Serum Concentration of Antibodies to Mumps, but Not Measles, Rubella, or Varicella, Is Associated with Intake of Dietary Fiber in the NHANES, 1999–2004
Abstract
1. Introduction
2. Materials and Methods
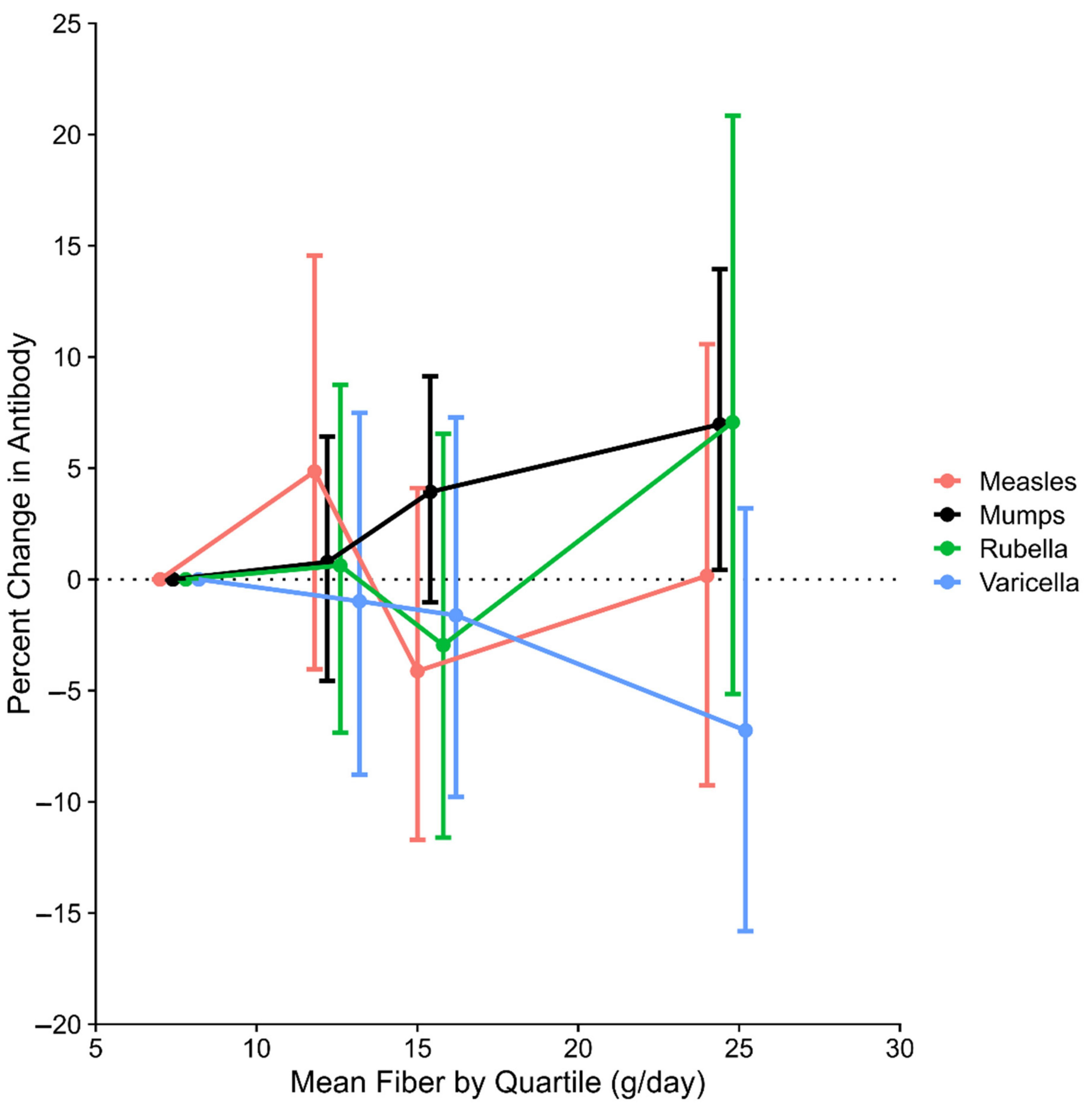
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IQR | interquartile range |
| MET | metabolic equivalents of task |
| NHANES | National Health and Nutrition Examination Survey |
| %∆ | percentage difference |
| SFCA | short-chain fatty acids |
| TDAR | T cell-dependent antibody response |
References
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Santos, J.I. Nutrition, infection, and immunocompetence. Infect. Dis. Clin. N. Am. 1994, 8, 243–267. [Google Scholar] [CrossRef]
- Schley, P.D.; Field, C.J. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 2002, 87 (Suppl. 2), 221–230. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. 29 October 2009. Available online: https://pubmed.ncbi.nlm.nih.gov/19865172/ (accessed on 29 October 2020).
