Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion
Abstract
1. Introduction
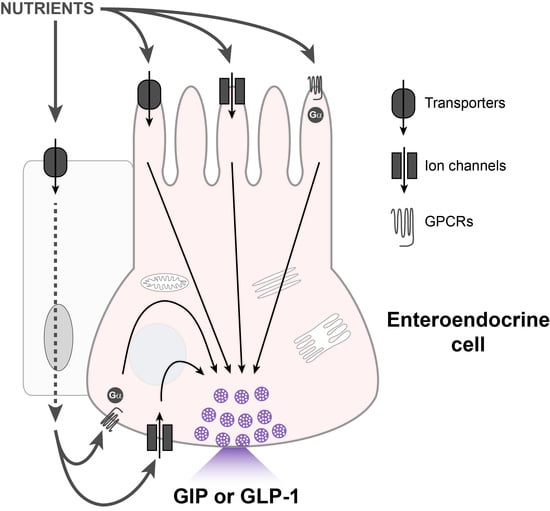
2. Enteroendocrine Cells That Release GLP-1 and GIP
3. Models to Study Nutrient-Sensing Mechanisms in the Gut
4. Cellular Mechanisms of Nutrient-Induced Gut Hormone Secretion
4.1. Carbohydrates
4.2. Proteins
4.3. Fats
4.4. Other: Bile Acids
4.5. Other: Short-Chain Fatty Acids (SCFAs)
5. Effect of GLP-1 and GIP on Food Intake and Weight Loss
6. Gut Hormone-Mediated Mechanisms of Satiety
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayliss, W.M.; Starling, E.H. The mechanism of pancreatic secretion. J. Physiol. 1902, 28, 325–353. [Google Scholar] [CrossRef]
- Bohórquez, D.V.; Chandra, R.; Samsa, L.A.; Vigna, S.R.; Liddle, R.A. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J. Mol. Histol. 2010, 42, 3–13. [Google Scholar] [CrossRef][Green Version]
- Bohórquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. An Enteroendocrine cell—enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881. [Google Scholar] [CrossRef]
- McSwiney, B.A.; Spurrell, W.R. Influence of osmotic pressure upon the emptying time of the stomach. J. Physiol. 1933, 79, 437–442. [Google Scholar] [CrossRef]
- Knapper, J.; Heath, A.; Fletcher, J.; Morgan, L.; Marks, V. GIP and GLP-1(7–36) amide secretion in response to intraduodenal infusions of nutrients in pigs. Comp. Biochem. Physiol. Part C Pharm. Toxicol. Endocrinol. 1995, 111, 445–450. [Google Scholar] [CrossRef]
- Rehfeld, J.F. The new biology of gastrointestinal hormones. Physiol. Rev. 1998, 78, 1087–1108. [Google Scholar] [CrossRef]
- Umar, S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, M.W. The incretin concept today. Diabetologia 1979, 16, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Elrick, H.; Stimmler, L.; Hlad, C.J.; Arai, Y. Plasma insulin response to oral and intravenous glucose administration. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.B.; Dirksen, C.; Bojsen-Møller, K.N.; Jacobsen, S.H.; Worm, D.; Hansen, D.L.; Kristiansen, V.B.; Naver, L.; Madsbad, S.; Holst, J.J. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with Type 2 diabetes. Diabetes 2013, 62, 3044–3052. [Google Scholar] [CrossRef]
- Hansen, K.B.; Vilsbøll, T.; Knop, F.K. Incretin mimetics: A novel therapeutic option for patients with type 2 diabetes—A review. Diabetes Metab. Syndr. Obes. Targets 2010, 3, 155–163. [Google Scholar]
- Mortensen, K.; Christensen, L.L.; Holst, J.J.; Orskov, C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003, 114, 189–196. [Google Scholar] [CrossRef]
- Theodorakis, M.J.; Carlson, O.; Michopoulos, S.; Doyle, M.E.; Juhaszova, M.; Petraki, K.; Egan, J.M. Human duodenal enteroendocrine cells: Source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Metab. 2006, 290, E550–E559. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; DeLorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nat. Cell Biol. 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Egerod, K.L.; Engelstoft, M.S.; Grunddal, K.V.; Nøhr, M.K.; Secher, A.; Sakata, I.; Pedersen, J.; Windeløv, J.A.; Füchtbauer, E.-M.; Olsen, J.; et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 2012, 153, 5782–5795. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Richards, P.; Cairns, L.S.; Rogers, G.J.; Bannon, C.A.M.; Parker, H.E.; Morley, T.C.E.; Yeo, G.S.H.; Reimann, F.; Gribble, F.M.; et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012, 153, 3054–3065. [Google Scholar] [CrossRef] [PubMed]
- Jorsal, T.; Rhee, N.A.; Pedersen, J.; Wahlgren, C.D.; Mortensen, B.; Jepsen, S.L.; Jelsing, J.; Dalbøge, L.S.; Vilmann, P.; Hassan, H.; et al. Enteroendocrine K and L cells in healthy and Type 2 diabetic individuals. Diabetologia 2018, 61, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.P.; Larraufie, P.; Richards, P.; Kay, R.G.; Galvin, S.G.; Miedzybrodzka, E.L.; Leiter, A.; Li, H.J.; Glass, L.L.; Ma, M.K.; et al. Comparison of human and murine enteroendocrine cells by transcriptomic and peptidomic profiling. Diabetes 2019, 68, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Sjölund, K.; Sandén, G.; Håkanson, R.; Sundler, F. Endocrine cells in human intestine: An immunocytochemical study. Gastroenterology 1983, 85, 1120–1130. [Google Scholar] [CrossRef]
- Bryant, M.G.; Bloom, S.R.; Polak, J.M.; Hobbs, S.; Domschke, W.; Mitznegg, P.; Ruppin, H.; Demling, L. Measurement of gut hormonal peptides in biopsies from human stomach and proximal small intestine. Gut 1983, 24, 114–119. [Google Scholar] [CrossRef]
- Buchan, A.M.J.; Polak, J.M.; Capella, C.; Solcia, E.; Pearse, A.G.E. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) im man. Histochem. Cell Biol. 1978, 56, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Eissele, R.; Göke, R.; Willemer, S.; Harthus, H.-P.; Vermeer, H.; Arnold, R.; Göke, B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Investig. 1992, 22, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Hlinka, M.; Tabrizi, Y.; Dimaso, N.; Raybould, H.E. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1293–R1307. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.; Ghatei, M.A.; Bloom, S.R. The inhibitory effects of peripheral administration of peptide YY3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal–brainstem–hypothalamic pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Walls, E.K.; Phillips, R.J.; Wang, F.B.; Holst, M.C.; Powley, T.L. Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R1410–R1419. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Panaro, B.L.; Yusta, B.; Matthews, D.; Koehler, J.A.; Song, Y.; Sandoval, D.A.; Drucker, D.J. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol. Metab. 2020, 37, 100990. [Google Scholar] [CrossRef]
- Buffa, R.; Solcia, E.; Go, V.L.W. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology 1976, 70, 528–532. [Google Scholar] [CrossRef]
- Hökfelt, T.; Herrera-Marschitz, M.; Seroogy, K.; Ju, G.; A Staines, W.; Holets, V.; Schalling, M.; Ungerstedt, U.; Post, C.; Rehfeld, J.F.; et al. Immunohistochemical studies on cholecystokinin (CCK)-immunoreactive neurons in the rat using sequence specific antisera and with special reference to the caudate nucleus and primary sensory neurons. J. Chem. Neuroanat. 1988, 1, 11–51. [Google Scholar]
- Rehfeld, J. Immunochemical studies on cholecystokinin. II: Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J. Biol. Chem. 1978, 253, 4022–4030. [Google Scholar] [CrossRef]
- Kerstens, P.; Lamers, C.; Jansen, J.; De Jong, A.; Hessels, M.; Hafkenscheid, J. Physiological plasma concentrations of cholecystokinin stimulate pancreatic enzyme secretion and gallbladder contraction in man. Life Sci. 1985, 36, 565–569. [Google Scholar] [CrossRef]
- Gibbs, J.; Smith, G.P. Cholecystokinin and satiety in rats and rhesus monkeys. Am. J. Clin. Nutr. 1977, 30, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 1973, 84, 488–495. [Google Scholar] [CrossRef]
- Saito, A.; Williams, J.A.; Goldfine, I.D. Alterations in brain cholecystokinin receptors after fasting. Nat. Cell Biol. 1981, 289, 599–600. [Google Scholar] [CrossRef]
- Little, T.J.; Horowitz, M.; Feinle-Bisset, C. Role of cholecystokinin in appetite control and body weight regulation. Obes. Rev. 2005, 6, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sykaras, A.G.; Demenis, C.; Cheng, L.; Pisitkun, T.; McLaughlin, J.T.; Fenton, R.A.; Smith, C.P. Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 2014, 155, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.A.; Kim, S.; Gordon, J.I. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am. J. Physiol. Liver Physiol. 1992, 263, G174–G180. [Google Scholar] [CrossRef]
- Gerspach, A.C.; Steinert, R.E.; Schönenberger, L.; Graber-Maier, A.; Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Metab. 2011, 301, E317–E325. [Google Scholar] [CrossRef]
- Hopman, W.P.M.; Jansen, J.B.M.J.; Lamers, C.B.H.W. Comparative Study of the effects of equal amounts of fat, protein, and starch on plasma cholecystokinin in man. Scand. J. Gastroenterol. 1985, 20, 843–847. [Google Scholar] [CrossRef]
- Liddle, R.A.; Goldfine, I.D.; Rosen, M.S.; Taplitz, R.A.; Williams, J.A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Investig. 1985, 75, 1144–1152. [Google Scholar] [CrossRef]
- Fothergill, L.J.; Callaghan, B.; Hunne, B.; Bravo, D.M.; Furness, J.B. Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 2017, 158, 2113–2123. [Google Scholar] [CrossRef]
- Rindi, G.; Ratineau, C.; Ronco, A.; E Candusso, M.; Tsai, M.; Leiter, A.B. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 1999, 126, 4149–4156. [Google Scholar]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Born, M.V.D.; Van Es, J.H.; Clevers, H.; et al. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat. Cell Biol. 2018, 20, 909–916. [Google Scholar] [CrossRef]
- Afroze, S.; Meng, F.; Jensen, K.; McDaniel, K.; Rahal, K.; Onori, P.; Gaudio, E.; Alpini, G.; Glaser, S.S. The physiological roles of secretin and its receptor. Ann. Transl. Med. 2013, 1, 29. [Google Scholar] [PubMed]
