Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms
Abstract
1. Introduction
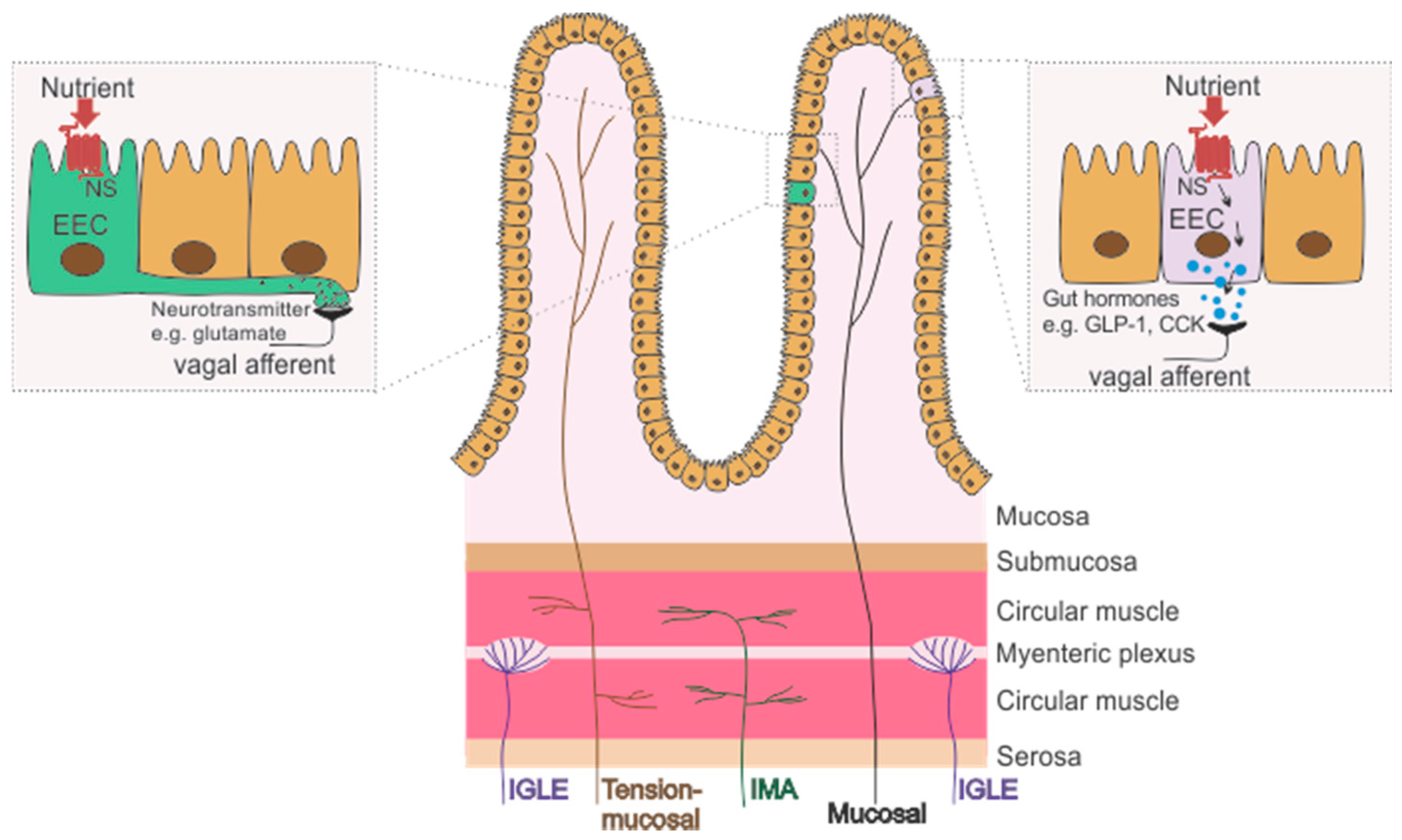
2. Gastrointestinal Vagal Afferents
2.1. Subtypes of Gastrointestinal Vagal Afferents
2.1.1. Mechanoreceptors
Tension or Stretch Receptors
Mucosal Receptors
Tension-Mucosal Receptors
2.1.2. Chemoreceptors
2.2. Gastrointestinal Vagal Afferents and Food Intake Regulation
2.2.1. Gastric Signals
2.2.2. Small Intestinal Signals
2.3. Plasticity of Gastrointestinal Afferents
3. Circadian Regulation of Food Intake
3.1. Circadian System and Food Intake Patterns
3.2. Circadian Vagal Afferent Signalling
3.2.1. Nutrient and Gut Hormone Signals
3.2.2. Gut Microbiota
3.2.3. Disrupted Circadian Signalling
High Fat Diet-Induced Obesity
Disrupted Light Cycle
3.2.4. Time Restricted Feeding
4. Conclusions
Funding
Conflicts of Interest
References
- Kentish, S.J.; Page, A.J. Plasticity of gastro-intestinal vagal afferent endings. Physiol. Behav. 2014, 136, 170–178. [Google Scholar] [CrossRef]
- Kentish, S.J.; Frisby, C.L.; Kennaway, D.J.; Wittert, G.A.; Page, A.J. Circadian variation in gastric vagal afferent mechanosensitivity. J. Neurosci. 2013, 33, 19238–19242. [Google Scholar] [CrossRef]
- Kentish, S.J.; Vincent, A.D.; Kennaway, D.J.; Wittert, G.A.; Page, A.J. High-Fat Diet-Induced Obesity Ablates Gastric Vagal Afferent Circadian Rhythms. J. Neurosci. 2016, 36, 3199–3207. [Google Scholar] [CrossRef]
- Armstrong, S. A chronometric approach to the study of feeding behavior. Neurosci. Biobehav. Rev. 1980, 4, 27–53. [Google Scholar] [CrossRef]
- Ma, M.A.; Morrison, E.H. Neuroanatomy, Nucleus Suprachiasmatic. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Kentish, S.J.; Page, A.J. The role of gastrointestinal vagal afferent fibres in obesity. J. Physiol. 2015, 593, 775–786. [Google Scholar] [CrossRef]
- Wang, Y.B.; De Lartigue, G.; Page, A.J. Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front. Physiol. 2020, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Powley, T.L. Tension and stretch receptors in gastrointestinal smooth muscle: Re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Rev. 2000, 34, 1–26. [Google Scholar] [CrossRef]
- Brookes, S.J.; Spencer, N.J.; Costa, M.; Zagorodnyuk, V.P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Martin, C.M.; Blackshaw, L.A. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J. Neurophysiol. 2002, 87, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Blackshaw, L.A. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J. Physiol. 1998, 512, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zagorodnyuk, V.P.; Chen, B.N.; Brookes, S.J.H. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 2001, 534, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Zagorodnyuk, V.P.; Brookes, S.J. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 2000, 20, 6249–6255. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143.e1123. [Google Scholar] [CrossRef]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Iggo, A. Tension receptors in the stomach and the urinary bladder. J. Physiol. 1955, 128, 593–607. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Powley, T.L. Vagal Afferent Innervation of the Rat Fundic Stomach: Morphological Characterization of the Gastric Tension Receptor. J. Comp. Neurol. 1992, 319, 261–276. [Google Scholar] [CrossRef]
- Fox, E.A.; Phillips, R.J.; Martinson, F.A.; Baronowsky, E.A.; Powley, T.L. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: Morphology and topography. J. Comp. Neurol. 2000, 428, 558–576. [Google Scholar] [CrossRef]
