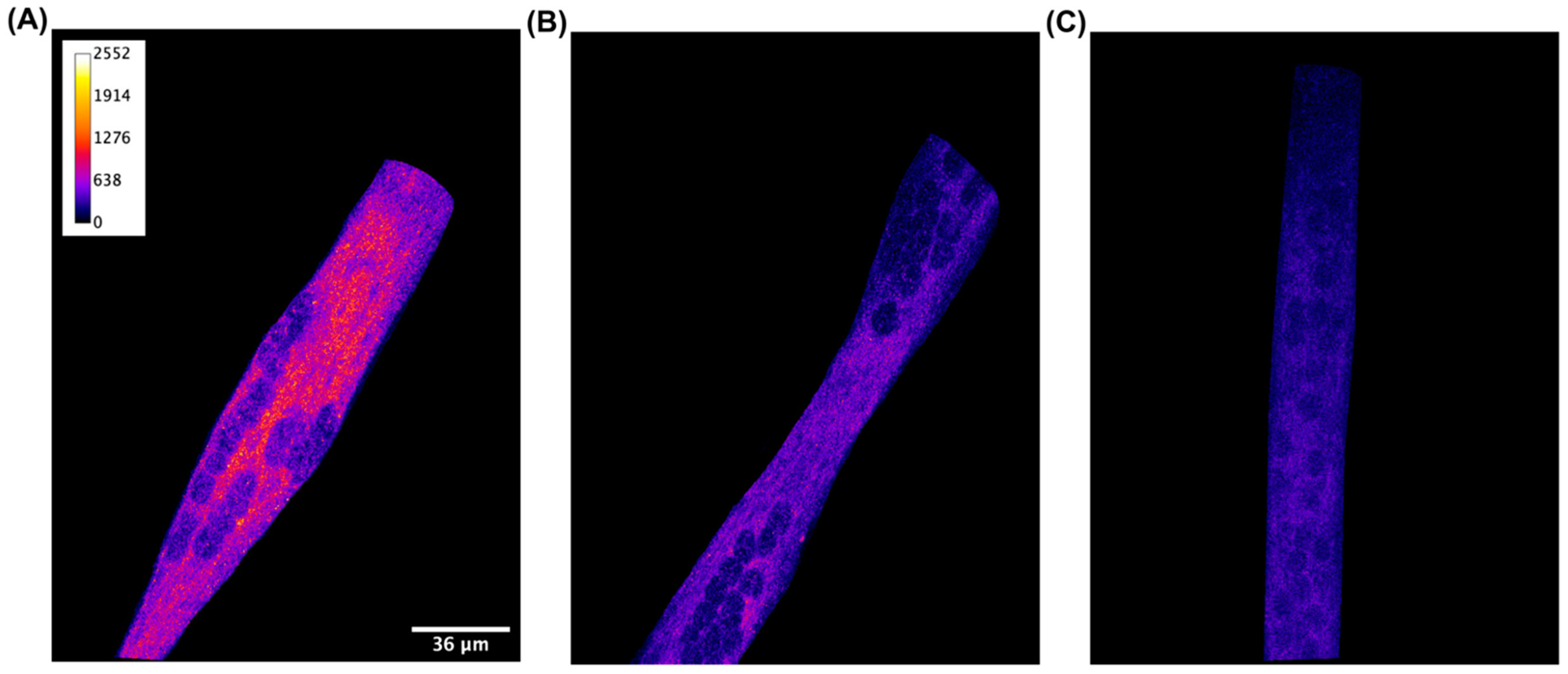
3.1. CB2 Receptor Is Expressed in C2C12 Skeletal Muscle Cells
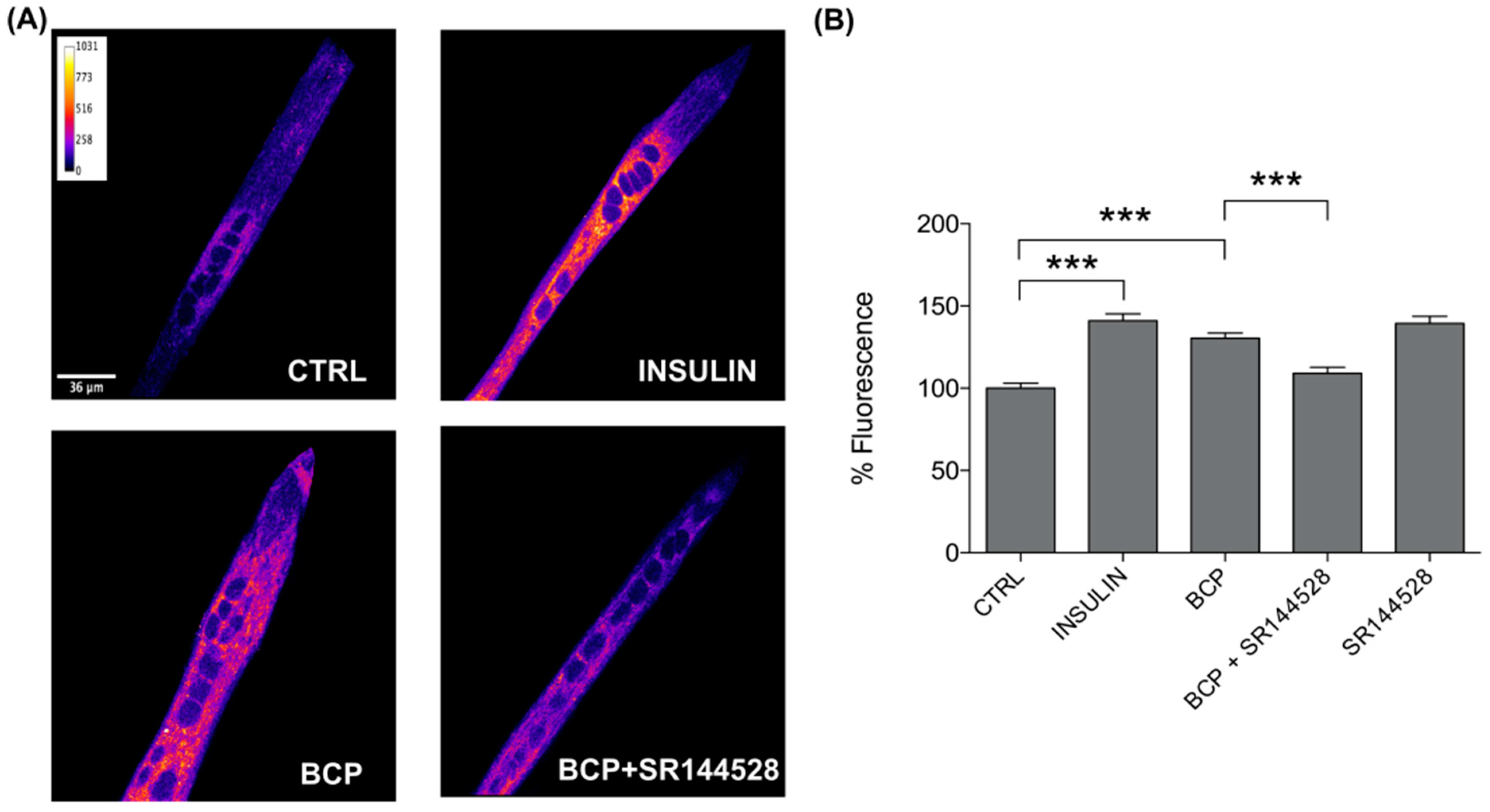
3.2. CB2 Receptor Mediates Glucose Uptake in C2C12 Myotubes
3.3. Trans-β-Caryophyllene (BCP) Improves Glycolytic Metabolism
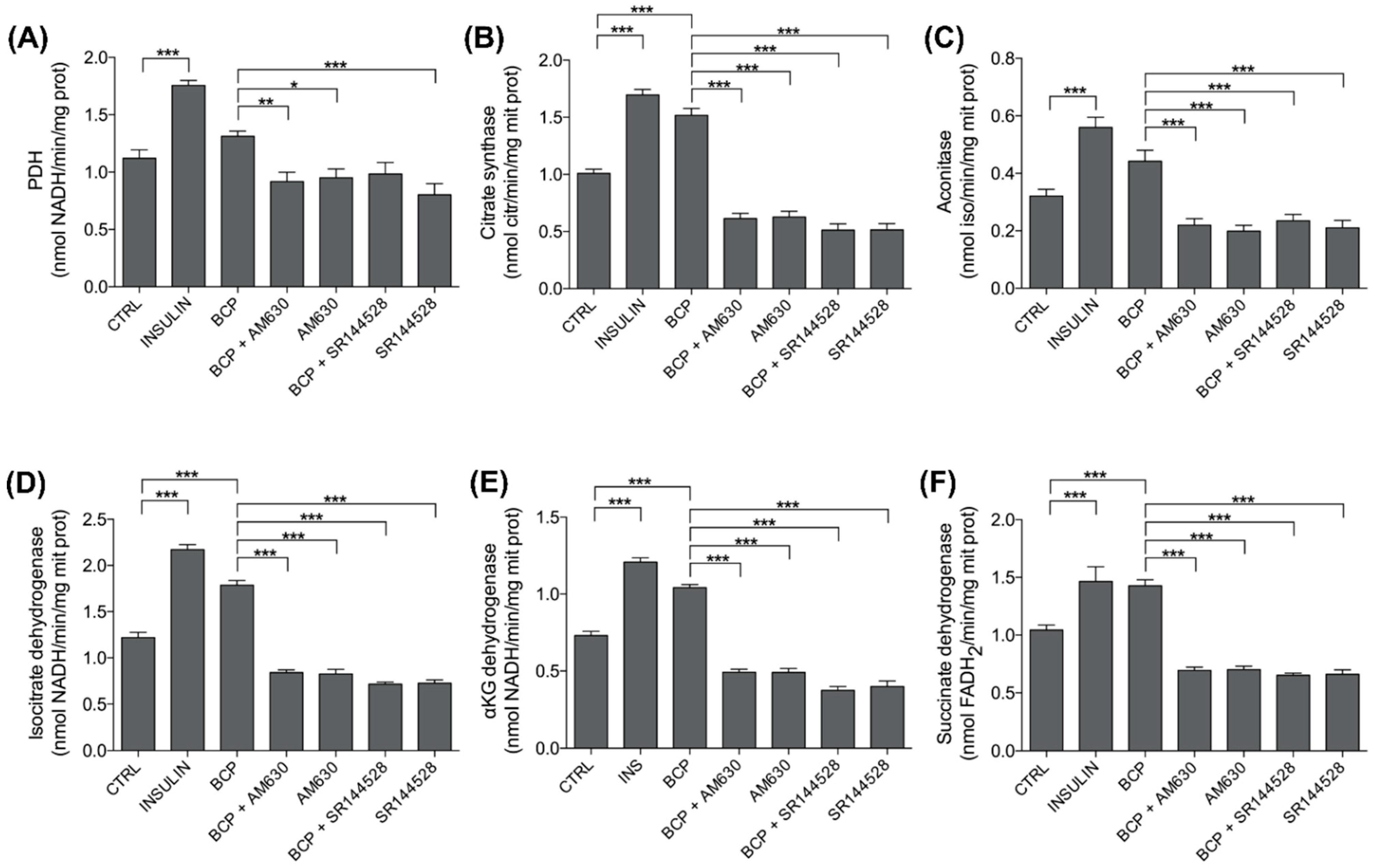
3.4. BCP Improves Mitochondrial Metabolism: PDH and TCA Enzyme Activity
3.5. BCP Improves Mitochondrial Metabolism: Electron Transport Chain (ETC) and Mitochondrial ATP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceco, E.; Weinberg, S.E.; Chandel, N.S.; Sznajder, J.I. Metabolism and skeletal muscle homeostasis in lung disease. Am. J. Respir. Cell Mol. Biol. 2017, 57, 28–34. [Google Scholar] [CrossRef]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle—A focus on the molecular mechanisms of insulin resistance. Mol. Cell. Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Deshmukh, A.S. Insulin-stimulated glucose uptake in healthy and insulin-resistant skeletal muscle. Horm. Mol. Biol. Clin. Investig. 2016, 26, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Alvim, R.O.; Cheuhen, M.R.; Machado, S.R.; Sousa, A.G.P.; Santos, P.C.J.L. General aspects of muscle glucose uptake. Acad. Bras. Ciênc. 2015, 87, 351–368. [Google Scholar] [CrossRef]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal muscle regulates metabolism via interorgan crosstalk: Roles in health and disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Javeed, N.; Matveyenko, A.V. Circadian etiology of Type 2 Diabetes mellitus. Physiology 2018, 33, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Looijaard, S.M.L.M.; te Lintel Hekkert, M.L.; Wüst, R.C.I.; Otten, R.H.J.; Meskers, C.G.M.; Maier, A.B. Pathophysiological mechanisms explaining poor clinical outcome of older cancer patients with low skeletal muscle mass. Acta Physiol. 