- Cheng, C.Y.Y.; Chu, J.Y.S.; Chow, B.K.C. Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 2010, 36, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Motojima, Y.; Kawasaki, M.; Matsuura, T.; Saito, R.; Yoshimura, M.; Hashimoto, H.; Ueno, H.; Maruyama, T.; Suzuki, H.; Ohnishi, H.; et al. Effects of peripherally administered cholecystokinin-8 and secretin on feeding/drinking and oxytocin-mRFP1 fluorescence in transgenic rats. Neurosci. Res. 2016, 109, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Y.S.; Cheng, C.Y.Y.; Sekar, R.; Chow, B.K.C. Vagal afferent mediates the anorectic effect of peripheral secretin. PLoS ONE 2013, 8, e64859. [Google Scholar] [CrossRef]
- Lopez, M.J.; Upchurch, B.H.; Rindi, G.; Leiter, A.B. Studies in Transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J. Biol. Chem. 1995, 270, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G.; Hamscher, G.; Kusiek, R.; Meyer, H.; Metzger, J. Identification of xenin, a xenopsin-related peptide, in the human gastric mucosa and its effect on exocrine pancreatic secretion. J. Biol. Chem. 1992, 267, 22305–22309. [Google Scholar] [CrossRef]
- Anlauf, M.; Weihe, E.; Hartschuh, W.; Hamscher, G.; Feurle, G.E. Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J. Histochem. Cytochem. 2000, 48, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G. Xenin—A review. Peptides 1998, 19, 609–615. [Google Scholar] [CrossRef]
- Feurle, G.E.; Ikonomu, S.; Partoulas, G.; Stoschus, B.; Hamscher, G. Xenin plasma concentrations during modified sham feeding and during meals of different composition demonstrated by radioimmunoassay and chromatography. Regul. Pept. 2003, 111, 153–159. [Google Scholar] [CrossRef]
- Wice, B.M.; Wang, S.; Crimmins, D.L.; Diggs-Andrews, K.A.; Althage, M.C.; Ford, E.L.; Tran, H.; Ohlendorf, M.; Griest, T.A.; Wang, Q.; et al. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J. Biol. Chem. 2010, 285, 19842–19853. [Google Scholar] [CrossRef]
- Feurle, G.E.; Heger, M.; Niebergall-Roth, E.; Teyssen, S.; Fried, M.; Eberle, C.; Singer, M.V.; Hamscher, G. Gastroenteropancreatic effects of xenin in the dog. J. Pept. Res. 2009, 49, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G.E.; Pfeiffer, A.; Schmidt, T.; Dominguez-Munoz, E.; Malfertheiner, P.; Hamscher, G. Phase III of the migrating motor complex: Associated with endogenous xenin plasma peaks and induced by exogenous xenin. Neurogastroenterol. Motil. 2001, 13, 237–246. [Google Scholar] [CrossRef]
- Cooke, J.H.; Patterson, M.; Patel, S.R.; Smith, K.L.; Ghatei, M.A.; Bloom, S.R.; Murphy, K.G. Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity 2009, 17, 1135–1143. [Google Scholar] [CrossRef]
- Kim, E.R.; Mizuno, T.M. Role of neurotensin receptor 1 in the regulation of food intake by neuromedins and neuromedin-related peptides. Neurosci. Lett. 2010, 468, 64–67. [Google Scholar] [CrossRef]
- Dakka, T.; Cuber, J.C.; Chayvialle, J.A. Functional coupling between the active transport of glucose and the secretion of intestinal neurotensin in rats. J. Physiol. 1993, 469, 753–765. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Gribble, F.M.; Hartmann, B.; Reimann, F.; Windeløv, J.A.; Rehfeld, J.F.; Holst, J.J. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Liver Physiol. 2014, 306, G622–G630. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.P.; Fujimura, M.; Sakamoto, T.; Greeley, G.H.; Townsend, C.M.; Thompson, J.C. Importance of the ileum in neurotensin released by fat. Surgery 1985, 98, 224–229. [Google Scholar]
- Hawkins, M.F. Central nervous system neurotensin and feeding. Physiol. Behav. 1986, 36, 1–8. [Google Scholar] [CrossRef]
- Luttinger, D.; King, R.A.; Sheppard, D.; Strupp, J.; Nemeroff, C.B.; Prange, A.J. The effect of neurotensin on food consumption in the rat. Eur. J. Pharm. 1982, 81, 499–503. [Google Scholar] [CrossRef]
- Remaury, A.; Vita, N.; Gendreau, S.; Jung, M.; Arnone, M.; Poncelet, M.; Culouscou, J.-M.; Le Fur, G.; Soubrié, P.; Caput, D.; et al. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002, 953, 63–72. [Google Scholar] [CrossRef]
- Polak, J.M.; Sullivan, S.N.; Bloom, S.R.; Buchan, A.M.J.; Facer, P.; Brown, M.R.; Pearse, A.G.E. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nat. Cell Biol. 1977, 270, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Grunddal, K.V.; Ratner, C.F.; Svendsen, B.; Sommer, F.; Engelstoft, M.S.; Madsen, A.N.; Pedersen, J.; Nøhr, M.K.; Egerod, K.L.; Nawrocki, A.R.; et al. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 2016, 157, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Dakin, C.L.; Gunn, I.; Small, C.J.; Edwards, C.M.B.; Hay, D.L.; Smith, D.M.; Ghatei, M.A.; Bloom, S.R. Oxyntomodulin inhibits food intake in the rat. Endocrinology 2001, 142, 4244–4250. [Google Scholar] [CrossRef]
- Dakin, C.L.; Small, C.J.; Batterham, R.L.; Neary, N.M.; Cohen, M.A.; Patterson, M.; Ghatei, M.A.; Bloom, S.R. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 2004, 145, 2687–2695. [Google Scholar] [CrossRef]
- Kosinski, J.R.; Hubert, J.; Carrington, P.E.; Chicchi, G.G.; Mu, J.; Miller, C.; Cao, J.; Bianchi, E.; Pessi, A.; Sinharoy, R.; et al. The glucagon receptor is involved in mediating the body weight-lowering effects of oxyntomodulin. Obesity 2012, 20, 1566–1571. [Google Scholar] [CrossRef]
- Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Batterham, R.L.; Park, A.; Patterson, M.M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocrinol. Metab. 2003, 88, 4696–4701. [Google Scholar] [CrossRef]
- Parker, V.E.R.; Robertson, D.; Wang, T.; Hornigold, D.C.; Petrone, M.; Cooper, A.T.; Posch, M.G.; Heise, T.; Plum-Moerschel, L.; Schlichthaar, H.; et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. J. Clin. Endocrinol. Metab. 2019, 105, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.; Ferri, G.-L.; Bacarese-Hamilton, A.; Fuessl, H.; Polak, J.; Bloom, S. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- Billing, L.J.; Smith, C.A.; Larraufie, P.; Goldspink, D.A.; Galvin, S.; Kay, R.G.; Howe, J.D.; Walker, R.; Pruna, M.; Glass, L.; et al. Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptide YY from murine and human colonic enteroendocrine cells. Mol. Metab. 2018, 16, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pedersen-Bjergaard, U.; Høt, U.; Kelbæk, H.; Schifter, S.; Rehfeld, J.F.; Faber, J.; Christensen, N.J. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand. J. Clin. Lab. Investig. 1996, 56, 497–503. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nat. Cell Biol. 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Halatchev, I.G.; Ellacott, K.L.J.; Fan, W.; Cone, R.D. Peptide YY3–36Inhibits food intake in mice through a melanocortin-4 Receptor-independent mechanism. Endocrinology 2004, 145, 2585–2590. [Google Scholar] [CrossRef]
- Lewis, J.E.; Miedzybrodzka, E.L.; Foreman, R.E.; Woodward, O.R.M.; Kay, R.G.; Goldspink, D.A.; Gribble, F.M.; Reimann, F. Selective stimulation of colonic L cells improves metabolic outcomes in mice. Diabetologia 2020, 63, 1396–1407. [Google Scholar] [CrossRef]
- Babu, M.; Purhonen, A.; Bansiewicz, T.; Mäkelä, K.; Walkowiak, J.; Miettinen, P.; Herzig, K. Effect of total colectomy and PYY infusion on food intake and body weight in rats. Regul. Pept. 2005, 131, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Boggiano, M.M.; Chandler, P.C.; Oswald, K.D.; Rodgers, R.J.; Blundell, J.E.; Ishii, Y.; Beattie, A.H.; Holch, P.; Allison, D.B.; Schindler, M.; et al. PYY3–36 as an anti-obesity drug target. Obes. Rev. 2005, 6, 307–322. [Google Scholar] [CrossRef]
- Tschöp, M.; Castañeda, T.R.; Joost, H.G.; Thöne-Reineke, C.; Ortmann, S.; Klaus, S.; Hagan, M.M.; Chandler, P.C.; Oswald, K.D.; Benoit, S.C.; et al. Does gut hormone PYY3–36 decrease food intake in rodents? Nat. Cell Biol. 2004, 430, 1–3. [Google Scholar] [CrossRef]
- Fu-Cheng, X.; Anini, Y.; Chariot, J.; Castex, N.; Galmiche, J.-P.; Rozé, C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: Neural regulation by proximal gut. Pflügers Arch. 1997, 433, 571–579. [Google Scholar] [CrossRef]
- Koda, S.; Date, Y.; Murakami, N.; Shimbara, T.; Hanada, T.; Toshinai, K.; Niijima, A.; Furuya, M.; Inomata, N.; Osuye, K.; et al. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology 2005, 146, 2369–2375. [Google Scholar] [CrossRef]
- Yang, H.; Tache, Y. PYY in brain stem nuclei induces vagal stimulation of gastric acid secretion in rats. Am. J. Physiol. Liver Physiol. 1995, 268, G943–G948. [Google Scholar] [CrossRef]
- Zeng, N.; Walsh, J.; Kang, T.; Wu, S.; Sachs, G. Peptide YY inhibition of rat gastric enterochromaffin-like cell function. Gastroenterology 1997, 112, 127–135. [Google Scholar] [CrossRef]
- Lin, H.C.; Zhao, X.T.; Wang, L.; Wong, H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology 1996, 110, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.P.; E Adrian, T.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef]
- Wiley, J.W.; Lu, Y.; Owyang, C. Mechanism of action of peptide YY to inhibit gastric motility. Gastroenterology 1991, 100, 865–872. [Google Scholar] [CrossRef]
- Grosse, J.; Heffron, H.; Burling, K.; Hossain, M.A.; Habib, A.M.; Rogers, G.J.; Richards, P.; Larder, R.; Rimmington, D.; Adriaenssens, A.A.; et al. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc. Natl. Acad. Sci. USA 2014, 111, 11133–11138. [Google Scholar] [CrossRef]
- Lee, Y.S.; De Vadder, F.; Tremaroli, V.; Wichmann, A.; Mithieux, G.; Bäckhed, F. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Mol. Metab. 2016, 5, 263–270. [Google Scholar] [CrossRef]