- Powley, T.L.; Hudson, C.N.; McAdams, J.L.; Baronowsky, E.A.; Phillips, R.J. Vagal Intramuscular Arrays: The Specialized Mechanoreceptor Arbors That Innervate the Smooth Muscle Layers of the Stomach Examined in the Rat. J. Comp. Neurol. 2016, 524, 713–737. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.M.; Kelly, K.A. Antral control of canine gastric emptying of solids. Am. J. Physiol. Gastrointest. Liver Physiol. 1983, 8, 334–338. [Google Scholar] [CrossRef]
- Andrews, P.L.; Wood, K.L. Vagally mediated gastric motor and emetic reflexes evoked by stimulation of the antral mucosa in anaesthetized ferrets. J. Physiol. 1988, 395, 1–16. [Google Scholar] [CrossRef]
- Brierley, S.M.; Jones, R.C.; Gebhart, G.F.; Blackshaw, L.A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004, 127, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Jones, R.C., 3rd; Xu, L.; Gebhart, G.F.; Blackshaw, L.A. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol. Motil. 2005, 17, 854–862. [Google Scholar] [CrossRef]
- Spencer, N.J.; Kyloh, M.; Beckett, E.A.; Brookes, S.; Hibberd, T. Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J. Comp. Neurol. 2016, 524, 3064–3083. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Phillips, R.J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 2004, 82, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Grabauskas, G.; Song, I.; Zhou, S.; Owyang, C. Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia. J. Physiol. 2010, 588, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Tehan, B.; Marshall, F. Pharmacology and physiology of gastrointestinal enteroendocrine cells. Pharmacol. Res. Perspect. 2015, 3, e00155. [Google Scholar] [CrossRef]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. Luminal sensing in the gut: An overview. J. Physiol. Pharmacol. 2003, 54, 9–17. [Google Scholar] [PubMed]
- Kaelberer, M.M.; Bohorquez, D.V. The now and then of gut-brain signaling. Brain Res. 2018, 1693, 192–196. [Google Scholar] [CrossRef]
- Bohorquez, D.V.; Chandra, R.; Samsa, L.A.; Vigna, S.R.; Liddle, R.A. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J. Mol. Histol. 2011, 42, 3–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bohorquez, D.V.; Liddle, R.A. Axon-like basal processes in enteroendocrine cells: Characteristics and potential targets. Clin. Transl. Sci. 2011, 4, 387–391. [Google Scholar] [CrossRef]
- Bohorquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881. [Google Scholar] [CrossRef]
- Bohorquez, D.V.; Shahid, R.A.; Erdmann, A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Feinle, C.; Grundy, D.; Read, N.W. Effects of duodenal nutrients on sensory and motor responses of the human stomach to distension. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G721–G726. [Google Scholar] [CrossRef]
- Wang, G.; Tomasi, D.; Backus, W. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008, 39, 1824–1831. [Google Scholar] [CrossRef]
- Distrutti, E.; Azpiroz, F.; Soldevilla, A.; Malagelada, J.R. Gastric wall tension determines perception of gastric distention. Gastroenterology 1999, 116, 1035–1042. [Google Scholar] [CrossRef]
- Kissileff, H.R.; Carretta, J.C.; Geliebter, A.; Pi-Sunyer, F.X. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R992–R998. [Google Scholar] [CrossRef]
- Melton, P.M.; Kissileff, H.R.; Pi-Sunyer, F.X. Cholecystokinin (CCK-8) affects gastric pressure and ratings of hunger and fullness in women. Am. J. Physiol. 1992, 263, R452–R456. [Google Scholar] [CrossRef]
- Geliebter, A.; Westreich, S.; Gage, D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am. J. Clin. Nutr. 1988, 48, 592–594. [Google Scholar] [CrossRef]
- Jones, K.L.; Doran, S.M.; Hveem, K. Relation between postprandial satiation and antral area in normal subjects. Am. J. Clin. Nutr. 1997, 66, 127–132. [Google Scholar] [CrossRef]