2021, 231, e13516. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; et al. RIFM fragrance ingredient safety assessment, β-Caryophyllene alcohol, CAS registry number 472-97-9. Food Chem. Toxicol. 2018, 122, S566–S572. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and therapeutic potential of β-Caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic Interaction of β-Caryophyllene with Aromadendrene Oxide 2 and Phytol Induces Apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β -Caryophyllene and β -Caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Da Machado, K.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of Beta-Caryophyllene: Neurobiological effects of β-Caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective effect of β-Caryophyllene on cerebral ischemia-reperfusion injury via regulation of necroptotic neuronal death and inflammation: In vivo and in vitro. Front. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef]
- Al-Taee, H.; Azimullah, S.; Meeran, M.F.N.; Alaraj Almheiri, M.K.; Al Jasmi, R.A.; Tariq, S.; AB Khan, M.; Adeghate, E.; Ojha, S. β-Caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid Type-2 (CB2) receptors in rats. Chem. Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-γ receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective effects of (E)-β-Caryophyllene (BCP) in chronic inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Kamikubo, R.; Kai, K.; Tsuji-Naito, K.; Akagawa, M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 Hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Geddo, F.; Scandiffio, R.; Antoniotti, S.; Cottone, E.; Querio, G.; Maffei, M.E.; Bovolin, P.; Gallo, M.P. PipeNig®-FL, a fluid extract of black pepper (Piper Nigrum L.) with a high standardized content of Trans-β-Caryophyllene, reduces lipid accumulation in 3T3-L1 preadipocytes and improves glucose uptake in C2C12 myotubes. Nutrients 2019, 11, 2788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B. Kinetic characterisation of phosphofructokinase purified from Setaria Cervi: A bovine filarial parasite. Enzym. Res. 2011, 2011, 939472. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical Methods, 2nd ed.; Grune & Stratton: New York, NY, USA, 1975. [Google Scholar]
- Campia, I.; Lussiana, C.; Pescarmona, G.; Ghigo, D.; Bosia, A.; Riganti, C. Geranylgeraniol Prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. Br. J. Pharm. 2009, 158, 1777–1786. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Marzo, V.D.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid Receptors and their ligands: Beyond CB1 and CB2. Pharm. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Bockus, L.B.; Matsuzaki, S.; Vadvalkar, S.S.; Young, Z.T.; Giorgione, J.R.; Newhardt, M.F.; Kinter, M.; Humphries, K.M. Cardiac insulin signaling regulates glycolysis through Phosphofructokinase 2 Content and activity. JAHA 2017, 6, e007159. [Google Scholar] [CrossRef]
- López-Alarcón, L.; Mojena, M.; Monge, L.; Felíu, J.E. Stimulation of pyruvate kinase phosphatase activity by insulin in isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 1986, 134, 292–298. [Google Scholar] [CrossRef]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, T.; Wang, X. Activation of Type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation through the SIRT1/PGC-1α pathway. Biochem. Biophys. Res. Commun. 2013, 436, 377–381. [Google Scholar] [CrossRef]
