Saffron Extract-Induced Improvement of Depressive-Like Behavior in Mice Is Associated with Modulation of Monoaminergic Neurotransmission
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
2.2. Oral Administration of Safr’InsideTM
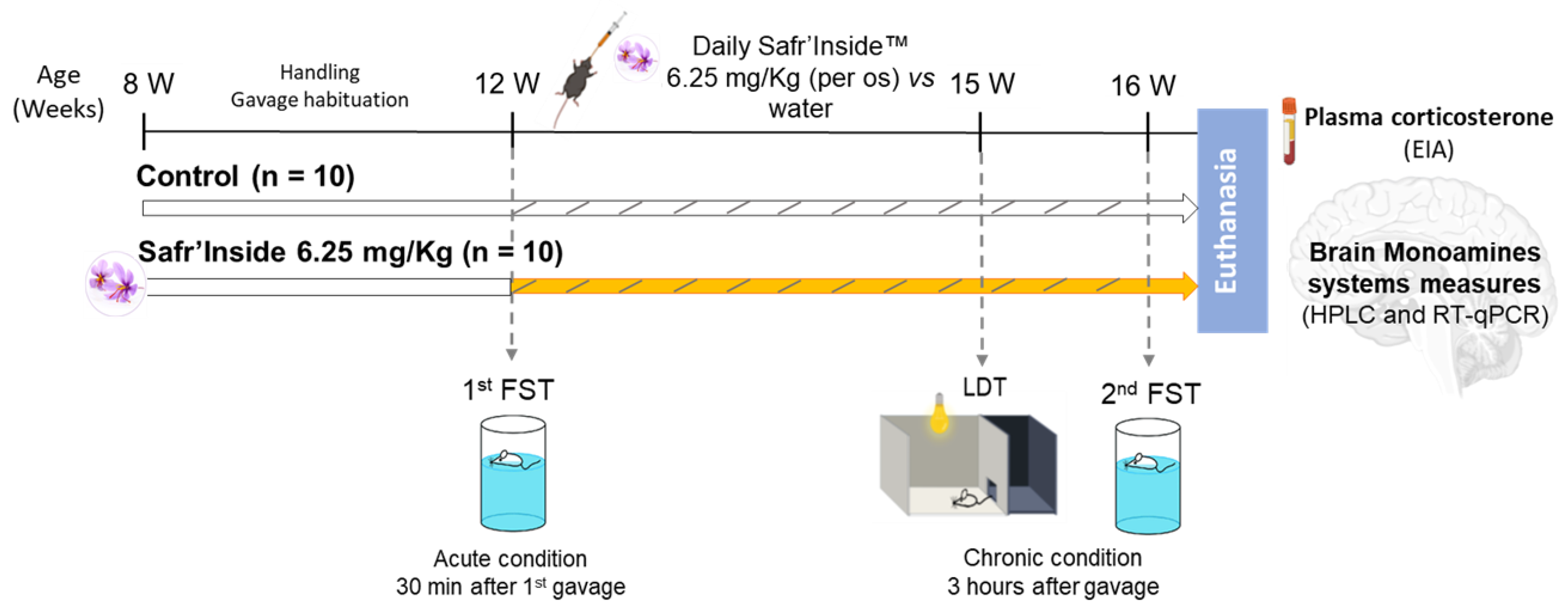
2.3. Experimental Design
2.4. Behavioral Tests
2.5. Tissue Sampling
2.6. Enzyme Immunoassays (EIA) for Corticosterone Dosage
2.7. Brain Monoamines and Metabolites Analysis by High Performance Liquid Chromatography Coupled to Electrochemical Detection (HPLC-EC)
2.8. Real-Time Quantitative PCR (RT-qPCR)
2.9. Statistical Analysis
3. Results
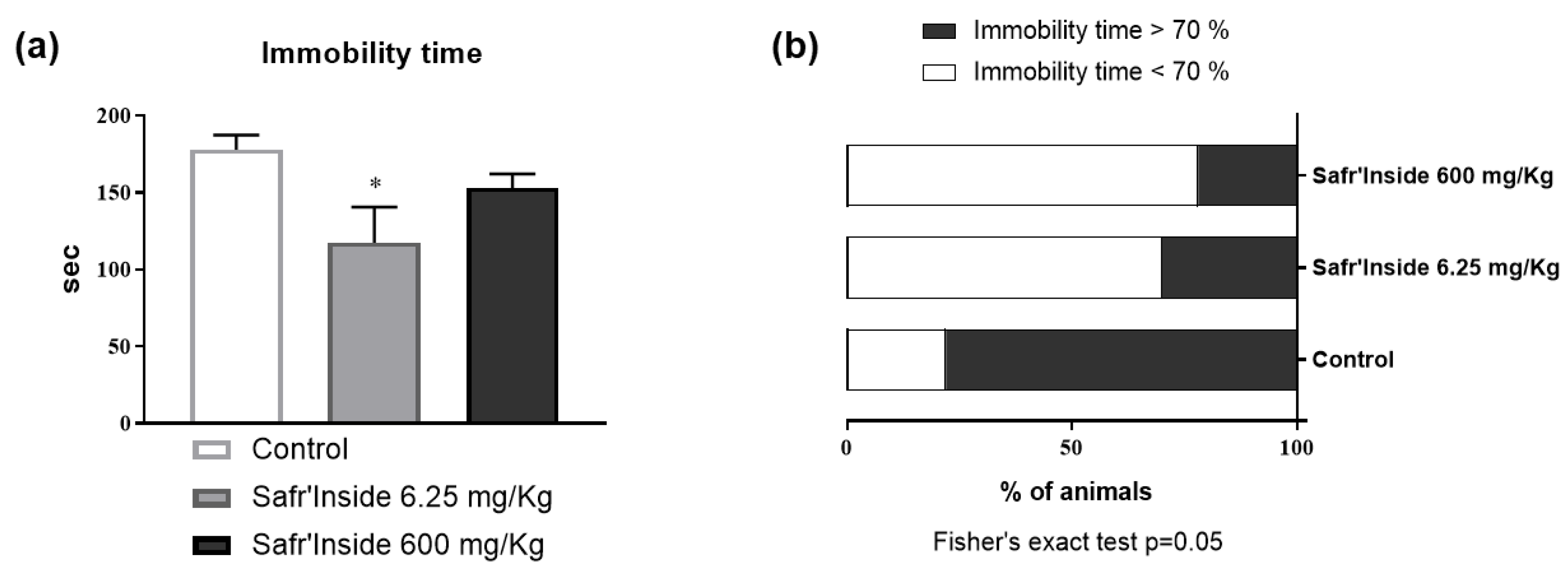
3.1. Efficient Dose of Acute Oral Administration of Safr’InsideTM in the FST
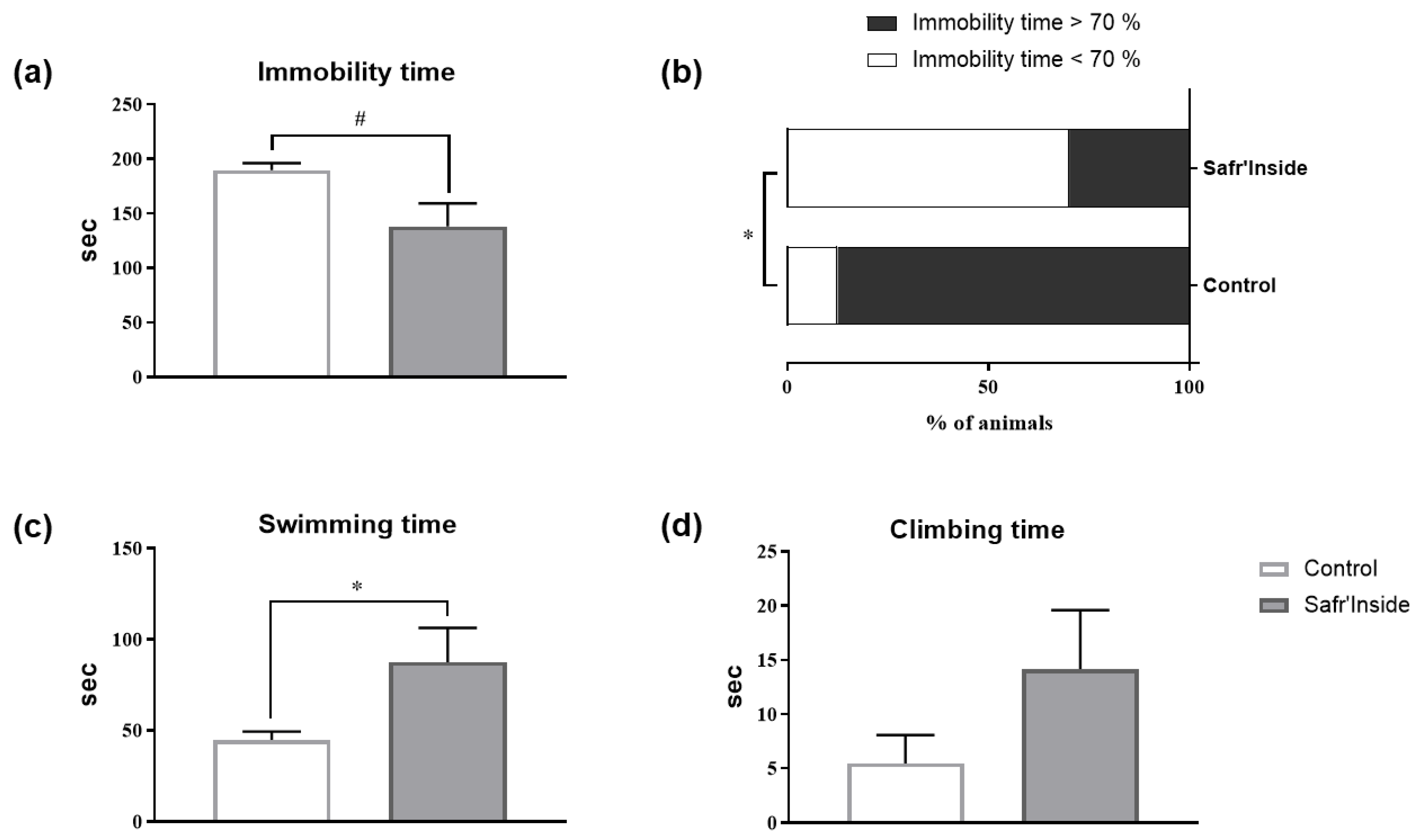
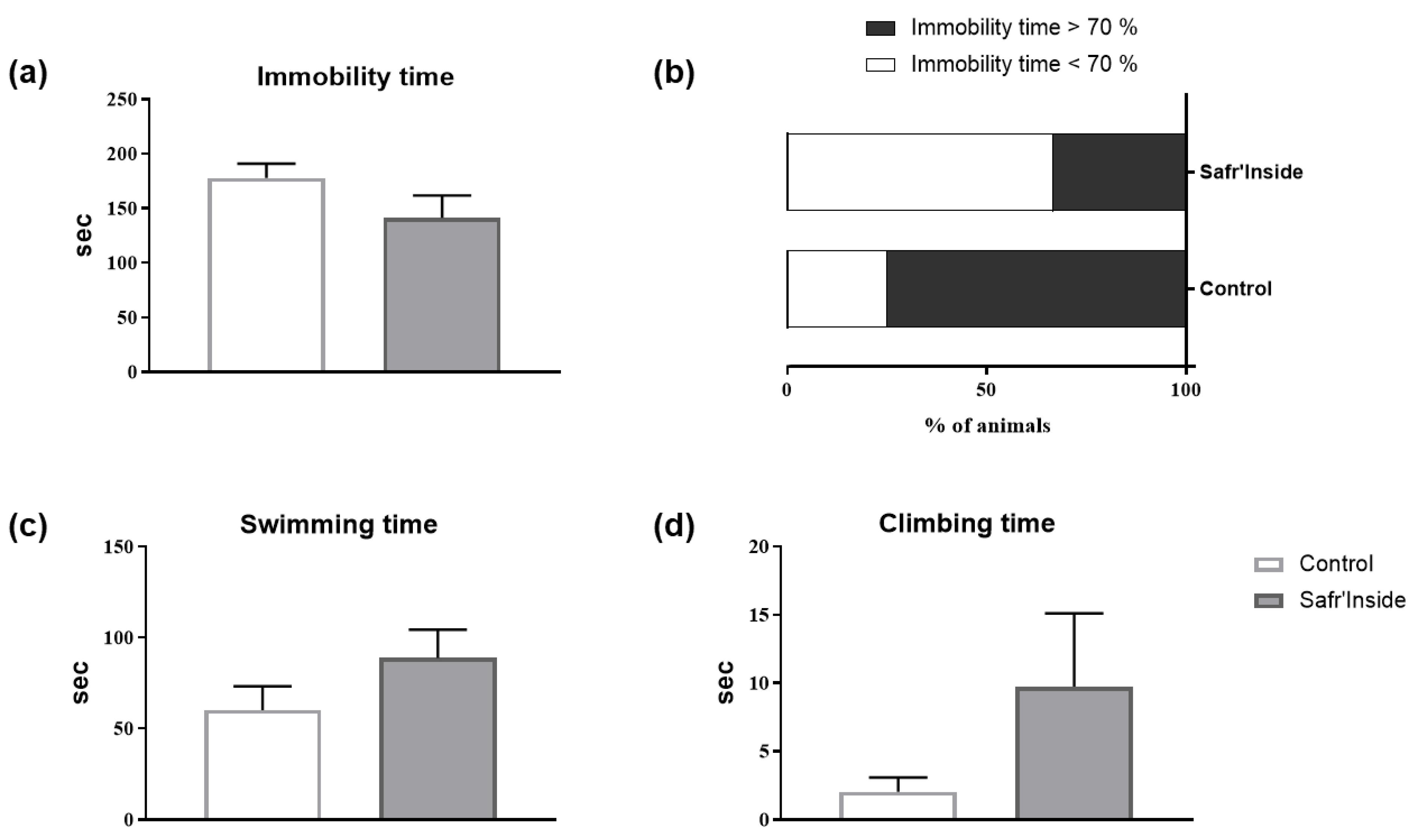
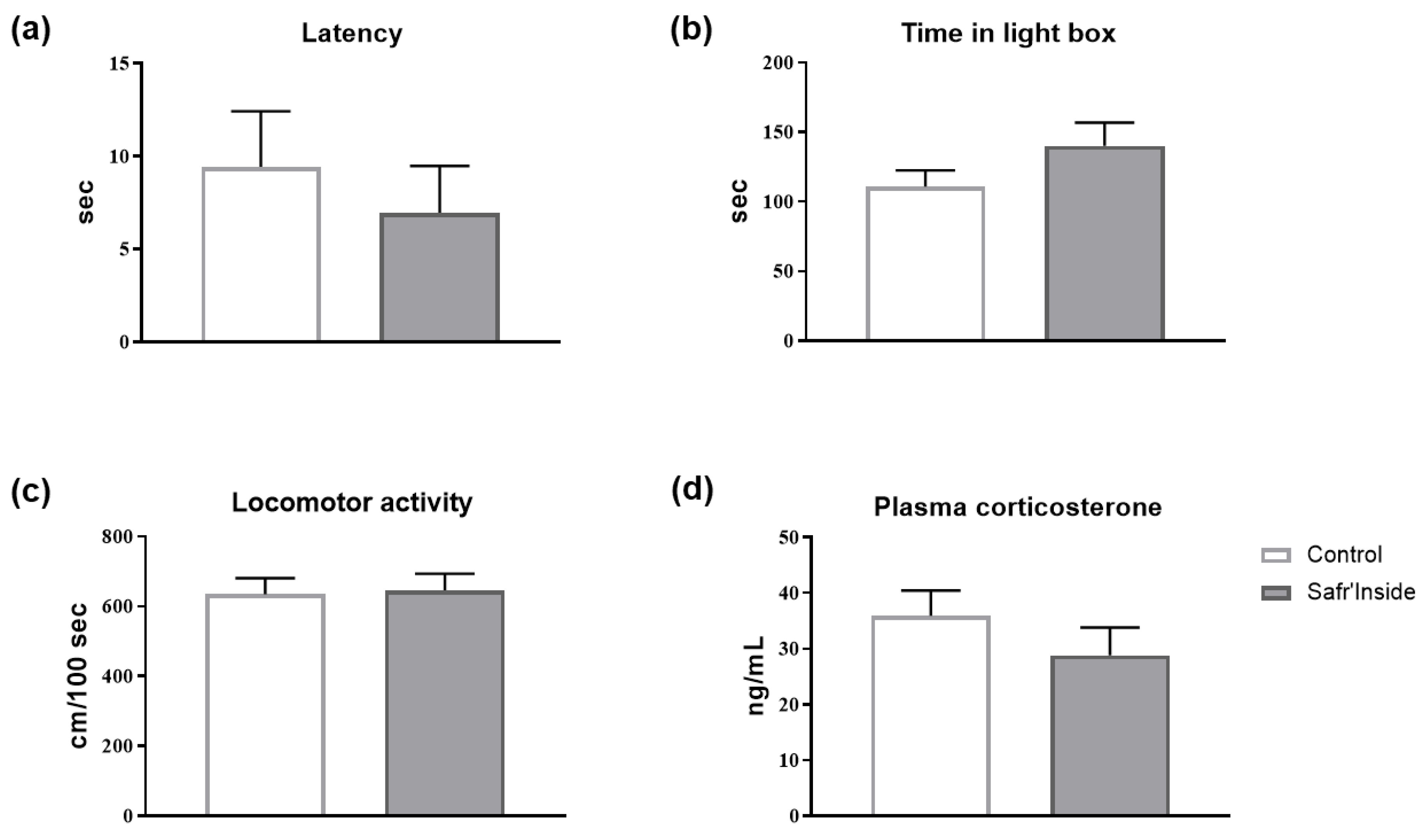
3.2. Impact of Acute and Chronic Oral Administration of Safr’InsideTM on Depressive-Like and Anxiety-Like Behaviors
3.3. Impact of Chronic Oral Administration of Safr’InsideTM on Monoaminergic Neurotransmission
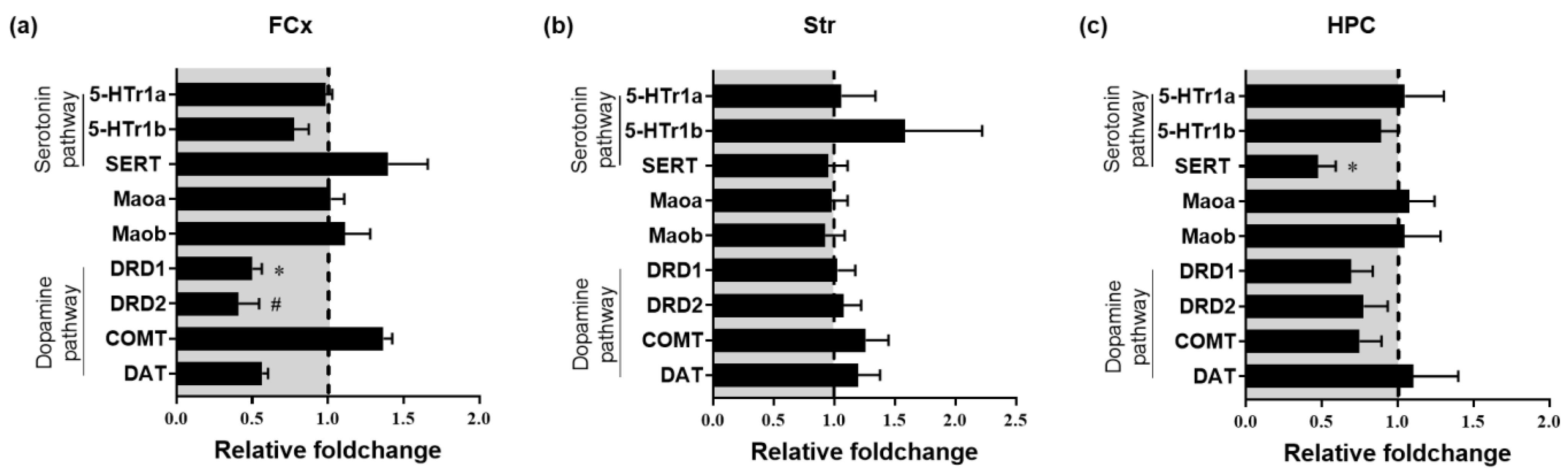
3.4. Impact of Chronic Oral Administration of Safr’InsideTM on Gene Expression of Markers of 5-HT and DA Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Depression and Other Common Mental Disorders. Available online: http://www.who.int/mental_health/management/depression/prevalence_global_health_estimates/en/ (accessed on 7 January 2021).