- Sturm, K.; Parker, B.; Wishart, J. Energy intake and appetite are related to antral area in healthy young and older subjects. Am. J. Clin. Nutr. 2004, 80, 656–667. [Google Scholar] [CrossRef]
- Jagger, A.; Grahn, J.; Ritter, R.C. Reduced vagal sensory innervation of the small intestinal myenteric plexus following capsaicin treatment of adult rats. Neurosci. Lett. 1997, 236, 103–106. [Google Scholar] [CrossRef]
- Liou, A.P.; Sei, Y.; Zhao, X. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to l-phenylalanine in acutely isolated intestinal I cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G538–G546. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J. Biol. Chem. 1978, 253, 4022–4030. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Zhang, X.; Young, R.L.; Bound, M. Comparative Effects of Proximal and Distal Small Intestinal Glucose Exposure on Glycemia, Incretin Hormone Secretion, and the Incretin Effect in Health and Type 2 Diabetes. Diabetes Care 2019, 42, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.; Christensen, L.L.; Holst, J.J.; Orskov, C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003, 114, 189–196. [Google Scholar] [CrossRef]
- Wu, T.; Rayner, C.K.; Watson, L.E.; Jones, K.L.; Horowitz, M.; Little, T.J. Comparative effects of intraduodenal fat and glucose on the gut-incretin axis in healthy males. Peptides 2017, 95, 124–127. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Cairns, L.S. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012, 153, 3054–3065. [Google Scholar] [CrossRef]
- Meier, J.J.; Nauck, M.A.; Kranz, D. Secretion, Degradation, and Elimination of Glucagon-Like Peptide 1 and Gastric Inhibitory Polypeptide in Patients with Chronic Renal Insufficiency and Healthy Control Subjects. Diabetes 2004, 53, 654–662. [Google Scholar] [CrossRef]
- Ballinger, A.B.; Clark, M.L. L-phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans: Evidence for a physiological role of CCK in control of eating. Metabolism 1994, 43, 735–738. [Google Scholar] [CrossRef]
- Dockray, G.J. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Thomas, S.R.; Kilroy, G.; Schwartz, G.J.; York, D.A. Enterostatin inhibition of dietary fat intake is dependent on CCK-A receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R321–R328. [Google Scholar] [CrossRef]
- Batterham, R.L.; Heffron, H.; Kapoor, S. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Burdyga, G.; De Lartigue, G.; Raybould, H.E. Cholecystokinin Regulates Expression of Y2 Receptors in Vagal Afferent Neurons Serving the Stomach. J. Neurosci. 2008, 28, 11583–11592. [Google Scholar] [CrossRef]
- Koda, S.; Date, Y.; Murakami, N. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 2005, 146, 2369–2375. [Google Scholar] [CrossRef]
- Abbott, C.R.; Monteiro, M.; Small, C.J. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef]
- Talsania, T.; Anini, Y.; Siu, S.; Drucker, D.J.; Brubaker, P.L. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005, 146, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Babic, T.; Holmes, G.M.; Swartz, E.; Travagli, R.A. A critical re-evaluation of the specificity of action of perivagal capsaicin. J. Physiol. 2013, 591, 1563–1580. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, M.A.; Stadlbauer, U.; Weber, E.; Arnold, M.; Langhans, W.; Pacheco-López, G. Vagal Afferents Mediate Early Satiation and Prevent Flavour Avoidance Learning in Response to Intraperitoneally Infused Exendin-4. J. Neuroendocrinol. 2012, 24, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Satake, H.; Nakabayashi, H. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci. 2004, 110, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 2016, 65, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Larue-Achagiotis, C.; Le Magnen, J. Changes of meal patterns induced by food deprivation: Metabolic correlates. Neurosci. Biobehav. Rev. 1980, 4, 25–27. [Google Scholar] [CrossRef]