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Janovská, A.; Game, P.; Wittert, G.A. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 2007, 364, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, I.; Lutz, B. Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues. Cell. Mol. Life Sci. 2019, 76, 1341–1363. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, V.S.; Kaur, G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, S.-K.; Tian, Z.-L.; Wang, M.; Zhao, R.; Wang, L.-L.; Li, S.-S.; Liu, M.; Li, J.-Y.; Zhang, M.-Z.; et al. CB2R orchestrates fibrogenesis through regulation of inflammatory response during the repair of skeletal muscle contusion. Int. J. Clin. Exp. Pathol. 2015, 8, 3491–3502. [Google Scholar]
- Zhang, M.; Zhang, M.; Wang, L.; Yu, T.; Jiang, S.; Jiang, P.; Sun, Y.; Pi, J.; Zhao, R.; Guan, D. Activation of cannabinoid Type 2 receptor protects skeletal muscle from ischemia-reperfusion injury partly via Nrf2 signaling. Life Sci. 2019, 230, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kobayashi, H.; Kawasaki, H.; Mineki, R.; Naito, H.; Ohmori, D. Glyceraldehyde-3-Phosphate dehydrogenase interacts with phosphorylated Akt resulting from increased blood glucose in rat cardiac muscle. FEBS Lett. 2010, 584, 2796–2800. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Sato, K.; Tsuchiya, N.; Thomas, S.; Fell, D.A.; Veech, R.L.; Passonneau, J.V. Control of glucose utilization in working perfused rat heart. J. Biol. Chem. 1994, 269, 25502–25514. [Google Scholar] [CrossRef]