- Drucker, D.J.; Jin, T.; Asa, S.L.; Young, T.A.; Brubaker, P.L. Activation of proglucagon gene transcription by protein kinase—A in a novel mouse enteroendocrine cell line. Mol. Endocrinol. 1994, 8, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- De Bruïne, A.P.; Dinjens, W.N.M.; Pijls, M.M.J.; Linden, E.P.M.V.D.; Rousch, M.J.M.; Moerkerk, P.T.; De Goeij, A.F.P.M.; Bosnian, F.T. NCI-H716 cells as a model for endocrine differentiation in colorectal cancer. Virchows Arch. B 1992, 62, 311–320. [Google Scholar] [CrossRef]
- Park, J.G.; Oie, H.K.; Sugarbaker, P.H.; Henslee, J.G.; Chen, T.R.; Johnson, B.E.; Gazdar, A. Characteristics of cell lines estab-lished from human colorectal carcinoma. Cancer Res. 1987, 47, 6710–6718. [Google Scholar] [PubMed]
- Brubaker, P.L.; Schloos, J.; Drucker, D.J. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag Enteroendocrine cell line. Endocrinology 1998, 139, 4108–4114. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Darimont, C.; Gremlich, S.; Nicolas-Métral, V.; Rüegg, U.T.; Macé, K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 2001, 142, 4522–4528. [Google Scholar] [CrossRef]
- Cao, X.; Flock, G.; Choi, C.; Irwin, D.M.; Drucker, D.J. Aberrant regulation of human intestinal proglucagon gene expression in the NCI-H716 cell line. Endocrinology 2003, 144, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Grant, S.G.; Yiangou, Y.; Ghatei, M.A.; Bloom, S.R.; Bautch, V.L.; Solcia, E.; Polak, J.M. Development of neuroen-docrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am. J. Pathol. 1990, 136, 1349–1363. [Google Scholar]
- Cheung, A.T.; Dayanandan, B.; Lewis, J.T.; Korbutt, G.S.; Rajotte, R.V.; Bryer-Ash, M.; Boylan, M.O.; Wolfe, M.M.; Kieffer, T.J. Glucose-dependent insulin release from genetically engineered K cells. Science 2000, 290, 1959–1962. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; Huang, Z.; McIntosh, C.H.; Buchan, A.M.; Brown, J.C.; Pederson, R.A. Gastric inhibitory polypeptide release from a tumor-derived cell line. Am. J. Physiol. Metab. 1995, 269, E316–E322. [Google Scholar] [CrossRef]
- Ramshur, E.B.; Rull, T.R.; Wice, B.M. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function In Vivo. J. Cell. Physiol. 2002, 192, 339–350. [Google Scholar] [CrossRef]
- Aponte, G.W.; Taylor, I.L.; Soll, A.H. Primary culture of PYY cells from canine colon. Am. J. Physiol. Liver Physiol. 1988, 254, G829–G836. [Google Scholar] [CrossRef]
- Kieffer, T.J.; Buchan, A.M.; Barker, H.; Brown, J.C.; Pederson, R.A. Release of gastric inhibitory polypeptide from cultured canine endocrine cells. Am. J. Physiol. Metab. 1994, 267, E489–E496. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L.; Vranic, M. Fetal rat intestinal cells in monolayer culture: A new In Vitro System to study the glucagon-like immunoreactive peptides. Endocrinology 1987, 120, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008, 8, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.E.; Habib, A.M.; Rogers, G.J.; Gribble, F.M.; Reimann, F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009, 52, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures In Vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, D.A.; Lu, V.B.; Billing, L.J.; Larraufie, P.; Tolhurst, G.; Gribble, F.M.; Reimann, F. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol. Metab. 2018, 7, 90–101. [Google Scholar] [CrossRef]
- Beumer, J.; Puschhof, J.; -Martinez, J.B.; Martínez-Silgado, A.; Elmentaite, R.; James, K.R.; Ross, A.; Hendriks, D.; Artegiani, B.; Busslinger, G.A.; et al. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell 2020, 182, 1062–1064. [Google Scholar] [CrossRef]
- Goldspink, D.A.; Lu, V.B.; Miedzybrodzka, E.L.; Smith, C.A.; Foreman, R.E.; Billing, L.J.; Kay, R.G.; Reimann, F.; Gribble, F.M. Labeling and characterization of human GLP-1-secreting L-cells in primary ileal organoid culture. Cell Rep. 2020, 31, 107833. [Google Scholar] [CrossRef]
- Ussing, H.H.; Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 1951, 23, 110–127. [Google Scholar] [CrossRef]
- Brighton, C.A.; Rievaj, J.; Kuhre, R.E.; Glass, L.L.; Schoonjans, K.; Holst, J.J.; Gribble, F.M.; Reimann, F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein—coupled bile acid receptors. Endocrinology 2015, 156, 3961–3970. [Google Scholar] [CrossRef]
- Joshi, S.; Tough, I.R.; Cox, H.M. Endogenous PYY and GLP-1 mediatel-glutamine responses in intestinal mucosa. Br. J. Pharm. 2013, 170, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Phillips, S.F.; Sarr, M.G.; Kost, L.J.; Holst, J.J. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am. J. Physiol. Liver Physiol. 1995, 269, G945–G952. [Google Scholar] [CrossRef]
- Ritzel, U.; Fromme, A.; Ottleben, M.; Leonhardt, U.; Ramadori, G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol. 1997, 34, 18–21. [Google Scholar] [CrossRef]
- Cook, C.G.; Andrews, J.M.; Jones, K.L.; Wittert, G.A.; Chapman, I.M.; Morley, J.E.; Horowitz, M. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am. J. Physiol. Content 1997, 273, R755–R761. [Google Scholar] [CrossRef]
- Lavin, J.H.; Wittert, G.A.; Andrews, J.; Yeap, B.; Wishart, J.M.; Morris, H.A.; Morley, J.E.; Horowitz, M.; Read, N.W. Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am. J. Clin. Nutr. 1998, 68, 591–598. [Google Scholar] [CrossRef]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1(7–36) amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef]
- Herman, G.A.; Bergman, A.; Stevens, C.; Kotey, P.; Yi, B.; Zhao, P.; Dietrich, B.; Golor, G.; Schrodter, A.; Keymeulen, B.; et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with Type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 4612–4619. [Google Scholar] [CrossRef]
- Schirra, J.; Katschinski, M.; Weidmann, C.; Schäfer, T.; Wank, U.; Arnold, R.; Göke, B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J. Clin. Investig. 1996, 97, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Yasharpour, S.; Lloyd, K.C.; Mirzayan, R.; Diamond, J.M. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol. Liver Physiol. 1990, 259, G822–G837. [Google Scholar] [CrossRef]
- Stephen, A.M.; Haddad, A.C.; Phillips, S.F. Passage of carbohydrate into the colon. Direct measurements in humans. Gastroenterology 1983, 85, 589–595. [Google Scholar] [CrossRef]
- Sun, E.W.; De Fontgalland, D.; Rabbitt, P.; Hollington, P.; Sposato, L.; Due, S.L.; Wattchow, D.A.; Rayner, C.K.; Deane, A.M.; Young, R.L.; et al. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes 2017, 66, 2144–2149. [Google Scholar] [CrossRef]
- Nauck, M.A.; Siemsgluss, J.; Orskov, C.; Holst, J.J. Release of glucagon-like peptide 1 (GLP-1 [7–36 amide]), gastric inhibi-tory polypeptide (GIP) and insulin in response to oral glucose after upper and lower intestinal resections. Zeitschrift Gastroenterologie 1996, 34, 159–166. [Google Scholar]
- Yoshikawa, T.; Inoue, R.; Matsumoto, M.; Yajima, T.; Ushida, K.; Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011, 135, 183–194. [Google Scholar] [CrossRef]
- Yoshida, A.; Takata, K.; Kasahara, T.; Aoyagi, T.; Saito, S.; Hirano, H. Immunohistochemical localization of Na(+)-dependent glucose transporter in the rat digestive tract. Histochem. J. 1995, 27, 420–426. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Hirayama, B.A.; Wright, E.M. Distribution of the SGLT1 Na+glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem. Biophys. Res. Commun. 1991, 181, 1208–1217. [Google Scholar] [CrossRef]
- Gribble, F.M.; Williams, L.; Simpson, A.K.; Reimann, F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 2003, 52, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sampedro, A.; Lostao, M.; Wright, E.; Hirayama, B. Glycoside binding and translocation in Na+-dependent glucose cotransporters: Comparison of SGLT1 and SGLT3. J. Membr. Biol. 2000, 176, 111–117. [Google Scholar] [CrossRef]
- Landau, B.R.; Bernstein, L.; Wilson, T.H. Hexose transport by hamster intestine in vitro. Am. J. Physiol. Content 1962, 203, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Csáky, T.Z.; Glenn, J.E. Urinary recovery of 3-methylglucose administered to rats. Am. J. Physiol. Content 1956, 188, 159–162. [Google Scholar] [CrossRef]
- Sykes, S.; Morgan, L.M.; English, J.; Marks, V. Evidence for preferential stimulation of gastric inhibitory polypeptide secretion in the rat by actively transported carbohydrates and their analogues. J. Endocrinol. 1980, 85, 201–207. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2011, 61, 187–196. [Google Scholar] [CrossRef]
- Powell, D.R.; Smith, M.; Greer, J.; Harris, A.; Zhao, S.; Dacosta, C.; Mseeh, F.; Shadoan, M.K.; Sands, A.; Zambrowicz, B.; et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)–mediated absorption of intestinal glucose. J. Pharm. Exp. 2013, 345, 250–259. [Google Scholar] [CrossRef]
- Díez-Sampedro, A.; Hirayama, B.A.; Osswald, C.; Gorboulev, V.; Baumgarten, K.; Volk, C.; Wright, E.M.; Koepsell, H. A glucose sensor hiding in a family of transporters. Proc. Natl. Acad. Sci. USA 2003, 100, 11753–11758. [Google Scholar] [CrossRef]
- Kellett, G.L.; Helliwell, P.A. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem. J. 2000, 350, 155–162. [Google Scholar] [CrossRef]