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroye, J.O.; Da Silva, D.M.; De Oliveira, D.R.; Costa, E.A. Treatment of Anxiety and Depression: Medicinal Plants in Retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Trindade, E.; Menon, D.; Topfer, L.A.; Coloma, C. Adverse Effects Associated with Selective Serotonin Reuptake Inhibitors and Tricyclic Antidepressants: A Meta-Analysis. CMAJ Can. Med. Assoc. J. J. Assoc. Med. Can. 1998, 159, 1245–1252. [Google Scholar]
- Blackwell, B. Adverse Effects of Antidepressant Drugs Part 1: Monoamine Oxidase Inhibitors and Tricyclics. Drugs 1981, 21, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-P.; Matsuoka, Y.; Pae, C.-U. Omega-3 Polyunsaturated Fatty Acids in Prevention of Mood and Anxiety Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal Medicine for Depression and Anxiety: A Systematic Review with Assessment of Potential Psycho-Oncologic Relevance. Phytother. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.-H.; Kassaian, S.E.; Noorbala Tafti, A.-A.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S.; et al. A Randomized, Double-Blind, Clinical Trial Comparing the Efficacy and Safety of Crocus sativus L. with Fluoxetine for Improving Mild to Moderate Depression in Post Percutaneous Coronary Intervention Patients. J. Affect. Disord. 2014, 155, 216–222. [Google Scholar] [CrossRef]
- Mazidi, M.; Shemshian, M.; Mousavi, S.H.; Norouzy, A.; Kermani, T.; Moghiman, T.; Sadeghi, A.; Mokhber, N.; Ghayour-Mobarhan, M.; Ferns, G.A.A. A Double-Blind, Randomized and Placebo-Controlled Trial of Saffron (Crocus sativus L.) in the Treatment of Anxiety and Depression. J. Complement. Integr. Med. 2016, 13. [Google Scholar] [CrossRef]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and Biological Properties of the World’s Most Expensive Spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Bathaie, S.Z. Historical Uses of Saffron: Identifying Potential New Avenues for Modern Research. Avicenna J. Phytomedicine 2011, 1, 57–66. [Google Scholar]