- Le Magnen, J.; Devos, M.; Larue-Achagiotis, C. Food deprivation induced parallel changes in blood glucose, plasma free fatty acids and feeding during two parts of the diurnal cycle in rats. Neurosci. Biobehav. Rev. 1980, 4, 17–23. [Google Scholar] [CrossRef]
- Kentish, S.; Li, H.; Philp, L.K. Diet-induced adaptation of vagal afferent function. J. Physiol. 2012, 590, 209–221. [Google Scholar] [CrossRef]
- Li, H.; Clarke, G.S.; Christie, S. Pregnancy-related plasticity of gastric vagal afferent signals in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G183–G192. [Google Scholar] [CrossRef]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef]
- Christie, S.; Vincent, A.D.; Li, H. A rotating light cycle promotes weight gain and hepatic lipid storage in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G932–G942. [Google Scholar] [CrossRef]
- Honma, K.; Hikosaka, M.; Mochizuki, K.; Goda, T. Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Metabolism 2016, 65, 482–491. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K.; Wirz-Justice, A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. 1994, 267, R819–R829. [Google Scholar] [CrossRef] [PubMed]
- Spengler, C.M.; Czeisler, C.A.; Shea, S.A. An endogenous circadian rhythm of respiratory control in humans. J. Physiol. 2000, 526, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef]
- Owens, D.S.; Macdonald, I.; Benton, D.; Sytnik, N.; Tucker, P.; Folkard, S. A preliminary investigation into individual differences in the circadian variation of meal tolerance: Effects on mood and hunger. Chronobiol. Int. 1996, 13, 435–447. [Google Scholar] [CrossRef]
- Sargent, C.; Zhou, X.; Matthews, R.W.; Darwent, D.; Roach, G.D. Daily Rhythms of Hunger and Satiety in Healthy Men during One Week of Sleep Restriction and Circadian Misalignment. Int. J. Environ. Res. Public Health 2016, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e1763. [Google Scholar] [CrossRef] [PubMed]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Gekakis, N.; Staknis, D.; Nguyen, H.B. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef]
- Reick, M.; Garcia, J.A.; Dudley, C.; McKnight, S.L. NPAS2: An analog of clock operative in the mammalian forebrain. Science 2001, 293, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Sato, T.K.; Panda, S.; Miraglia, L.J. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, A. Clock genes in mammalian peripheral tissues. Cell Tissue Res. 2002, 309, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Boivin, D.B. The regulation of central and peripheral circadian clocks in humans. Obes. Rev. 2009, 10, 25–36. [Google Scholar] [CrossRef]
- Zhang, E.E.; Kay, S.A. Clocks not winding down: Unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 2010, 11, 764–776. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Pitts, S.; Perone, E.; Silver, R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R57–R67. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.F.; Weitz, C.J. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc. Natl. Acad. Sci. USA 2009, 106, 6808–6813. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, Y.; Dentin, R. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef]
- Iijima, M.; Yamaguchi, S.; Van der Horst, G.T.; Bonnefont, X.; Okamura, H.; Shibata, S. Altered food-anticipatory activity rhythm in Cryptochrome-deficient mice. Neurosci. Res. 2005, 52, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Organization of the mammalian circadian system. Ciba Found. Symp. 1995, 183, 88–99; discussion 100–106. [Google Scholar] [PubMed]