- Affleck, J.A.; Helliwell, P.A.; Kellett, G.L. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J. Histochem. Cytochem. 2003, 51, 1567–1574. [Google Scholar] [CrossRef]
- Scow, J.S.; Iqbal, C.W.; Jones, T.W.; Qandeel, H.G.; Zheng, Y.; Duenes, J.A.; Nagao, M.; Madhavan, S.; Sarr, M.G.; Madhaven, S.; et al. Absence of evidence of translocation of GLUT2 to the apical membrane of enterocytes in everted intestinal sleeves. J. Surg. Res. 2011, 167, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Parker, H.E.; Adriaenssens, A.; Rogers, G.; Richards, P.; Koepsell, H.; Reimann, F.; Gribble, F.M. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 2012, 55, 2445–2455. [Google Scholar] [CrossRef]
- Jetton, T.L.; Liang, Y.; Pettepher, C.C.; Zimmerman, E.C.; Cox, F.G.; Horvath, K.; Matschinsky, F.M.; Magnuson, M.A. Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroen-docrine cells in the brain and gut. J. Biol. Chem. 1994, 269, 3641–3654. [Google Scholar] [CrossRef]
- Murphy, R.; Tura, A.; Clark, P.M.; Holst, J.J.; Mari, A.; Hattersley, A.T. Glucokinase, the pancreatic glucose sensor, is not the gut glucose sensor. Diabetologia 2008, 52, 154–159. [Google Scholar] [CrossRef]
- Rorsman, P. The pancreatic beta-cell as a fuel sensor: An electrophysiologist’s viewpoint. Diabetologia 1997, 40, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.B.; Ploug, K.B.; Swift, P.; Ørskov, C.; Jansen-Olesen, I.; Chiarelli, F.; Holst, J.J.; Hougaard, P.; Pörksen, S.; Holl, R.; et al. Co-localisation of the Kir6.2/SUR1 channel complex with glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide expression in human ileal cells and implications for glycaemic control in new onset type 1 diabetes. Eur. J. Endocrinol. 2007, 156, 663–671. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F.M. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 2002, 51, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Frost, C.R.; Svendsen, B.; Holst, J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 2014, 64, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Mangel, A.W.; Prpic, V.; Snow, N.D.; Basavappa, S.; Hurst, L.J.; Sharara, A.I.; Liddle, R.A. Regulation of cholecystokinin secretion by ATP-sensitive potassium channels. Am. J. Physiol. Liver Physiol. 1994, 267, G595–G600. [Google Scholar] [CrossRef] [PubMed]
- Tsukiyama, K.; Yamada, Y.; Miyawaki, K.; Hamasaki, A.; Nagashima, K.; Hosokawa, M.; Fujimoto, S.; Takahashi, A.; Toyoda, K.; Toyokuni, S.; et al. Gastric inhibitory polypeptide is the major insulinotropic factor in K(ATP) null mice. Eur. J. Endocrinol. 2004, 151, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.; Bodvarsdottir, T.; Wareham, K.; Prior, S.; Bracken, R.; Lowe, G.; Rumley, A.; Dunseath, G.; Luzio, S.; Deacon, C.; et al. Effects of short-term therapy with glibenclamide and repaglinide on incretin hormones and oxidative damage associated with postprandial hyperglycaemia in people with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 94, 199–206. [Google Scholar] [CrossRef] [PubMed]
- El-Ouaghlidi, A.; Rehring, E.; Holst, J.J.; Schweizer, A.; Foley, J.; Holmes, D.; Nauck, M.A. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide-induced hypoglycemia but reduces glucose-induced glucagon-like peptide 1 and gastric inhibitory polypeptide secretion. J. Clin. Endocrinol. Metab. 2007, 92, 4165–4171. [Google Scholar] [CrossRef]
- Moran, T.H.; McHugh, P.R. Distinctions among three sugars in their effects on gastric emptying and satiety. Am. J. Physiol. Integr. Comp. Physiol. 1981, 241, R25–R30. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.K.; Park, H.S.; Wishart, J.M.; Kong, M.-F.; Doran, S.M.; Horowitz, M. Effects of intraduodenal glucose and fructose on antropyloric motility and appetite in healthy humans. Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, R360–R366. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.-F.; Chapman, I.; Goble, E.; Wishart, J.; Wittert, G.; Morris, H.; Horowitz, M. Effects of oral fructose and glucose on plasma GLP-1 and appetite in normal subjects. Peptides 1999, 20, 545–551. [Google Scholar] [CrossRef]
- Shima, K.; Suda, T.; Nishimoto, K.; Yoshimoto, S. Relationship between molecular structures of sugars and their ability to stimulate the release of glucagon-like peptide-1 from canine ileal loops. Eur. J. Endocrinol. 1990, 123, 464–470. [Google Scholar] [CrossRef]
- Burant, C.; Takeda, J.; Brot-Laroche, E.; Bell, G.; Davidson, N. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef]
- Rand, E.B.; De Paoli, A.M.; Davidson, N.O.; Bell, G.I.; Burant, C.F. Sequence, tissue distribution, and functional characterization of the rat fructose transporter Glutam. J. Physiol. Liver Physiol. 1993, 264, G1169–G1176. [Google Scholar] [CrossRef]
- Seino, Y.; Ogata, H.; Maekawa, R.; Izumoto, T.; Iida, A.; Harada, N.; Miki, T.; Seino, S.; Inagaki, N.; Tsunekawa, S.; et al. Fructose induces glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1 and insulin secretion: Role of adenosine triphosphate-sensitive K+channels. J. Diabetes Investig. 2015, 6, 522–526. [Google Scholar] [CrossRef]
- Wong, G.T.; Gannon, K.S.; Margolskee, R.F. Transduction of bitter and sweet taste by gustducin. Nat. Cell Biol. 1996, 381, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An amino-acid taste receptor. Nat. Cell Biol. 2002, 416, 199–202. [Google Scholar] [CrossRef]
- Rozengurt, N.; Wu, S.V.; Chen, M.C.; Huang, C.; Sternini, C.; Rozengurt, E. Colocalization of the α-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am. J. Physiol. Liver Physiol. 2006, 291, G792–G802. [Google Scholar] [CrossRef]
- Kokrashvili, Z.; Mosinger, B.; Margolskee, R.F. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am. J. Clin. Nutr. 2009, 90, 822S–825S. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Fujita, Y.; Wideman, R.D.; Speck, M.; Asadi, A.; King, D.S.; Webber, T.D.; Haneda, M.; Kieffer, T.J. Incretin release from gut is acutely enhanced by sugar but not by sweeteners In Vivo. Am. J. Physiol. Metab. 2009, 296, E473–E479. [Google Scholar] [CrossRef] [PubMed]
- Bezençon, C.; Le Coutre, J.; Damak, S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 2007, 32, 41–49. [Google Scholar] [CrossRef]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.H.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chang, J.; Checklin, H.L.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br. J. Nutr. 2010, 104, 803–806. [Google Scholar] [CrossRef]
- Saltiel, M.Y.; Kuhre, R.E.; Christiansen, C.B.; Eliasen, R.; Conde-Frieboes, K.W.; Rosenkilde, M.M.; Holst, J.J. Sweet taste receptor activation in the gut is of limited importance for glucose-stimulated GLP-1 and GIP secretion. Nutrients 2017, 9, 418. [Google Scholar] [CrossRef]
- Thomas, F.; Sinar, D.; Mazzaferri, E.; Cataland, S.; Mekhjian, H.; Caldwell, J.; Fromkes, J. Selective release of gastric inhibitory polypeptide by intraduodenal amino acid perfusion in man. Gastroenterology 1978, 74, 1261–1265. [Google Scholar] [CrossRef]
- Cordier-Bussat, M.; Bernard, C.; Levenez, F.; Klages, N.; Laser-Ritz, B.; Philippe, J.; Chayvialle, J.A.; Cuber, J.C. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 1998, 47, 1038–1045. [Google Scholar] [CrossRef]
- Reimer, R.A. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J. Endocrinol. 2006, 191, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F.; et al. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef]
- Matsumura, K.; Miki, T.; Jhomori, T.; Gonoi, T.; Seino, S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem. Biophys. Res. Commun. 2005, 336, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rose, A.J.; Sijmonsma, T.P.; Bröer, A.; Pfenninger, A.; Herzig, S.; Schmoll, D.; Bröer, S. Mice lacking neutral amino acid transporter B0AT1 (Slc6a19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol. Metab. 2015, 4, 406–417. [Google Scholar] [CrossRef]
- Pais, R.; Gribble, F.M.; Reimann, F. Signalling pathways involved in the detection of peptones by murine small intestinal enteroendocrine L-cells. Peptides 2016, 77, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef]
- Liou, A.P.; Sei, Y.; Zhao, X.; Feng, J.; Lu, X.; Thomas, C.; Pechhold, S.; Raybould, H.E.; Wank, S.A. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to l-phenylalanine in acutely isolated intestinal I cells. Am. J. Physiol. Liver Physiol. 2011, 300, G538–G546. [Google Scholar] [CrossRef] [PubMed]
- Rey, O.; Young, S.H.; Jacamo, R.; Moyer, M.P.; Rozengurt, E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J. Cell. Physiol. 2010, 225, 73–83. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Holst, J.J. Peptone-mediated glucagon-like peptide-1 secretion depends on intestinal absorption and activation of basolaterally located Calcium-sensing receptors. Physiol. Rep. 2019, 7, e14056. [Google Scholar] [CrossRef]
- Choi, S.; Lee, M.; Shiu, A.L.; Yo, S.J.; Halldén, G.; Aponte, G.W. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am. J. Physiol. Liver Physiol. 2007, 292, G1366–G1375. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a New G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597. [Google Scholar] [CrossRef]
- Rudenko, O.; Shang, J.; Munk, A.; Ekberg, J.P.; Petersen, N.; Engelstoft, M.S.; Egerod, K.L.; Hjorth, S.A.; Wu, M.; Feng, Y.; et al. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol. Metab. 2019, 19, 49–64. [Google Scholar] [CrossRef]