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.; Lee, J.C.; Yau, Y.F.; Ansell, J.; Theis, S.; et al. Developments in understanding and applying prebiotics in research and practice—An ISAPP conference paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-L.; Shih, P.-C.; Liu, S.-J.; Lin, C.-H.; Liu, J.-M.; Lei, W.-T.; Lin, C.-Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: A systematic review and meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef] [PubMed]
- NHANES 2003–2004: Mumps Antibody—Serum (Surplus) Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/SSMUMP_C.htm (accessed on 29 October 2020).
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 1 2013, 56, 1–37. [Google Scholar]
- Anonymous. Laboratory Procedure Manual. Measles, Rubella, and Varicella-Zoster Antibodies in Serum. California State Department of Health Services. 2003–2004. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l19_c_met_mrv.pdf (accessed on 26 February 2021).
- Food Surveys Research Group. USDA ARS. 2018. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/ (accessed on 29 October 2020).
- Phillips, K.M.; Haytowitz, D.B.; Pehrsson, P.R. Implications of two different methods for analyzing total dietary fiber in foods for food composition databases. J. Food Compos. Anal. 2019, 84, 103253. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Edwards, M.K.; Crush, E.; Ikuta, T.; Del Arco, A. Dose-Response Association Between Physical Activity and Cognitive Function in a National Sample of Older Adults. Am. J. Health Promot. 2018, 32, 554–560. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. 4), 1220S–1228S. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Patel, S.Y.; Carbone, J.; Jolles, S. The Expanding Field of Secondary Antibody Deficiency: Causes, Diagnosis, and Management. Front. Immunol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; Available online: http://www.nap.edu/catalog/11537 (accessed on 30 October 2020).
- Hyde, T.B.; Kruszon-Moran, D.; McQuillan, G.M.; Cossen, C.; Forghani, B.; Reef, S.E. Rubella Immunity Levels in the United States Population: Has the Threshold of Viral Elimination Been Reached? Clin. Infect. Dis. 2006, 43, S146–S150. [Google Scholar] [CrossRef]
- Kutty, P.K.; Kruszon-Moran, D.M.; Dayan, G.H.; Alexander, J.P.; Williams, N.J.; Garcia, P.E.; Hickman, C.J.; McQuillan, G.M.; Bellini, W.J. Seroprevalence of Antibody to Mumps Virus in the US Population, 1999–2004. J. Infect. Dis. 2010, 202, 667–674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McQuillan, G.M.; Kruszon-Moran, D.; Hyde, T.B.; Forghani, B.; Bellini, W.; Dayan, G.H. Seroprevalence of Measles Antibody in the US Population, 1999–2004. J. Infect. Dis. 2007, 196, 1459–1464. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Kruszon-Moran, D.; Jumaan, A.; Schmid, D.S.; McQuillan, G.M. Varicella Seroprevalence in the U.S.: Data from the National Health and Nutrition Examination Survey, 1999–2004. Public Health Rep. 2010, 125, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Lebo, E.J.; Kruszon-Moran, D.M.; Marin, M.; Bellini, W.J.; Schmid, S.; Bialek, S.R.; Wallace, G.S.; McLean, H.Q. Seroprevalence of Measles, Mumps, Rubella and Varicella Antibodies in the United States Population, 2009–2010. Open Forum Infect. Dis. 2015, 2, ofv006. [Google Scholar] [CrossRef]
- Antia, A.; Ahmed, H.; Handel, A.; Carlson, N.E.; Amanna, I.J.; Antia, R.; Slifka, M. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018, 16, e2006601. [Google Scholar] [CrossRef]