- Dando, I.; Donadelli, M.; Costanzo, C.; Dalla Pozza, E.; D’Alessandro, A.; Zolla, L.; Palmieri, M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 2013, 4, e664. [Google Scholar] [CrossRef]
- Orozco, J.M.; Krawczyk, P.A.; Scaria, S.M.; Cangelosi, A.L.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. Dihydroxyacetone phosphate signals glucose availability to MTORC1. Nat. Metab. 2020, 2, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Suginohara, T.; Wakabayashi, K.; Ato, S.; Ogasawara, R. Effect of 2-Deoxyglucose-mediated inhibition of glycolysis on the regulation of MTOR signaling and protein synthesis before and after high-intensity muscle contraction. Metabolism 2021, 114, 154419. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Thoudam, T.; Choi, E.J.; Kim, M.-J.; Harris, R.A.; Lee, I.-K. Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases. J. Diabetes Investig. 2021, 12, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Mogensen, M.; Petersen, I.; Højlund, K.; Levin, K.; Sahlin, K.; Beck-Nielsen, H.; Gaster, M. Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with Type 2 diabetes: Evidence for an intrinsic oxidative enzyme defect. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1741, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gallardo, R.V.; Noriega-Cisneros, R.; Esquivel-Gutiérrez, E.; Calderón-Cortés, E.; Cortés-Rojo, C.; Manzo-Avalos, S.; Campos-García, J.; Salgado-Garciglia, R.; Montoya-Pérez, R.; Boldogh, I.; et al. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. J. Bioenerg. Biomembr. 2014, 46, 511–518. [Google Scholar] [CrossRef]