- Bathaie, S.Z.; Hoshyar, R.; Miri, H.; Sadeghizadeh, M. Anticancer Effects of Crocetin in Both Human Adenocarcinoma Gastric Cancer Cells and Rat Model of Gastric Cancer. Biochem. Cell Biol. 2013, 91, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H. Saffron: A Promising Natural Medicine in the Treatment of Metabolic Syndrome: Saffron as a Natural Medicine in Metabolic Syndrome. J. Sci. Food Agric. 2017, 97, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and Hypnotic Effect of Crocus sativus Aqueous Extract and Its Constituents, Crocin and Safranal, in Mice. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Noorbala, A.A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-Alcoholic Extract of Crocus sativus L. versus Fluoxetine in the Treatment of Mild to Moderate Depression: A Double-Blind, Randomized Pilot Trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Talaei, A.; Hassanpour Moghadam, M.; Sajadi Tabassi, S.A.; Mohajeri, S.A. Crocin, the Main Active Saffron Constituent, as an Adjunctive Treatment in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. J. Affect. Disord. 2015, 174, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Saha, D.; Dubyak, P.J.; Anton, S.D. Saffron (Crocus sativus L.) and Major Depressive Disorder: A Meta-Analysis of Randomized Clinical Trials. J. Integr. Med. 2013, 11, 377–383. [Google Scholar] [CrossRef]