- Lin, H.V.; Efanov, A.M.; Fang, X.; Beavers, L.S.; Wang, X.; Wang, J.; Valcarcel, I.C.G.; Ma, T. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS ONE 2016, 11, e0157298. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Inoue, T.; Higashiyama, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D.; Akiba, Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J. Pharm. Exp. 2011, 339, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Blonde, G.D.; Travers, S.P.; Spector, A.C. Taste sensitivity to a mixture of monosodium glutamate and inosine 5′-monophosphate by mice lacking both subunits of the T1R1 + T1R3 amino acid receptor. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R802–R810. [Google Scholar] [CrossRef]
- Lin, W.; Kinnamon, S.C. Physiological evidence for ionotropic and metabotropic glutamate receptors in rat taste cells. J. Neurophysiol. 1999, 82, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Bigiani, A.; De Lay, R.J.; Chaudhari, N.; Kinnamon, S.C.; Roper, S.D. Responses to glutamate in rat taste cells. J. Neurophysiol. 1997, 77, 3048–3059. [Google Scholar] [CrossRef]
- Akiba, Y.; Watanabe, C.; Mizumori, M.; Kaunitz, J.D. Luminal l-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am. J. Physiol. Liver Physiol. 2009, 297, G781–G791. [Google Scholar] [CrossRef]
- Wellendorph, P.; Hansen, K.B.; Balsgaard, A.; Greenwood, J.R.; Egebjerg, J.; Bräuner-Osborne, H. Deorphanization of GPRC6A: A promiscuous l-α-Amino acid receptor with preference for basic amino acids. Mol. Pharm. 2004, 67, 589–597. [Google Scholar] [CrossRef]
- Pi, M.; Quarles, L.D. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 2012, 153, 2062–2069. [Google Scholar] [CrossRef]
- Mizokami, A.; Yasutake, Y.; Higashi, S.; Kawakubo-Yasukochi, T.; Chishaki, S.; Takahashi, I.; Takeuchi, H.; Hirata, M. Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone 2014, 69, 68–79. [Google Scholar] [CrossRef]
- Wang, H.; Murthy, K.S.; Grider, J.R. Expression patterns of l-amino acid receptors in the murine STC-1 enteroendocrine cell line. Cell Tissue Res. 2019, 378, 471–483. [Google Scholar] [CrossRef]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J. Biol. Chem. 2013, 288, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.I.; Jones, K.L.; Horowitz, M.; Sun, W.M.; Hebbard, G.; Edelbroek, M.A.L. Stereospecific effects of tryptophan on gastric emptying and hunger in humans. J. Gastroenterol. Hepatol. 1994, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Luscombe-Marsh, N.D.; Little, T.J.; Standfield, S.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal infusion of L-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. J. Clin. Endocrinol. Metab. 2014, 99, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- McVeay, C.; Fitzgerald, P.C.E.; Ullrich, S.S.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal administration of lauric acid and L-tryptophan, alone and combined, on gut hormones, pyloric pressures, and energy intake in healthy men. Am. J. Clin. Nutr. 2019, 109, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.T.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22, 1191. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Williams, L.; Xavier, G.D.S.; Rutter, G.A.; Gribble, F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 2004, 47, 1592–1601. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Farooqi, I.S.; Keogh, J.M.; Henning, E.; Habib, A.M.; Blackwood, A.; Reimann, F.; Holst, J.J.; Gribble, F.M. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and Type 2 diabetic subjects. Am. J. Clin. Nutr. 2008, 89, 106–113. [Google Scholar] [CrossRef]
- Chang, J.; Wu, T.; Greenfield, J.R.; Samocha-Bonet, D.; Horowitz, M.; Rayner, C.K. Effects of intraduodenal glutamine on incretin hormone and insulin release, the glycemic response to an intraduodenal glucose infusion, and antropyloroduodenal motility in health and Type 2 diabetes. Diabetes Care 2013, 36, 2262–2265. [Google Scholar] [CrossRef]
- Tolhurst, G.; Zheng, Y.; Parker, H.E.; Habib, A.M.; Reimann, F.; Gribble, F.M. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 2011, 152, 405–413. [Google Scholar] [CrossRef]
- Mamillapalli, R.; Wysolmerski, J. The calcium-sensing receptor couples to Gαs and regulates PTHrP and ACTH secretion in pituitary cells. J. Endocrinol. 2009, 204, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.G.; A Cheng, C.; Lawson, C.; Pappas, T.N. The effect of caloric load and nutrient composition on induction of small intestinal satiety in dogs. Physiol. Behav. 1997, 62, 39–42. [Google Scholar] [CrossRef]
- Chapman, I.M.; A Goble, E.; A Wittert, G.; Horowitz, M. Effects of small-intestinal fat and carbohydrate infusions on appetite and food intake in obese and nonobese men. Am. J. Clin. Nutr. 1999, 69, 6–12. [Google Scholar] [CrossRef]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.-C.; Arnold, R.; Göke, B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Rasmussen, O.; Lousen, T.; Holst, J.J.; Fenselau, S.; Schrezenmeir, J.; Hermansen, K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.S.; Brubaker, P.L. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 1995, 136, 5593–5599. [Google Scholar] [CrossRef]
- Kwasowski, P.; Flatt, P.R.; Bailey, C.J.; Marks, V. Effects of fatty acid chain length and saturation on gastric inhibitory polypeptide release in obese hyperglycaemic (ob/ob) mice. Biosci. Rep. 1985, 5, 701–705. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.P.M.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef]
- Maggio, C.A.; Koopmans, H.S. Food intake after intragastric meals of short-, medium-, or long-chain triglyceride. Physiol. Behav. 1982, 28, 921–926. [Google Scholar] [CrossRef]
- Pilichiewicz, A.; O’Donovan, D.; Feinle, C.; Lei, Y.; Wishart, J.M.; Bryant, L.; Meyer, J.H.; Horowitz, M.; Jones, K.L. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in Type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- Enç, F.Y.; Öneş, T.; Akın, H.L.; DeDe, F.; Turoğlu, H.T.; Ülfer, G.; Bekiroğlu, N.; Haklar, G.; Rehfeld, J.F.; Holst, J.J.; et al. Orlistat accelerates gastric emptying and attenuates GIP release in healthy subjects. Am. J. Physiol. Liver Physiol. 2009, 296, G482–G489. [Google Scholar] [CrossRef]
- Ellrichmann, M.; Kapelle, M.; Ritter, P.R.; Holst, J.J.; Herzig, K.-H.; Schmidt, W.E.; Schmitz, F.; Meier, J.J. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7–36)-Amide-1, cholecystokinin, and peptide YY concentrations. J. Clin. Endocrinol. Metab. 2008, 93, 3995–3998. [Google Scholar] [CrossRef] [PubMed]
- Beglinger, S.; Drewe, J.; Schirra, J.; Göke, B.; D’Amato, M.; Beglinger, C. Role of fat hydrolysis in regulating glucagon-like peptide-1 secretion. J. Clin. Endocrinol. Metab. 2010, 95, 879–886. [Google Scholar] [CrossRef]
- Rajalahti, T.; Lin, C.; Mjøs, S.A.; Kvalheim, O.M. Serum fatty acid and lipoprotein subclass concentrations and their associations in prepubertal healthy Norwegian children. Metabolomics 2016, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Larraufie, P.F.; Goldspink, D.A.; Gribble, F.M.; Reimann, F. Chylomicrons stimulate incretin secretion in mouse and human cells. Diabetologia 2017, 60, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Fatma, S.; Yakubov, R.; Anwar, K.; Hussain, M.M. Pluronic L81 enhances triacylglycerol accumulation in the cytosol and inhibits chylomicron secretion. J. Lipid Res. 2006, 47, 2422–2432. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Fukuoka, D.; Suzuki, J.; Fujii, Y.; Mizuno, T.; Meguro, S.; Tokimitsu, I.; Hase, T. Coingestion of acylglycerols differentially affects glucose-induced insulin secretion via glucose-dependent insulinotropic polypeptide in C57BL/6J mice. Endocrinology 2009, 150, 2118–2126. [Google Scholar] [CrossRef]
- Lu, W.J.; Yang, Q.; Yang, L.; Lee, D.; D’Alessio, D.; Tso, P. Chylomicron formation and secretion is required for lipid-stimulated release of incretins GLP-1 and GIP. Lipids 2012, 47, 571–580. [Google Scholar] [CrossRef]
- Okawa, M.; Fujii, K.; Ohbuchi, K.; Okumoto, M.; Aragane, K.; Sato, H.; Tamai, Y.; Seo, T.; Itoh, Y.; Yoshimoto, R. Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem. Biophys. Res. Commun. 2009, 390, 377–381. [Google Scholar] [CrossRef]
- Liu, J.; McLaren, D.G.; Chen, D.; Kan, Y.; Stout, S.J.; Shen, X.; Murphy, B.A.; Forrest, G.; Karanam, B.; Sonatore, L.; et al. Potential mechanism of enhanced postprandial glucagon-like peptide-1 release following treatment with a diacylglycerol acyltransferase 1 inhibitor. Pharm. Res. Perspect. 2015, 3, e00193. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K.; Schwartz, G.J. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol. Behav. 2003, 78, 285–294. [Google Scholar] [CrossRef]
- Tamura, C.S.; Ritter, R.C. Intestinal capsaicin transiently attenuates suppression of sham feeding by oleate. Am. J. Physiol. Integr. Comp. Physiol. 1994, 267, R561–R568. [Google Scholar] [CrossRef]