- Mullen, E.; Ohlendieck, K. Proteomic profiling of non-obese Type 2 diabetic skeletal muscle. Int. J. Mol. Med. 2010, 25, 445–458. [Google Scholar] [CrossRef]
- Spallotta, F.; Cencioni, C.; Atlante, S.; Garella, D.; Cocco, M.; Mori, M.; Mastrocola, R.; Kuenne, C.; Guenther, S.; Nanni, S.; et al. Stable oxidative cytosine modifications accumulate in cardiac mesenchymal cells from Type2 Diabetes patients: Rescue by α-Ketoglutarate and TET-TDG functional reactivation. Circ. Res. 2018, 122, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Protective effect of theaflavin on glycoprotein components and TCA cycle enzymes in high-fat diet and streptozotocin-induced diabetic rats. J. Basic Appl. Zool. 2019, 80, 1–9. [Google Scholar] [CrossRef]
- Zou, K.; Turner, K.; Zheng, D.; Hinkley, J.M.; Kugler, B.A.; Hornby, P.J.; Lenhard, J.; Jones, T.E.; Pories, W.J.; Dohm, G.L.; et al. Impaired glucose partitioning in primary myotubes from severely obese women with Type 2 Diabetes. Am. J. Physiol. Cell Physiol. 2020, 319, C1011–C1019. [Google Scholar] [CrossRef]
- Gaster, M.; Nehlin, J.O.; Minet, A.D. Impaired TCA cycle flux in mitochondria in skeletal muscle from Type 2 diabetic subjects: Marker or maker of the diabetic phenotype? Arch. Physiol. Biochem. 2012, 118, 156–189. [Google Scholar] [CrossRef]
- Nair, K.S. Aging muscle. Am. J. Clin. Nutr. 2005, 81, 953–963. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-Y.; Ju, C.; Anthony Jalin, A.M.A.; Lee, D.I.; Prather, P.L.; Kim, W.-K. Activation of cannabinoid CB2 receptor–mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am. J. Pathol. 2013, 182, 928–939. [Google Scholar] [CrossRef] [PubMed]

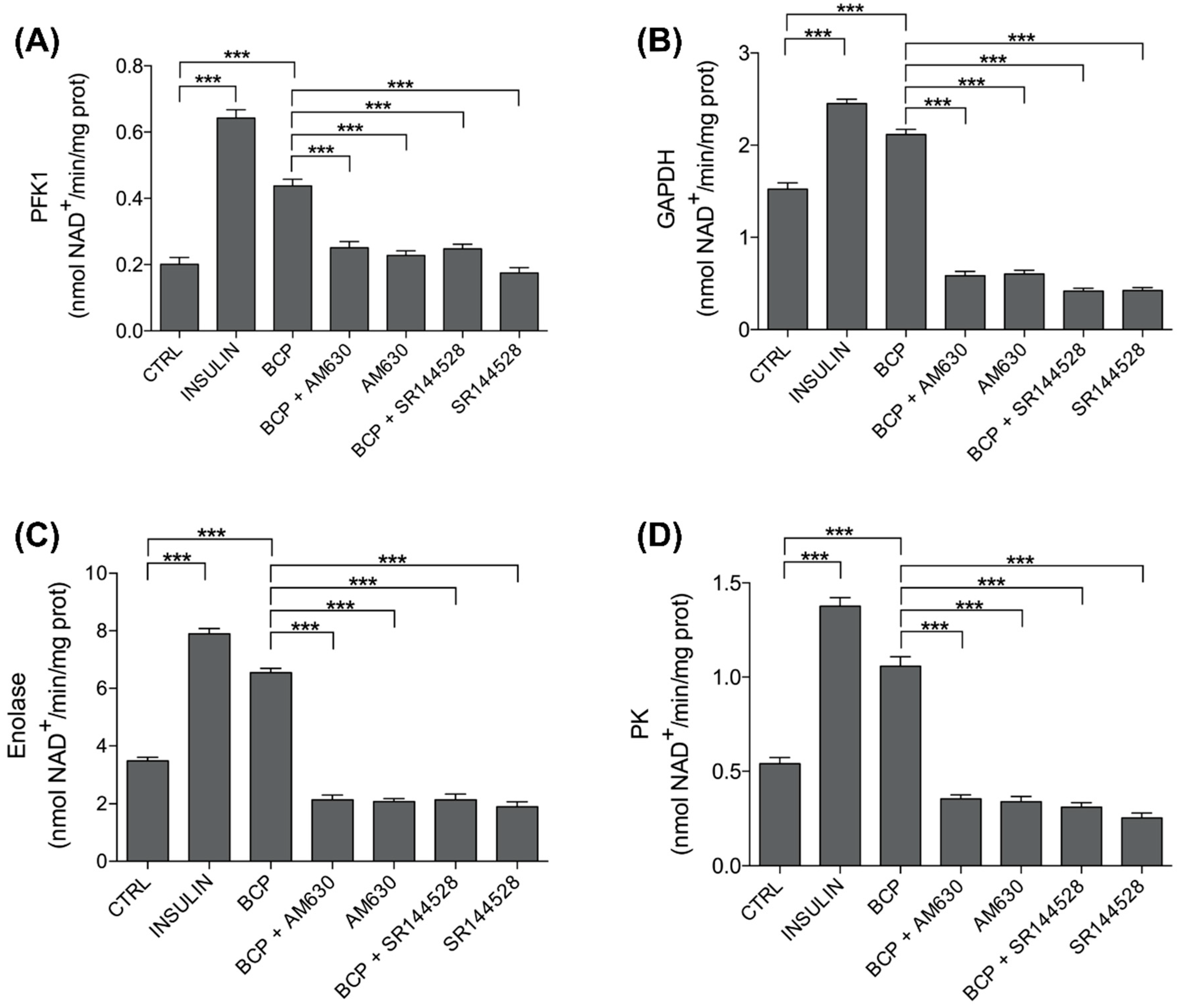