- Wang, Y.; Han, T.; Zhu, Y.; Zheng, C.-J.; Ming, Q.-L.; Rahman, K.; Qin, L.-P. Antidepressant Properties of Bioactive Fractions from the Extract of Crocus sativus L. J. Nat. Med. 2010, 64, 24–30. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant Effect of Crocus sativus L. Stigma Extracts and their Constituents, Crocin and Safranal, in Mice. Acta Hortic. 2004, 435–445. [Google Scholar] [CrossRef]
- Amin, B.; Nakhsaz, A.; Hosseinzadeh, H. Evaluation of the Antidepressant-like Effects of Acute and Sub-Acute Administration of Crocin and Crocetin in Mice. Avicenna J. Phytomed. 2015, 5, 458–468. [Google Scholar] [PubMed]
- Pitsikas, N.; Boultadakis, A.; Georgiadou, G.; Tarantilis, P.A.; Sakellaridis, N. Effects of the Active Constituents of Crocus sativus L., Crocins, in an Animal Model of Anxiety. Phytomedicine 2008, 15, 1135–1139. [Google Scholar] [CrossRef]
- Hollon, S.D.; Thase, M.E.; Markowitz, J.C. Treatment and Prevention of Depression. Psychol. Sci. Public Interest 2002, 3, 39–77. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.P.R.; Medvedev, I.O.; Caron, M.G. The 5-HT Deficiency Theory of Depression: Perspectives from a Naturalistic 5-HT Deficiency Model, the Tryptophan Hydroxylase 2Arg439His Knockin Mouse. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2444–2459. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.; Keshavan, M. The Neurobiology of Depression: An Integrated View. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Dopamine and Depression: A Review of Recent Evidence. I. Empirical Studies. Brain Res. Rev. 1983, 6, 211–224. [Google Scholar] [CrossRef]