- Greenberg, D.; Smith, G.P.; Gibbs, J. Intraduodenal infusions of fats elicit satiety in sham-feeding rats. Am. J. Physiol. Integr. Comp. Physiol. 1990, 259, R110–R118. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, K.; Nilsson, N.E.; Flodgren, E.; Owman, C.; Olde, B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem. Biophys. Res. Commun. 2003, 301, 406–410. [Google Scholar] [CrossRef]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Lückmann, M.; et al. GPR40 (FFAR1)—Combined Gs and Gq signaling In Vitro is associated with robust incretin secretagogue action Ex Vivo and In Vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gu, Y.; Wu, C.; Yu, F.; Chen, Y.; Zhu, J.; Yao, X.; Bei, C.; Zhu, Q. Agonist-induced activation of human FFA1 receptor signals to extracellular signal-regulated kinase 1 and 2 through Gq- and Gi-coupled signaling cascades. Cell. Mol. Biol. Lett. 2017, 22, 1–12. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshida, M.; Ito, K.; Dezaki, K.; Yada, T.; Ishikawa, S.-E.; Kakei, M. Potentiation of glucose-stimulated insulin secretion by the GPR40–PLC–TRPC pathway in pancreatic β-Cells. Sci. Rep. 2016, 6, 25912. [Google Scholar] [CrossRef]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Tanaka, T.; Yano, T.; Adachi, T.; Koshimizu, T.-A.; Hirasawa, A.; Tsujimoto, G. Cloning and characterization of the rat free fatty acid receptor GPR120: In Vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic β cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Harada, N.; Sasaki, K.; Yamane, S.; Iida, K.; Suzuki, K.; Hamasaki, A.; Nasteska, D.; Shibue, K.; Joo, E.; et al. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 2015, 156, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.H.; Hauge, M.; Kristensen, L.V.; Madsen, A.N.; Engelstoft, M.S.; Husted, A.-S.; Sichlau, R.; Egerod, K.L.; Timshel, P.; Kowalski, T.J.; et al. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology 2016, 157, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Sankoda, A.; Harada, N.; Iwasaki, K.; Yamane, S.; Murata, Y.; Shibue, K.; Thewjitcharoen, Y.; Suzuki, K.; Harada, T.; Kanemaru, Y.; et al. Long-chain free fatty acid receptor GPR120 mediates oil-induced GIP secretion through CCK in male mice. Endocrinology 2017, 158, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Engelstoft, M.S.; Park, W.-M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nøhr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef]
- Stone, V.M.; Dhayal, S.; Brocklehurst, K.J.; Lenaghan, C.; Winzell, M.S.; Hammar, M.; Xu, X.; Smith, D.M.; Morgan, N.G. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia 2014, 57, 1182–1191. [Google Scholar] [CrossRef]
- Iakoubov, R.; Izzo, A.; Yeung, A.; Whiteside, C.I.; Brubaker, P.L. Protein kinase Cζ is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 2007, 148, 1089–1098. [Google Scholar] [CrossRef]
- Shah, B.P.; Liu, P.; Yu, T.; Hansen, D.R.; Gilbertson, T.A. TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am. J. Physiol. Physiol. 2012, 302, C210–C219. [Google Scholar] [CrossRef] [PubMed]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Fu, J.; Astarita, G.; Li, X.; Gaetani, S.; Campolongo, P.; Cuomo, V.; Piomelli, D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008, 8, 281–288. [Google Scholar] [CrossRef]
- Hansen, K.B.; Rosenkilde, M.M.; Knop, F.K.; Wellner, N.; Diep, T.A.; Rehfeld, J.F.; Andersen, U.B.; Holst, J.J.; Hansen, H.S. 2-oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 2011, 96, E1409–E1417. [Google Scholar] [CrossRef]
- Chu, Z.-L.; Carroll, C.; Chen, R.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Xing, C.; Sebring, K.; Zhou, J.; et al. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol. Endocrinology 2010, 24, 161–170. [Google Scholar] [CrossRef]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.-I.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Lan, H.; Vassileva, G.; Corona, A.; Liu, L.; Baker, H.; Golovko, A.; Abbondanzo, S.J.; Hu, W.; Yang, S.; Ning, Y.; et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J. Endocrinol. 2009, 201, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Oveisi, F.; Gaetani, S.; Lin, E.; Piomelli, D. Oleoylethanolamide, an endogenous PPAR-α agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology 2005, 48, 1147–1153. [Google Scholar] [CrossRef]
- Fu, J.; Gaetani, S.; Oveisi, F.; Verme, J.L.; Serrano, A.; De Fonseca, F.R.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nat. Cell Biol. 2003, 425, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.-L.; Carroll, C.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Pedraza, M.; Mondala, H.; Gao, H.; Bagnol, D.; et al. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 2008, 149, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- De Fonseca, F.R.; Navarro, M.; Gómez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodríguez, E.; Giuffrida, A.; LoVerme, J.; Gaetani, S.; et al. An anorexic lipid mediator regulated by feeding. Nature 2001, 414, 209–212. [Google Scholar] [CrossRef]
- Fu, J.; Astarita, G.; Gaetani, S.; Kim, J.; Cravatt, B.F.; Mackie, K.; Piomelli, D. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J. Biol. Chem. 2007, 282, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Overton, H.A.; Babbs, A.J.; Doel, S.M.; Fyfe, M.C.; Gardner, L.S.; Griffin, G.; Jackson, H.C.; Procter, M.J.; Rasamison, C.M.; Tang-Christensen, M.; et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175. [Google Scholar] [CrossRef]
- Moss, C.E.; Glass, L.L.; Diakogiannaki, E.; Pais, R.; Lenaghan, C.; Smith, D.M.; Wedin, M.; Bohlooly, M.-Y.; Gribble, F.M.; Reimann, F. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 2016, 77, 16–20. [Google Scholar] [CrossRef]
- Katz, L.B.; Gambale, J.J.; Rothenberg, P.L.; Vanapalli, S.R.; Vaccaro, N.; Xi, L.; Sarich, T.C.; Stein, P.P. Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with Type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.W.; Kuhre, R.E.; Janus, C.; Svendsen, B.; Holst, J.J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol. Rep. 2015, 3, e12551. [Google Scholar] [CrossRef] [PubMed]
- Tough, I.R.; Forbes, S.; Herzog, H.; Jones, R.M.; Schwartz, T.W.; Cox, H.M. Bidirectional GPR119 agonism requires peptide YY and glucose for activity in mouse and human colon mucosa. Endocrinology 2018, 159, 1704–1717. [Google Scholar] [CrossRef]
- Simons, P.J.; Kummer, J.A.; Luiken, J.J.; Boon, L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 2011, 113, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T.; Kawada, T.; Tsuruta, M.; Hiraoka, T.; Iwanaga, T.; Sugimoto, E.; Fushiki, T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997, 414, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, Y.; Braunstein, E.; Georgeson, K.E.; Harmon, C.M. Gut expression and regulation of FAT/CD36: Possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Metab. 2001, 281, E916–E923. [Google Scholar] [CrossRef]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.-P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef]
- Drover, V.A.; Nguyen, D.V.; Bastie, C.C.; Darlington, Y.F.; Abumrad, N.A.; Pessin, J.E.; London, E.; Sahoo, D.; Phillips, M.C. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 2008, 283, 13108–13115. [Google Scholar] [CrossRef]
- Nauli, A.M.; Nassir, F.; Zheng, S.; Yang, Q.; Lo, C.; Von Lehmden, S.B.; Lee, D.; Jandacek, R.J.; Abumrad, N.A.; Tso, P.; et al. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 2006, 131, 1197–1207. [Google Scholar] [CrossRef]
- Nassir, F.; Wilson, B.; Han, X.; Gross, R.W.; Abumrad, N.A. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 2007, 282, 19493–19501. [Google Scholar] [CrossRef]
- Martin, C.; Passilly-Degrace, P.; Gaillard, D.; Merlin, J.-F.; Chevrot, M.; Besnard, P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: Impact on spontaneous fat preference. PLoS ONE 2011, 6, e24014. [Google Scholar] [CrossRef]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B.; et al. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Namba, M.; Matsuyama, T.; Nonaka, K.; Tarui, S. Effect of intraluminal bile or bile acids on release of gut glucagon-like immunoreactive materials in the dog. Horm. Metab. Res. 1983, 15, 82–84. [Google Scholar] [CrossRef]
- Namba, M.; Matsuyama, T.; Itoh, H.; Imai, Y.; Horie, H.; Tarui, S. Inhibition of pentagastrin-stimulated gastric acid secretion by intraileal administration of bile and elevation of plasma concentrations of gut glucagon-like immunoreactivity in anesthetized dogs. Regul. Pept. 1986, 15, 121–128. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Parker, H.E.; Wallis, K.; Le Roux, C.W.; Wong, K.Y.; Reimann, F.; Gribble, F.M. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharm. 2011, 165, 414–423. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Gedulin, B.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes. Metab. 2012, 15, 474–477. [Google Scholar] [CrossRef]