| Treatments | PKF1 | GAPDH | Enolase | PK |
|---|---|---|---|---|
| CTRL | 0.2011 ± 0.0202 | 1.5244 ± 0.0675 | 3.4756 ± 0.1327 | 0.5411 ± 0.0322 |
| INS | 0.6422 ± 0.0257 | 2.4522 ± 0.0455 | 7.8967 ± 0.1827 | 1.3767 ± 0.0455 |
| BCP | 0.4378 ± 0.0201 | 2.1156 ± 0.0566 | 6.5489 ± 0.1505 | 1.0578 ± 0.0499 |
| BCP + AM630 | 0.2511 ± 0.0184 | 0.5833 ± 0.0489 | 2.1411 ± 0.1623 | 0.3556 ± 0.0214 |
| AM630 | 0.2278 ± 0.0138 | 0.6033 ± 0.0402 | 2.0811 ± 0.0946 | 0.3411 ± 0.0274 |
| BCP + SR144528 | 0.2475 ± 0.0140 | 0.4188 ± 0.0311 | 2.1413 ± 0.1923 | 0.3113 ± 0.0246 |
| SR144528 | 0.1750 ± 0.0157 | 0.4238 ± 0.0317 | 1.8925 ± 0.1796 | 0.2550 ± 0.0251 |
| Treatments | PDH | Citrate Synth | Aconitase | IsocitrateDH | aKGDH | SuccinateDH |
|---|---|---|---|---|---|---|
| CTRL | 1.2111 ± 0.0723 | 1.0100 ± 0.0350 | 0.3211 ± 0.0232 | 1.220 ± 0.0554 | 0.7300 ± 0.0281 | 1.044 ± 0.0423 |
| INS | 1.7566 ± 0.0427 | 1.6955 ± 0.0490 | 0.5600 ± 0.0348 | 2.1711 ± 0.0542 | 1.2078 ± 0.0282 | 1.4644 ± 0.1284 |
| BCP | 1.3122 ± 0.0457 | 1.5177 ± 0.0604 | 0.4422 ± 0.0379 | 1.7855 ± 0.0513 | 1.0422 ± 0.0196 | 1.4277 ± 0.0522 |
| BCP + AM630 | 0.9166 ± 0.0824 | 0.6133 ± 0.0455 | 0.2200 ± 0.0224 | 0.8411 ± 0.0296 | 0.4922 ± 0.0196 | 0.6944 ± 0.0278 |
| AM630 | 0.9511 ± 0.0771 | 0.6277 ± 0.0488 | 0.1989 ± 0.0200 | 0.8266 ± 0.0493 | 0.4911 ± 0.0260 | 0.7011 ± 0.0293 |
| BCP + SR144528 | 0.9837 ± 0.0997 | 0.5125 ± 0.0549 | 0.2350 ± 0.0220 | 0.7162 ± 0.0213 | 0.3738 ± 0.0260 | 0.6525 ± 0.0184 |
| SR144528 | 0.8025 ± 0.0970 | 0.5150 ± 0.0534 | 0.2100 ± 0.0266 | 0.7250 ± 0.0367 | 0.4000 ± 0.0354 | 0.6612 ± 0.0384 |
| Treatments | ETC | ATP |
|---|---|---|
| CTRL | 0.5722 ± 0.0305 | 12.5833 ± 0.6169 |