- Dailly, E.; Chenu, F.; Renard, C.E.; Bourin, M. Dopamine, Depression and Antidepressants. Fundam. Clin. Pharmacol. 2004, 18, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Nemeroff, C.B. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry 2007, 64, 327. [Google Scholar] [CrossRef]
- Chong, T.T.-J.; Husain, M. The Role of Dopamine in the Pathophysiology and Treatment of Apathy. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 229, pp. 389–426. ISBN 978-0-444-63701-7. [Google Scholar]
- Salamone, J.D.; Correa, M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef]
- Cooper, B.R.; Hester, T.J.; Maxwell, R.A. Behavioral and Biochemical Effects of the Antidepressant Bupropion (Wellbutrin): Evidence for Selective Blockade of Dopamine Uptake in Vivo. J. Pharmacol. Exp. Ther. 1980, 215, 127–134. [Google Scholar]
- Capuron, L.; Castanon, N. Role of Inflammation in the Development of Neuropsychiatric Symptom Domains: Evidence and Mechanisms. Curr. Top. Behav. Neurosci. 2017, 31, 31–44. [Google Scholar] [CrossRef]
- Moras, B.; Loffredo, L.; Rey, S. Quality Assessment of Saffron (Crocus sativus L.) Extracts via UHPLC-DAD-MS Analysis and Detection of Adulteration Using Gardenia Fruit Extract (Gardenia Jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- USFDA. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Adult Healthy Volunteer; US Food and Drug Administration: Rockville, MD, USA, 2005.
- Dinel, A.-L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Cognitive and Emotional Alterations Are Related to Hippocampal Inflammation in a Mouse Model of Metabolic Syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef]