- Adrian, T.E.; Ballantyne, G.H.; Longo, W.E.; Bilchik, A.J.; Graham, S.; Basson, M.D.; Tierney, R.P.; Modlin, I.M. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut 1993, 34, 1219–1224. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Albrechtsen, N.J.W.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- Burhol, P.G.; Lygren, I.; Waldum, H.; Jorde, R. The effect of duodenal infusion of bile on plasma VIP, GIP, and secretin and on duodenal bicarbonate secretion. Scand. J. Gastroenterol. 1980, 15, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.L.; Sorrentino, G.; Egerod, K.L.; Kroone, C.; Mortensen, B.; Knop, F.K.; Reimann, F.; Gribble, F.M.; Drucker, D.J.; De Koning, E.J.; et al. L-cell differentiation is induced by bile acids through GPBAR1 and paracrine GLP-1 and serotonin signaling. Diabetes 2020, 69, 614–623. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Trammell, S.A.J.; Albrechtsen, N.J.W.; Schoonjans, K.; Albrechtsen, R.; Gillum, M.P.; Kuhre, R.E.; Holst, J.J. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am. J. Physiol. Liver Physiol. 2019, 316, G574–G584. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Wring, S.A.; Polli, J.E. Interaction of native bile acids with human apical sodium-dependent bile acid transporter (hASBT): Influence of steroidal hydroxylation pattern and C-24 conjugation. Pharm. Res. 2006, 23, 1451–1459. [Google Scholar] [CrossRef]
- Trabelsi, M.-S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef]
- Poole, D.P.; Godfrey, C.; Cattaruzza, F.; Cottrell, G.S.; Kirkland, J.G.; Pelayo, J.C.; Bunnett, N.W.; Corvera, C.U. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol. Motil. 2010, 22, 814–e228. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Karaki, S.-I.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.-I.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T.; et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M.; et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor FFAR2. Diabetes 2011, 61, 364–371. [Google Scholar] [CrossRef]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Karaki, S.-I.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef]
- Nøhr, M.; Egerod, K.; Christiansen, S.; Gille, A.; Offermanns, S.; Schwartz, T.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Won, Y.-J.; Lu, V.B.; Puhl, H.L.; Ikeda, S.R. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 2013, 33, 19314–19325. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.J.; A Bewick, G.; Hanyaloglu, A.C.; A Ghatei, M.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Forbes, S.; Stafford, S.; Coope, G.; Heffron, H.; Real, K.; Newman, R.; Davenport, R.; Barnes, M.; Grosse, J.; Cox, H.; et al. Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes 2015, 64, 3763–3771. [Google Scholar] [CrossRef]
- Bolognini, D.; Moss, C.E.; Nilsson, K.; Petersson, A.U.; Donnelly, I.; Sergeev, E.; König, G.M.; Kostenis, E.; Kurowska-Stolarska, M.; Miller, A.; et al. A novel allosteric activator of free fatty acid 2 receptor displays unique gi-functional bias. J. Biol. Chem. 2016, 291, 18915–18931. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; Van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Høverstad, T.; Bjørneklett, A.; Midtvedt, T.; Fausa, O.; Bøhmer, T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J. Gastroenterol. 1984, 19, 1053–1058. [Google Scholar]
- Lee, E.-Y.; Zhang, X.; Miyamoto, J.; Kimura, I.; Taknaka, T.; Furusawa, K.; Jomori, T.; Fujimoto, K.; Uematsu, S.; Miki, T.; et al. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J. Endocrinol. 2018, 239, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J.; et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Reimann, F.; Bartfeld, S.; Farin, H.F.; Ringnalda, F.C.; Vries, R.G.J.; Brink, S.V.D.; Clevers, H.; Gribble, F.M.; De Koning, E.J.P.; et al. Generation of L cells in mouse and human small intestine organoids. Diabetes 2014, 63, 410–420. [Google Scholar] [CrossRef]
- Arora, T.; Akrami, R.; Pais, R.; Bergqvist, L.; Johansson, B.R.; Schwartz, T.W.; Reimann, F.; Gribble, F.M.; Bäckhed, F. Microbial regulation of the L cell transcriptome. Sci. Rep. 2018, 8, 1207. [Google Scholar] [CrossRef]
- Wichmann, A.; Allahyar, A.; Greiner, T.U.; Plovier, H.; Östergren, G.L.; Larsson, T.; Drucker, D.J.; Delzenne, N.M.; Cani, P.; Bäckhed, F.; et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 2013, 14, 582–590. [Google Scholar] [CrossRef]
- De Fonseca, F.R.; Navarro, M.; Alvarez, E.; Roncero, I.; Chowen, J.A.; Maestre, O.; Gómez, R.; Muñoz, R.M.; Eng, J.; Blázquez, E.; et al. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 2000, 49, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Näslund, E.; Barkeling, B.; King, N.; Gutniak, M.; Blundell, J.; Holst, J.; Rössner, S.; Hellström, P. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. 1999, 23, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Gutzwiller, J.-P.; Göke, B.; Drewe, J.; Hildebrand, P.; Ketterer, S.; Handschin, D.; Winterhalder, R.; Conen, D.; Beglinger, C. Glucagon-like peptide-1: A potent regulator of food intake in humans. Gut 1999, 44, 81–86. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; MacConell, L.; Zhuang, N.; Kothare, P.A.; Trautmann, M.; Fineman, M.; Taylor, K. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with Type 2 diabetes. Diabetes Care 2007, 30, 1487–1493. [Google Scholar] [CrossRef]
- Holst, J.J. Incretin hormones and the satiation signal. Int. J. Obes. 2013, 37, 1161–1168. [Google Scholar] [CrossRef]
- Astrup, A.; Rössner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef]
- Finan, B.; Ma, T.; Ottaway, N.; Müller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013, 5, 209. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Meessen, E.C.E.; Andersen, M.M.; Bergmann, S.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.B.; et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: A randomised, crossover study. Diabetologia 2019, 62, 665–675. [Google Scholar] [CrossRef]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef]
- Althage, M.C.; Ford, E.L.; Wang, S.; Tso, P.; Polonsky, K.S.; Wice, B.M. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J. Biol. Chem. 2008, 283, 18365–18376. [Google Scholar] [CrossRef]
- Pathak, V.; Gault, V.A.; Flatt, P.R.; Irwin, N. Antagonism of gastric inhibitory polypeptide (GIP) by palmitoylation of GIP analogues with N- and C-terminal modifications improves obesity and metabolic control in high fat fed mice. Mol. Cell. Endocrinol. 2015, 401, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tanimoto, H.; Mizuno, Y.; Okamoto, M.; Takeuchi, M.; Tsubamoto, Y.; Noda, H. Gastric inhibitory polypeptide receptor antagonist, SKL-14959, suppressed body weight gain on diet-induced obesity mice. Obes. Sci. Pr. 2018, 4, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Nian, C.; Karunakaran, S.; Clee, S.M.; Isales, C.M.; McIntosh, C.H.S. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS ONE 2012, 7, e40156. [Google Scholar] [CrossRef]
- Geary, N.; Smith, G.P. Pancreatic glucagon and postprandial satiety in the rat. Physiol. Behav. 1982, 28, 313–322. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Figlewicz, D.P.; Woods, S.C.; Porte, D.; Baskin, D.G. Insulin, neuropeptide Y, and food intake. Ann. N. Y. Acad. Sci. 1993, 692, 60–71. [Google Scholar] [CrossRef]
- Pamir, N.; Lynn, F.C.; Buchan, A.M.J.; Ehses, J.; Hinke, S.A.; Pospisilik, J.A.; Miyawaki, K.; Yamada, Y.; Seino, Y.; McIntosh, C.H.S.; et al. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am. J. Physiol. Metab. 2003, 284, E931–E939. [Google Scholar] [CrossRef]
- Sparre-Ulrich, A.H.; Hansen, L.S.; Svendsen, B.; Christensen, M.S.; Knop, F.K.; Hartmann, B.; Holst, J.J.; Rosenkilde, M.M. Species-specific action of (Pro3)GIP—A full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. Br. J. Pharm. 2015, 173, 27–38. [Google Scholar] [CrossRef]
- Gault, V.A.; O’Harte, F.P.; Harriott, P.; Flatt, P.R. Characterization of the cellular and metabolic effects of a novel enzyme-resistant antagonist of glucose-dependent insulinotropic polypeptide. Biochem. Biophys. Res. Commun. 2002, 290, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.A.; Finan, B.; Gelfanov, V.; Yang, B.; Tschöp, M.H.; DiMarchi, R.D.; Perez-Tilve, D. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol. Metab. 2019, 20, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Nørregaard, P.K.; Deryabina, M.A.; Shelton, P.T.; Fog, J.U.; Daugaard, J.R.; Eriksson, P.; Larsen, L.F.; Jessen, L. A novel GIP analogue, ZP 4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes. Metab. 2018, 20, 60–68. [Google Scholar] [CrossRef]
- Gault, V.A.; Kerr, B.D.; Harriott, P.; Flatt, P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin. Sci. 2011, 121, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Portron, A.; Jadidi, S.; Sarkar, N.; Di Marchi, R.; Schmitt, C. Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects. Diabetes Obes. Metab. 2017, 19, 1446–1453. [Google Scholar] [CrossRef]
- Schmitt, C.; Portron, A.; Jadidi, S.; Sarkar, N.; Di Marchi, R. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with Type 2 diabetes mellitus. Diabetes Obes. Metab. 2017, 19, 1436–1445. [Google Scholar] [CrossRef]
- Frias, J.P.; Bastyr, E.J.; Vignati, L.; Tschöp, M.H.; Schmitt, C.; Owen, K.; Christensen, R.H.; DiMarchi, R.D. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090–2746, in patients with Type 2 diabetes. Cell Metab. 2017, 26, 343–352.e2. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with Type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of Type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Welch, I.; Saunders, K.; Read, N. Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology 1985, 89, 1293–1297. [Google Scholar] [CrossRef]