- Stephan, F.K.; Berkley, K.J.; Moss, R.L. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience 1981, 6, 2625–2641. [Google Scholar] [CrossRef]
- Vrang, N.; Larsen, P.J.; Moller, M.; Mikkelsen, J.D. Topographical organization of the rat suprachiasmatic-paraventricular projection. J. Comp. Neurol. 1995, 353, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Herzog, E.D.; Yamazaki, S. Circadian rhythms in isolated brain regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Guilding, C.; Hughes, A.T.; Brown, T.M.; Namvar, S.; Piggins, H.D. A riot of rhythms: Neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol. Brain 2009, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.W.; Elmquist, J.K. From neuroanatomy to behavior: Central integration of peripheral signals regulating feeding behavior. Nat. Neurosci. 2012, 15, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Akabayashi, A.; Levin, N.; Paez, X.; Alexander, J.T.; Leibowitz, S.F. Hypothalamic neuropeptide Y and its gene expression: Relation to light/dark cycle and circulating corticosterone. Mol. Cell Neurosci. 1994, 5, 210–218. [Google Scholar] [CrossRef]
- Steiner, R.A.; Kabigting, E.; Lent, K.; Clifton, D.K. Diurnal rhythm in proopiomelanocortin mRNA in the arcuate nucleus of the male rat. J. Neuroendocrinol. 1994, 6, 603–608. [Google Scholar] [CrossRef]
- Xu, B.; Kalra, P.S.; Farmerie, W.G.; Kalra, S.P. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: Effects of food restriction. Endocrinology 1999, 140, 2868–2875. [Google Scholar] [CrossRef]
- Li, A.J.; Wiater, M.F.; Oostrom, M.T. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1313–R1326. [Google Scholar] [CrossRef]
- Wiater, M.F.; Mukherjee, S.; Li, A.J. Circadian integration of sleep-wake and feeding requires NPY receptor-expressing neurons in the mediobasal hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1569–R1583. [Google Scholar] [CrossRef] [PubMed]
- Edelsbrunner, M.E.; Painsipp, E.; Herzog, H.; Holzer, P. Evidence from knockout mice for distinct implications of neuropeptide-Y Y2 and Y4 receptors in the circadian control of locomotion, exploration, water and food intake. Neuropeptides 2009, 43, 491–497. [Google Scholar] [CrossRef]
- Richard, C.D.; Tolle, V.; Low, M.J. Meal pattern analysis in neural-specific proopiomelanocortin-deficient mice. Eur. J. Pharmacol. 2011, 660, 131–138. [Google Scholar] [CrossRef]
- Yang, S.; Liu, A.; Weidenhammer, A. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 2009, 150, 2153–2160. [Google Scholar] [CrossRef]
- Bechtold, D.A.; Loudon, A.S. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci. 2013, 36, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S. Chronotherapeutic strategy: Rhythm monitoring, manipulation and disruption. Adv. Drug Deliv. Rev. 2010, 62, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M.; Boulos, Z.; Terman, M. Circadian organization of food intake and meal patterns in the rat. Physiol. Behav. 1981, 27, 33–39. [Google Scholar] [CrossRef]
- Gschossmann, J.M.; Buenger, L.; Adam, B. Diurnal variation of abdominal motor responses to colorectal distension and plasma cortisol levels in rats. Neurogastroenterol. Motil. 2001, 13, 585–589. [Google Scholar] [CrossRef]
- Bodosi, B.; Gardi, J.; Hajdu, I.; Szentirmai, E.; Obal, F., Jr.; Krueger, J.M. Rhythms of ghrelin, leptin, and sleep in rats: Effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1071–R1079. [Google Scholar] [CrossRef]