- André, C.; Dinel, A.-L.; Ferreira, G.; Layé, S.; Castanon, N. Diet-Induced Obesity Progressively Alters Cognition, Anxiety-like Behavior and Lipopolysaccharide-Induced Depressive-like Behavior: Focus on Brain Indoleamine 2,3-Dioxygenase Activation. Brain Behav. Immun. 2014, 41, 10–21. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal Models of Neuropsychiatric Disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The Mouse Forced Swim Test. J. Vis. Exp. 2011, 3638. [Google Scholar] [CrossRef]
- Fourrier, C.; Kropp, C.; Aubert, A.; Sauvant, J.; Vaysse, C.; Chardigny, J.-M.; Layé, S.; Joffre, C.; Castanon, N. Rapeseed Oil Fortified with Micronutrients Improves Cognitive Alterations Associated with Metabolic Syndrome. Brain Behav. Immun. 2020, 84, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the Gut Microbiota Enhances Anxiety-like Behavior and Neuroendocrine Response to Acute Stress in Rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef] [PubMed]
- De Cossío, L.F.; Fourrier, C.; Sauvant, J.; Everard, A.; Capuron, L.; Cani, P.D.; Layé, S.; Castanon, N. Impact of Prebiotics on Metabolic and Behavioral Alterations in a Mouse Model of Metabolic Syndrome. Brain Behav. Immun. 2017, 64, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.-D.; Milman, A.; Monnoye, M.; Douard, V.; Philippe, C.; Aubert, A.; Castanon, N.; Vancassel, S.; Guérineau, N.C.; Naudon, L.; et al. The Gut Microbiota Metabolite Indole Increases Emotional Responses and Adrenal Medulla Activity in Chronically Stressed Male Mice. Psychoneuroendocrinology 2020, 119, 104750. [Google Scholar] [CrossRef] [PubMed]
- Outlier Calculator. Available online: https://www.graphpad.com/quickcalcs/grubbs1/ (accessed on 9 March 2021).