- Lavin, J.H.; Wittert, G.; Sun, W.M.; Horowitz, M.; Morley, J.E.; Read, N.W. Appetite regulation by carbohydrate: Role of blood glucose and gastrointestinal hormones. Am. J. Physiol. Metab. 1996, 271, E209–E214. [Google Scholar] [CrossRef]
- Naslund, E.; Grybäck, P.; Bäckman, L.; Jacobsson, H.; Holst, J.J.; Theodorsson, H.E.; Hellstrom, P.M.; Juul, J. Distal small bowel hormones (Correlation with fasting antroduodenal motility and gastric emptying). Dig. Dis. Sci. 1998, 43, 945–952. [Google Scholar] [CrossRef]
- Imeryüz, N.; Yeğen, B.Ç.; Bozkurt, A.; Coşkun, T.; Villanueva-Peñacarrillo, M.L.; Ulusoy, N.B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am. J. Physiol. Liver Physiol. 1997, 273, G920–G927. [Google Scholar] [CrossRef]
- Schirra, J.; Nicolaus, M.; Woerle, H.J.; Struckmeier, C.; Katschinski, M.; Göke, B. GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol. Motil. 2009, 21, 609–e22. [Google Scholar] [CrossRef]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef]
- Fitzgerald, P.C.E.; Manoliu, B.; Herbillon, B.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Effects of L-phenylalanine on energy intake and glycaemia—impacts on appetite perceptions, gastrointestinal hormones and gastric emptying in healthy males. Nutrients 2020, 12, 1788. [Google Scholar] [CrossRef]
- Layer, P.; Holst, J.J.; Grandt, D.; Goebell, H. Ileal release of glucagon-like peptide-1 (GLP-1). Dig. Dis. Sci. 1995, 40, 1074–1082. [Google Scholar] [CrossRef]
- Wettergren, A.; Wojdemann, M.; Meisner, S.; Stadil, F.; Holst, J.J. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7–36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut 1997, 40, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Villar, H.V.; Fender, H.R.; Rayford, P.L.; Bloom, S.R.; Ramus, N.I.; Thompson, J.C. Suppression of gastrin release and gastric secretion by gastric inhibitory polypeptide (GIP) and vasoactive intestinal polypeptide (VIP). Ann. Surg. 1976, 184, 97–102. [Google Scholar] [CrossRef]
- Tolessa, T.; Gutniak, M.; Holst, J.J.; Efendic, S.; Hellström, P.M. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. J. Clin. Investig. 1998, 102, 764–774. [Google Scholar] [CrossRef]
- Halim, A.; Degerblad, M.; Sundbom, M.; Karlbom, U.; Holst, J.J.; Webb, D.-L.; Hellström, P.M. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J. Clin. Endocrinol. Metab. 2017, 103, 575–585. [Google Scholar] [CrossRef]
- Ogawa, E.; Hosokawa, M.; Harada, N.; Yamane, S.; Hamasaki, A.; Toyoda, K.; Fujimoto, S.; Fujita, Y.; Fukuda, K.; Tsukiyama, K.; et al. The effect of gastric inhibitory polypeptide on intestinal glucose absorption and intestinal motility in mice. Biochem. Biophys. Res. Commun. 2011, 404, 115–120. [Google Scholar] [CrossRef]
- Edholm, T.; Cejvan, K.; Efendic, S.; Schmidt, P.T.; Hellström, P.M.; Abdel-Halim, S.M. The incretin hormones GIP and GLP-1 in diabetic rats: Effects on insulin secretion and small bowel motility. Neurogastroenterol. Motil. 2009, 21, 313–321. [Google Scholar] [CrossRef]
- Powley, T.L.; Phillips, R.J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 2004, 82, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kupari, J.; Häring, M.; Agirre, E.; Castelo-Branco, G.; Ernfors, P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019, 27, 2508–2523.e4. [Google Scholar] [CrossRef]
- Egerod, K.L.; Petersen, N.; Timshel, P.N.; Rekling, J.C.; Wang, Y.; Liu, Q.; Schwartz, T.W.; Gautron, L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol. Metab. 2018, 12, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Bucinskaite, V.; Tolessa, T.; Pedersen, J.; Rydqvist, B.; Zerihun, L.; Holst, J.J.; Hellström, P.M. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil. 2009, 21, e78. [Google Scholar] [CrossRef] [PubMed]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef]
- Jensen, C.B.; Pyke, C.; Rasch, M.G.; Dahl, A.B.; Knudsen, L.B.; Secher, A. Characterization of the glucagonlike peptide-1 receptor in male mouse brain using a novel antibody and In Situ hybridization. Endocrinology 2017, 159, 665–675. [Google Scholar] [CrossRef]
- Ast, J.; Arvaniti, A.; Fine, N.H.F.; Nasteska, D.; Ashford, F.B.; Stamataki, Z.; Koszegi, Z.; Bacon, A.; Jones, B.J.; Lucey, M.A.; et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Yox, D.P.; Stokesberry, H.; Ritter, R.C. Fourth ventricular capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am. J. Physiol. Integr. Comp. Physiol. 1991, 260, R681–R687. [Google Scholar] [CrossRef]
- Yox, D.P.; Ritter, R.C. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am. J. Physiol. Integr. Comp. Physiol. 1988, 255, R569–R574. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Tang-Christensen, M.; Holst, J.J.; Ørskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997, 77, 257–270. [Google Scholar] [CrossRef]
- Hisadome, K.; Reimann, F.; Gribble, F.M.; Trapp, S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius. Diabetes 2010, 59, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Langhans, W.; Krause, E.; et al. Central and peripheral GLP-1 systems independently and additively suppress eating. bioRxiv 2020. [Google Scholar] [CrossRef]
- Powley, T.L.; Spaulding, R.A.; Haglof, S.A. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: Chemoreceptor and mechanoreceptor architecture. J. Comp. Neurol. 2010, 519, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, 5236. [Google Scholar] [CrossRef]
- Lu, V.B.; Rievaj, J.; O’Flaherty, E.A.; Smith, C.A.; Pais, R.; Pattison, L.A.; Tolhurst, G.; Leiter, A.B.; Bulmer, D.C.; Gribble, F.M.; et al. Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat. Commun. 2019, 10, 1029. [Google Scholar] [CrossRef]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 2008, 150, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.B.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nat. Cell Biol. 1996, 379, 69–72. [Google Scholar] [CrossRef]
- Tang-Christensen, M.; Larsen, P.J.; Göke, R.; Fink-Jensen, A.; Jessop, D.S.; Möller, M.; Sheikh, S.P. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol. Integr. Comp. Physiol. 1996, 271, R848–R856. [Google Scholar] [CrossRef]
- Schick, R.R.; Zimmermann, J.P.; Walde, T.V.; Schusdziarra, V. Glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1427–R1435. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Huang, Q.; Brown, T.J.; Drucker, D.J. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004, 127, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, C.; Kim, M.S.; Jang, B.-T.; Lee, Y.H.; Cho, Y.-M.; Choi, H.J. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem. Biophys. Res. Commun. 2017, 490, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, A.E.; Biggs, E.K.; Darwish, T.; Tadross, J.; Sukthankar, T.; Girish, M.; Polex-Wolf, J.; Lam, B.Y.; Zvetkova, I.; Pan, W.; et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019, 30, 987–996.e6. [Google Scholar] [CrossRef]
- Holt, M.K.; Richards, J.E.; Cook, D.R.; Brierley, D.I.; Williams, D.L.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes 2019, 68, 21–33. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Ortinski, P.I.; Rupprecht, L.E.; Olivos, D.R.; Alhadeff, A.L.; Pierce, R.C.; Hayes, M.R. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. Metab. 2013, 305, E1367–E1374. [Google Scholar] [CrossRef]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The Glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef]
- Dossat, A.M.; Diaz, R.; Gallo, L.; Panagos, A.; Kay, K.; Williams, D.L. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am. J. Physiol. Metab. 2013, 304, E1314. [Google Scholar] [CrossRef]
- Kentish, S.; Li, H.; Philp, L.K.; O’Donnell, T.A.; Isaacs, N.J.; Young, R.L.; Wittert, G.A.; Blackshaw, L.A.; Page, A.J. Diet-induced adaptation of vagal afferent function. J. Physiol. 2011, 590, 209–221. [Google Scholar] [CrossRef]
- Jepsen, S.L.; Grunddal, K.V.; Albrechtsen, N.J.W.; Engelstoft, M.S.; Gabe, M.B.N.; Jensen, E.P.; Ørskov, C.; Poulsen, S.S.; Rosenkilde, M.M.; Pedersen, J.; et al. Paracrine crosstalk between intestinal L- and D-cells controls secretion of glucagon-like peptide-1 in mice. Am. J. Physiol. Metab. 2019, 317, E1081–E1093. [Google Scholar] [CrossRef]
- Adriaenssens, A.; Lam, B.Y.H.; Billing, L.; Skeffington, K.; Sewing, S.; Reimann, F.; Gribble, F. A transcriptome-led exploration of molecular mechanisms regulating somatostatin-producing D-Cells in the gastric epithelium. Endocrinology 2015, 156, 3924–3936. [Google Scholar] [CrossRef]
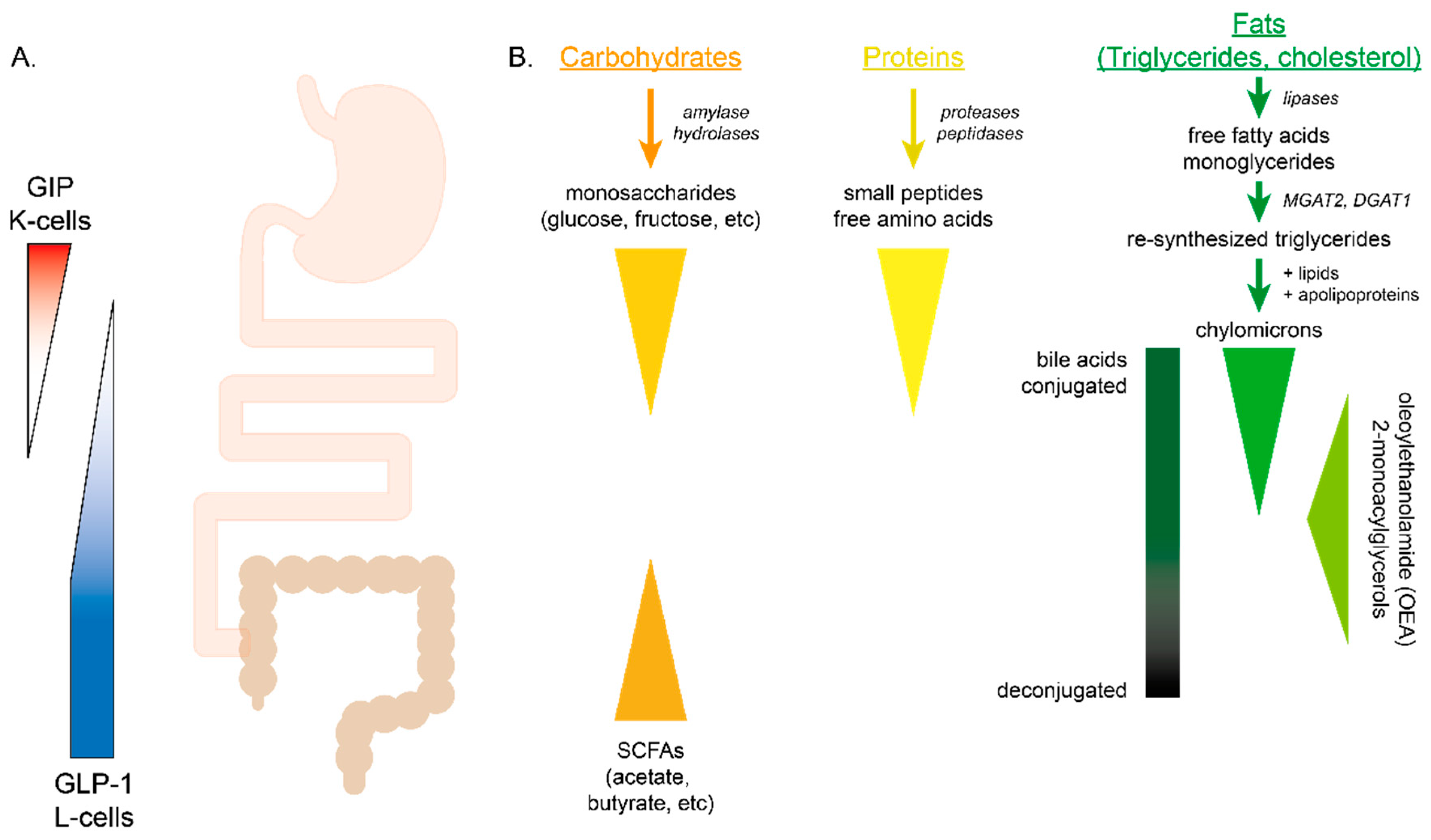
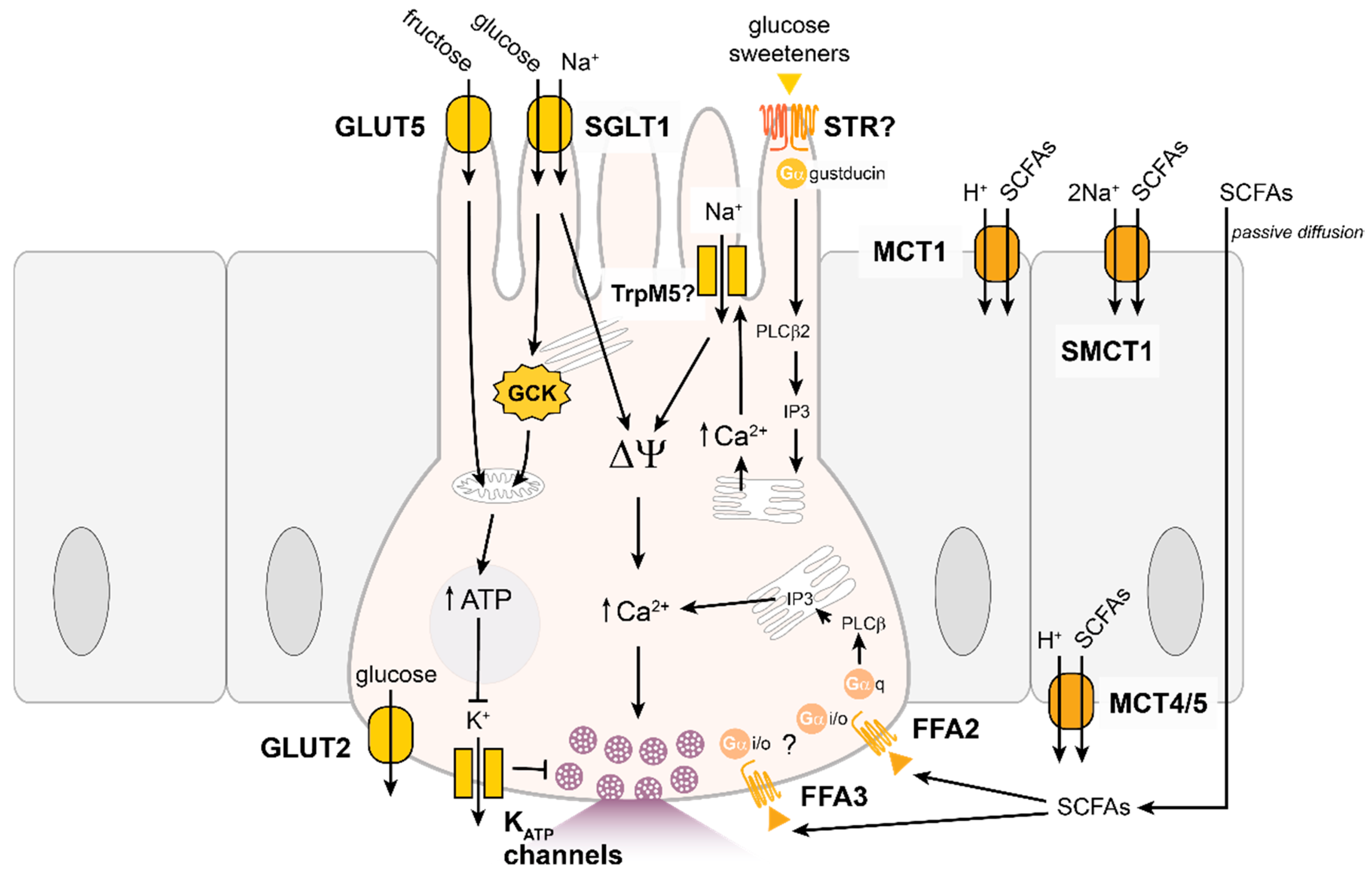
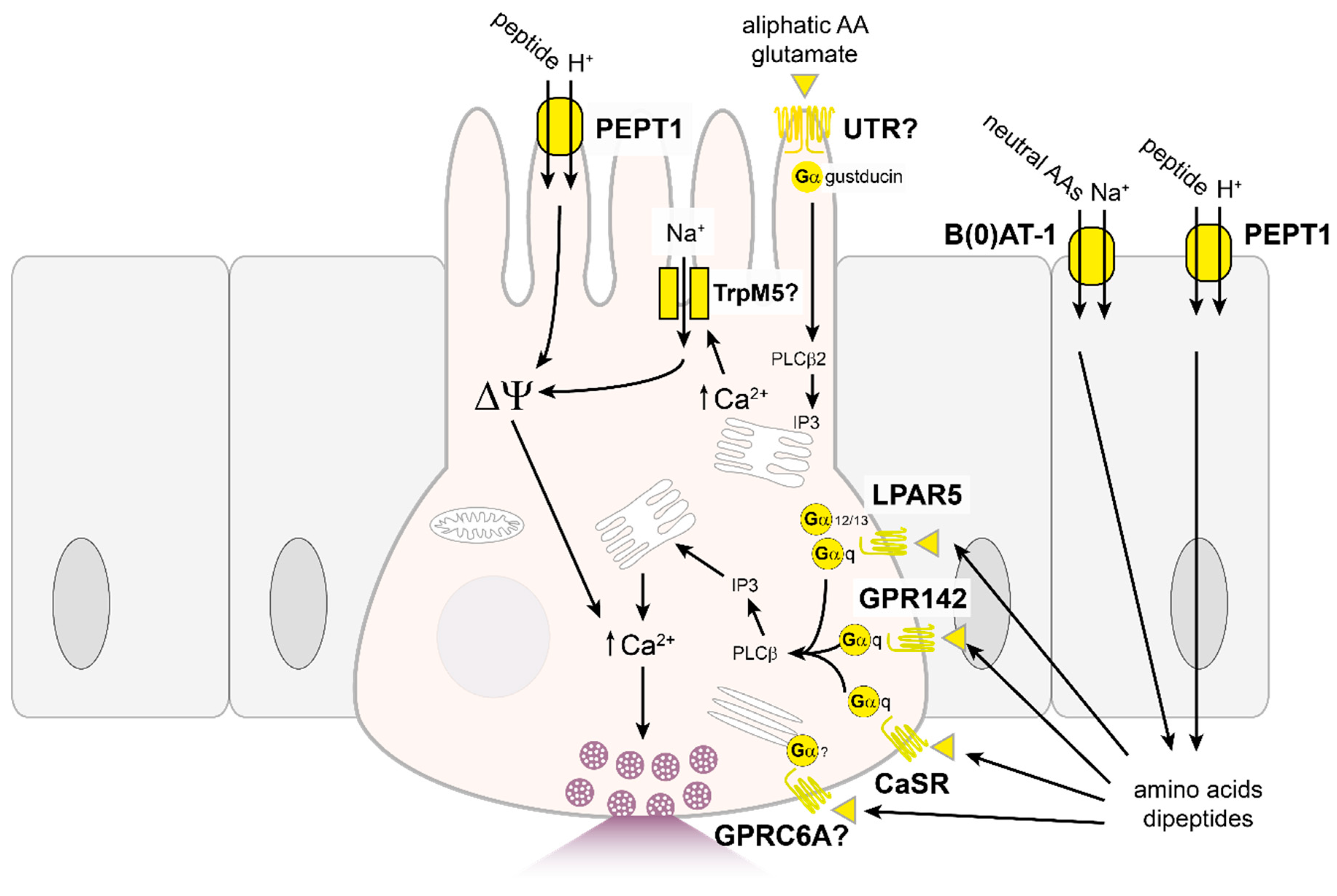
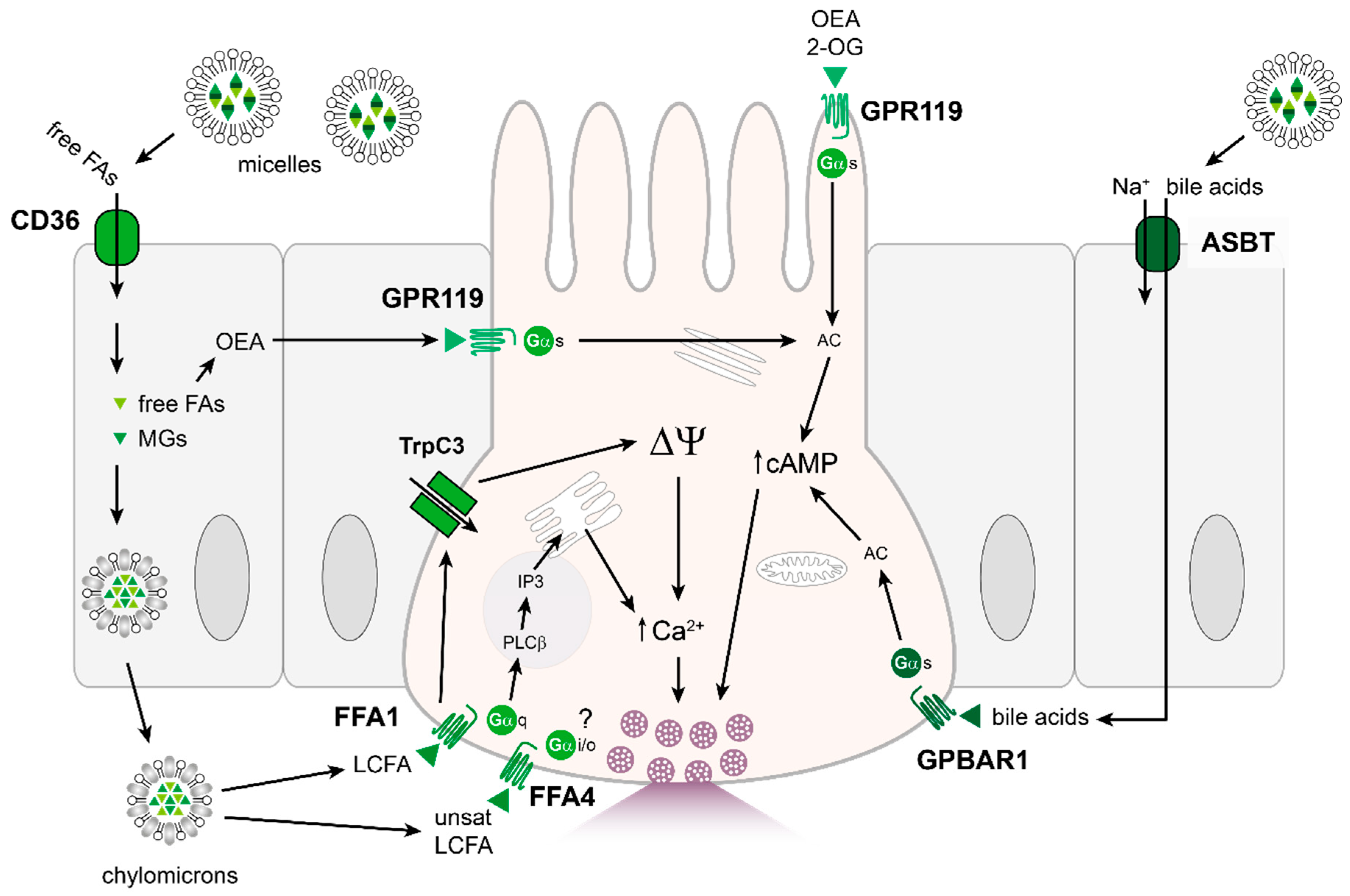
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, V.B.; Gribble, F.M.; Reimann, F. Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion. Nutrients 2021, 13, 883. https://doi.org/10.3390/nu13030883
Lu VB, Gribble FM, Reimann F. Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion. Nutrients. 2021; 13(3):883. https://doi.org/10.3390/nu13030883
Chicago/Turabian StyleLu, Van B., Fiona M. Gribble, and Frank Reimann. 2021. "Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion" Nutrients 13, no. 3: 883. https://doi.org/10.3390/nu13030883
APA StyleLu, V. B., Gribble, F. M., & Reimann, F. (2021). Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion. Nutrients, 13(3), 883. https://doi.org/10.3390/nu13030883