- Shiiya, T.; Nakazato, M.; Mizuta, M. Plasma Ghrelin Levels in Lean and Obese Humans and the Effect of Glucose on Ghrelin Secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Goo, R.H.; Moore, J.G.; Greenberg, E.; Alazraki, N.P. Circadian variation in gastric emptying of meals in humans. Gastroenterology 1987, 93, 515–518. [Google Scholar] [CrossRef]
- Page, A.J.; Christie, S.; Symonds, E.; Li, H. Circadian regulation of appetite and time restricted feeding. Physiol. Behav. 2020, 220, 112873. [Google Scholar] [CrossRef]
- Steinert, R.E.; Gerspach, A.C.; Gutmann, H.; Asarian, L.; Drewe, J.; Beglinger, C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin. Nutr. 2011, 30, 524–532. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Moghadam, A.A.; Moran, T.H.; Dailey, M.J. Alterations in circadian and meal-induced gut peptide levels in lean and obese rats. Exp. Biol. Med. 2017, 242, 1786–1794. [Google Scholar] [CrossRef]
- Bhutta, H.; Deelman, T.; Ashley, S.; Rhoads, D.; Tavakkoli, A. Disrupted Circadian Rhythmicity of the Intestinal Glucose Transporter SGLT1 in Zucker Diabetic Fatty Rats. Dig. Dis. Sci. 2013, 58, 1537–1545. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Yamazaki, M.; Sukigara, H. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Segers, A.; Desmet, L.; Thijs, T.; Verbeke, K.; Tack, J.; Depoortere, I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol. 2018, e13193. [Google Scholar] [CrossRef]
- Kentish, S.J.; Hatzinikolas, G.; Li, H.; Frisby, C.L.; Wittert, G.A.; Page, A.J. Time-Restricted Feeding Prevents Ablation of Diurnal Rhythms in Gastric Vagal Afferent Mechanosensitivity Observed in High-Fat Diet-Induced Obese Mice. J. Neurosci. 2018, 38, 5088–5095. [Google Scholar] [CrossRef]
- Daly, D.M.; Park, S.J.; Valinsky, W.C.; Beyak, M.J. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J. Physiol. 2011, 589, 2857–2870. [Google Scholar] [CrossRef]
- Kentish, S.J.; O’Donnell, T.A.; Frisby, C.L.; Li, H.; Wittert, G.A.; Page, A.J. Altered gastric vagal mechanosensitivity in diet-induced obesity persists on return to normal chow and is accompanied by increased food intake. Int. J. Obes. 2014, 38, 636–642. [Google Scholar] [CrossRef]
- Tomasi, D.; Wang, G.-J.; Wang, R. Association of Body Mass and Brain Activation during Gastric Distention: Implications for Obesity. PLoS ONE 2009, 4, e6847. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Chen, K.; Salbe, A.D. Persistence of abnormal neural responses to a meal in postobese individuals. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, D.; Suquet, J.P.; Aubert, R.; De Gasquet, P.; Pequignot, E. Metabolism of the mouse made obese by a high-fat diet. Diabete Metab. 1975, 1, 77–85. [Google Scholar]
- Levin, B.E.; Dunn-Meynell, A.A. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R231–R237. [Google Scholar] [CrossRef]
- Ravussin, Y.; Gutman, R.; Diano, S. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1352–R1362. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.B. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef]
- Di Lorenzo, L.; De Pergola, G.; Zocchetti, C. Effect of shift work on body mass index: Results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ogawa, T.; Hitosugi, S. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 2011, 6, e25231. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Dochi, M.; Sakata, K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity 2008, 16, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Agnes, P.T.; Isabella, B.; Jorge, C. Sixth European Working Conditions Survey—Overview Report; Eurofound: Brussels, Belgium, 2016. [Google Scholar]
- Bureau of Labor Statistics. Workers on Flexible and Shift Schedules in 2004 Summary; US Bureau of Labor Statistics: Washington, DC, USA, 2005.