- Bourin, M.; Hascoët, M. The Mouse Light/Dark Box Test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain Structural and Functional Abnormalities in Mood Disorders: Implications for Neurocircuitry Models of Depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, T.; Abnous, K.; Vahdati, F.; Mehri, S.; Razavi, B.; Hosseinzadeh, H. Antidepressant Effect of Crocus sativus Aqueous Extract and Its Effect on CREB, BDNF, and VGF Transcript and Protein Levels in Rat Hippocampus. Drug Res. 2014, 65, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. “Behavioural Despair” in Rats and Mice: Strain Differences and the Effects of Imipramine. Eur. J. Pharmacol. 1978, 51, 291–294. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Ballesta, A.; Martin, R.; Gomez de Heras, R. Antianhedonic and Antidepressant Effects of Affron®, a Standardized Saffron (Crocus sativus L.) Extract. Molecules 2020, 25, 3207. [Google Scholar] [CrossRef] [PubMed]
- Kazavchinsky, L.; Dafna, A.; Einat, H. Individual Variability in Female and Male Mice in a Test-Retest Protocol of the Forced Swim Test. J. Pharmacol. Toxicol. Methods 2019, 95, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Lucki, I. Antidepressant-like Behavioral Effects Mediated by 5-Hydroxytryptamine2C Receptors. J. Pharmacol. Exp. Ther. 2000, 295, 1120–1126. [Google Scholar]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Hooshmandi, Z.; Rohani, A.H.; Eidi, A.; Fatahi, Z.; Golmanesh, L.; Sahraei, H. Reduction of Metabolic and Behavioral Signs of Acute Stress in Male Wistar Rats by Saffron Water Extract and Its Constituent Safranal. Pharm. Biol. 2011, 49, 947–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halataei, B.-S.; Khosravi, M.; Arbabian, S.; Sahraei, H.; Golmanesh, L.; Zardooz, H.; Jalili, C.; Ghoshooni, H. Saffron (Crocus sativus) Aqueous Extract and Its Constituent Crocin Reduces Stress-Induced Anorexia in Mice. Phytother. Res. PTR 2011, 25, 1833–1838. [Google Scholar] [CrossRef]
- Ghadrdoost, B.; Vafaei, A.A.; Rashidy-Pour, A.; Hajisoltani, R.; Bandegi, A.R.; Motamedi, F.; Haghighi, S.; Sameni, H.R.; Pahlvan, S. Protective Effects of Saffron Extract and Its Active Constituent Crocin against Oxidative Stress and Spatial Learning and Memory Deficits Induced by Chronic Stress in Rats. Eur. J. Pharmacol. 2011, 667, 222–229. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Leonard, B.E. SSRI Differentiation: Pharmacology and Pharmacokinetics. Hum. Psychopharmacol. 1995, 10 (Suppl. 3), S149–S158. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.; Pehrson, A.L.; Jeyarajah, T.; Li, Y.; Leiser, S.C.; Smagin, G.; Olsen, C.K.; Sanchez, C. Effects of Serotonin in the Hippocampus: How SSRIs and Multimodal Antidepressants Might Regulate Pyramidal Cell Function. CNS Spectr. 2016, 21, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.C.; Gould, G.G.; Koek, W.; Daws, L.C. Ontogeny of SERT Expression and Antidepressant-like Response to Escitalopram in Wild-Type and SERT Mutant Mice. J. Pharmacol. Exp. Ther. 2016, 358, 271–281. [Google Scholar] [CrossRef]
- Thakker, D.R.; Natt, F.; Hüsken, D.; van der Putten, H.; Maier, R.; Hoyer, D.; Cryan, J.F. SiRNA-Mediated Knockdown of the Serotonin Transporter in the Adult Mouse Brain. Mol. Psychiatry 2005, 10, 782–789. [Google Scholar] [CrossRef]
- Malynn, S.; Campos-Torres, A.; Moynagh, P.; Haase, J. The Pro-Inflammatory Cytokine TNF-α Regulates the Activity and Expression of the Serotonin Transporter (SERT) in Astrocytes. Neurochem. Res. 2013, 38, 694–704. [Google Scholar] [CrossRef]