| INS | 1.0856 ± 0.0312 | 25.0311 ± 0.9975 |
| BCP | 0.9778 ± 0.0310 | 20.0367 ± 0.6576 |
| BCP + AM630 | 0.3767 ± 0.0291 | 7.4978 ± 0.2913 |
| AM630 | 0.3689 ± 0.0126 | 7.2322 ± 0.4469 |
| BCP + SR144528 | 0.2963 ± 0.0210 | 7.1350 ± 0.3379 |
| SR144528 | 0.3063 ± 0.0240 | 6.4625 ± 0.2724 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddo, F.; Antoniotti, S.; Querio, G.; Salaroglio, I.C.; Costamagna, C.; Riganti, C.; Gallo, M.P. Plant-Derived Trans-β-Caryophyllene Boosts Glucose Metabolism and ATP Synthesis in Skeletal Muscle Cells through Cannabinoid Type 2 Receptor Stimulation. Nutrients 2021, 13, 916. https://doi.org/10.3390/nu13030916
Geddo F, Antoniotti S, Querio G, Salaroglio IC, Costamagna C, Riganti C, Gallo MP. Plant-Derived Trans-β-Caryophyllene Boosts Glucose Metabolism and ATP Synthesis in Skeletal Muscle Cells through Cannabinoid Type 2 Receptor Stimulation. Nutrients. 2021; 13(3):916. https://doi.org/10.3390/nu13030916
Chicago/Turabian StyleGeddo, Federica, Susanna Antoniotti, Giulia Querio, Iris Chiara Salaroglio, Costanzo Costamagna, Chiara Riganti, and Maria Pia Gallo. 2021. "Plant-Derived Trans-β-Caryophyllene Boosts Glucose Metabolism and ATP Synthesis in Skeletal Muscle Cells through Cannabinoid Type 2 Receptor Stimulation" Nutrients 13, no. 3: 916. https://doi.org/10.3390/nu13030916
APA StyleGeddo, F., Antoniotti, S., Querio, G., Salaroglio, I. C., Costamagna, C., Riganti, C., & Gallo, M. P. (2021). Plant-Derived Trans-β-Caryophyllene Boosts Glucose Metabolism and ATP Synthesis in Skeletal Muscle Cells through Cannabinoid Type 2 Receptor Stimulation. Nutrients, 13(3), 916. https://doi.org/10.3390/nu13030916