- Australian Bureau of Statistics. Working Time Arrangements, Australia, November 2012; ABS: Canberra, Australia, 2012. [Google Scholar]
- Knutsson, A. Health disorders of shift workers. Occup. Med. 2003, 53, 103–108. [Google Scholar] [CrossRef]
- Vener, K.J.; Szabo, S.; Moore, J.G. The effect of shift work on gastrointestinal (GI) function: A review. Chronobiologia 1989, 16, 421–439. [Google Scholar]
- Pasqua, I.C.; Moreno, C.R. The nutritional status and eating habits of shift workers: A chronobiological approach. Chronobiol. Int. 2004, 21, 949–960. [Google Scholar] [CrossRef]
- Lennernas, M.; Hambraeus, L.; Akerstedt, T. Shift related dietary intake in day and shift workers. Appetite 1995, 25, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Cayanan, E.A.; Eyre, N.A.B.; Lao, V. Is 24-h energy intake greater during night shift compared to non-night shift patterns? A systematic review. Chronobiol. Int. 2019, 36, 1599–1612. [Google Scholar] [CrossRef]
- Shaw, E.; Dorrian, J.; Coates, A.M. Temporal pattern of eating in night shift workers. Chronobiol. Int. 2019, 36, 1613–1625. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Opperhuizen, A.L.; Van Kerkhof, L.W.; Proper, K.I.; Rodenburg, W.; Kalsbeek, A. Rodent models to study the metabolic effects of shiftwork in humans. Front. Pharmacol. 2015, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.L.; Husse, J.; Bode, B. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE 2012, 7, e37150. [Google Scholar] [CrossRef]
- De Oliveira, E.M.; Visniauskas, B.; Sandri, S. Late effects of sleep restriction: Potentiating weight gain and insulin resistance arising from a high-fat diet in mice. Obesity 2015, 23, 391–398. [Google Scholar] [CrossRef]
- Fonken, L.K.; Lieberman, R.A.; Weil, Z.M.; Nelson, R.J. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 2013, 154, 3817–3825. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Aubrecht, T.G.; Jenkins, R.; Nelson, R.J. Dim light at night increases body mass of female mice. Chronobiol. Int. 2015, 32, 557–560. [Google Scholar] [CrossRef]
- Kentish, S.J.; Christie, S.; Vincent, A.; Li, H.; Wittert, G.A.; Page, A.J. Disruption of the light cycle ablates diurnal rhythms in gastric vagal afferent mechanosensitivity. Neurogastroenterol. Motil. 2019, 31, e13711. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Metabolism and circadian rhythms—Implications for obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Sakurai, M.; Ippoushi, K.; Kobori, M. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem. Biophys. Res. Commun. 2015, 465, 556–561. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, A.J. Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms. Nutrients 2021, 13, 844. https://doi.org/10.3390/nu13030844
Page AJ. Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms. Nutrients. 2021; 13(3):844. https://doi.org/10.3390/nu13030844
Chicago/Turabian StylePage, Amanda J. 2021. "Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms" Nutrients 13, no. 3: 844. https://doi.org/10.3390/nu13030844
APA StylePage, A. J. (2021). Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms. Nutrients, 13(3), 844. https://doi.org/10.3390/nu13030844