- Houwing, D.J.; Buwalda, B.; Van Der Zee, E.A.; De Boer, S.F.; Olivier, J.D.A. The Serotonin Transporter and Early Life Stress: Translational Perspectives. Front. Cell. Neurosci. 2017, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Ettehadi, H.; Mojabi, S.N.; Ranjbaran, M.; Shams, J.; Sahraei, H.; Hedayati, M.; Asefi, F. Aqueous Extract of Saffron (Crocus sativus) Increases Brain Dopamine and Glutamate Concentrations in Rats. J. Behav. Brain Sci. 2013, 3, 315–319. [Google Scholar] [CrossRef]
- González, B.; Gancedo, S.N.; Garazatua, S.A.J.; Roldán, E.; Vitullo, A.D.; González, C.R. Dopamine Receptor D1 Contributes to Cocaine Epigenetic Reprogramming of Histone Modifications in Male Germ Cells. Front. Cell Dev. Biol. 2020, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Morales-Mulia, S.; Magdaleno-Madrigal, V.M.; Nicolini, H.; Genis-Mendoza, A.; Morales-Mulia, M. Orexin-A up-Regulates Dopamine D2 Receptor and MRNA in the Nucleus Accumbens Shell. Mol. Biol. Rep. 2020, 47, 9689–9697. [Google Scholar] [CrossRef] [PubMed]
- Forbes, E.E.; Hariri, A.R.; Martin, S.L.; Silk, J.S.; Moyles, D.L.; Fisher, P.M.; Brown, S.M.; Ryan, N.D.; Birmaher, B.; Axelson, D.A.; et al. Altered Striatal Activation Predicting Real-World Positive Affect in Adolescent Major Depressive Disorder. Am. J. Psychiatry 2009, 166, 64–73. [Google Scholar] [CrossRef] [PubMed]

| FCx | Str | HPC | ||||
|---|---|---|---|---|---|---|
| (pmoles/g) | Control | Safr’Inside | Control | Safr’Inside | Control | Safr’Inside |
| [DA] | 8988.1 ± 2875.0 | 4316.1 ± 1474.8 | 54,369.3 ± 8573.8 | 81,815.8 ± 9730.2 # | 244.8 ± 39.9 | 232.0 ± 60.2 |
| [DOPAC] | 1337.8 ± 141.9 | 914.1 ± 112.9 * | 7237.3 ± 1295.3 | 9209.1 ± 1498.6 | n.d | n.d |
| [HVA] | 1915.0 ± 242.8 | 1287.4 ± 118.2 * | 3135.9 ± 468.5 | 3979.2 ± 538.0 | n.d | n.d |
| [5-HT] | 1955.0 ± 324.3 | 1890.2 ±217.8 | 3536.3 ± 290.0 | 3282.2 ± 161.2 | 2101.3 ± 257.8 | 2069.6 ± 341.7 |
| [5-HIAA] | 464.1 ± 137.8 | 253.9 ± 29.5 | 749.7 ± 67.4 | 764.4 ± 62.0 | 442.5 ± 35.4 | 491.8 ± 103.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monchaux De Oliveira, C.; Pourtau, L.; Vancassel, S.; Pouchieu, C.; Capuron, L.; Gaudout, D.; Castanon, N. Saffron Extract-Induced Improvement of Depressive-Like Behavior in Mice Is Associated with Modulation of Monoaminergic Neurotransmission. Nutrients 2021, 13, 904. https://doi.org/10.3390/nu13030904
Monchaux De Oliveira C, Pourtau L, Vancassel S, Pouchieu C, Capuron L, Gaudout D, Castanon N. Saffron Extract-Induced Improvement of Depressive-Like Behavior in Mice Is Associated with Modulation of Monoaminergic Neurotransmission. Nutrients. 2021; 13(3):904. https://doi.org/10.3390/nu13030904
Chicago/Turabian StyleMonchaux De Oliveira, Camille, Line Pourtau, Sylvie Vancassel, Camille Pouchieu, Lucile Capuron, David Gaudout, and Nathalie Castanon. 2021. "Saffron Extract-Induced Improvement of Depressive-Like Behavior in Mice Is Associated with Modulation of Monoaminergic Neurotransmission" Nutrients 13, no. 3: 904. https://doi.org/10.3390/nu13030904
APA StyleMonchaux De Oliveira, C., Pourtau, L., Vancassel, S., Pouchieu, C., Capuron, L., Gaudout, D., & Castanon, N. (2021). Saffron Extract-Induced Improvement of Depressive-Like Behavior in Mice Is Associated with Modulation of Monoaminergic Neurotransmission. Nutrients, 13(3), 904. https://doi.org/10.3390/nu13030904