- Stam, J.; Van Stuijvenberg, M.; Garssen, J.; Knipping, K.; Sauer, P.J.J. A mixture of three prebiotics does not affect vaccine specific antibody responses in healthy term infants in the first year of life. Vaccine 2011, 29, 7766–7772. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.P.; Westerbeek, E.A.M.; Van Der Klis, F.R.M.; Berbers, G.A.M.; Lafeber, H.N.; Van Elburg, R.M. Neutral and Acidic Oligosaccharides Supplementation Does Not Increase the Vaccine Antibody Response in Preterm Infants in a Randomized Clinical Trial. PLoS ONE 2013, 8, e70904. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.P.; Westerbeek, E.A.M.; van der Klis, F.R.M.; Sanders, E.A.M.; Berbers, G.A.M.; van Elburg, R.M. Response on Pneumococcal Vaccine in Preterm Infants After Neutral and Acidic Oligosaccharides Supplementation. Pediatric Infect. Dis. J. 2015, 34, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Van Hoffen, E.; Ruiter, B.; Faber, J.; M’Rabet, L.; Knol, E.; Stahl, B.; Arslanoglu, S.; Moro, G.; Boehm, G.; Garssen, J. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 2009, 64, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Karvetti, R.L.; Knuts, L.R. Validity of the 24-hour dietary recall. J. Am. Diet. Assoc. 1985, 85, 1437–1442. [Google Scholar]
- Slavin, J. Impact of the proposed definition of dietary fiber on nutrient databases. J. Food Compos. Anal. 2003, 16, 287–291. [Google Scholar] [CrossRef]
- Terada, K.; Hagihara, K.; Oishi, T.; Miyata, I.; Akaike, H.; Ogita, S.; Ohno, N.; Ouchi, K. Cellular and humoral immunity after vaccination or natural mumps infection. Pediatric Int. 2017, 59, 885–890. [Google Scholar] [CrossRef]
- De Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.-Y.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Dzierlenga, M.W.; Keast, D.R.; Longnecker, M.P. The concentration of several perfluoroalkyl acids in serum appears to be reduced by dietary fiber. Environ. Int. 2021, 146, 106292. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.I.; Portier, C.; Pait, D.G.; Rosenthal, G.J.; Germolec, D.R.; Corsini, E.; Blaylock, B.L.; Pollock, P.; Kouchi, Y.; Craig, W. Risk Assessment in Immunotoxicology II. Relationships between Immune and Host Resistance Tests. Fundam. Appl. Toxicol. 1993, 21, 71–82. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Abraham, K.; Mielke, H.; Fromme, H.; Völkel, W.; Menzel, J.; Peiser, M.; Zepp, F.; Willich, S.N.; Weikert, C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. [Google Scholar] [CrossRef]
- Stein, C.R.; McGovern, K.J.; Pajak, A.M.; Maglione, P.J.; Wolff, M.S. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatric Res. 2016, 79, 348–357. [Google Scholar] [CrossRef]
- Lin, P.-I.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.-F.; Fleisch, A.F.; Calafat, A.M.; Sanchez-Guerra, M.; Osorio-Yáñez, C.; et al. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: Cross-sectional results from the Diabetes Prevention Program Trial. Environ. Int. 2020, 137, 105217. [Google Scholar] [CrossRef]
| Characteristic | Median (and Quartiles), or Percent (n = 13225) |
|---|---|
| Age | 28 (17, 39) |
| Sex | |
| Female | 48.2 |
| Male | 51.8 |
| Race/Ethnicity | |
| Mexican American | 10.1 |
| Other Hispanic | 5.9 |
| Non-Hispanic White | 66.6 |
| Non-Hispanic Black | 12.1 |
| Other Race | 5.3 |
| Education | |
| <9th grade | 16.6 |
| Grades 9 to 11 | 16.6 |
| High School or GED (includes those in Grade 12) | 21.2 |
| Some College | 26.1 |
| College | 19.5 |
| Income-Poverty Ratio | 2.6 (1.3, 4.5) |
| Survey Year | |
| 1999–2000 | 29.9 |
| 2001–2002 | 35.9 |
| 2003–2004 | 34.2 |
| BMI (kg/m2) | 24.9 (21.0, 29.6) |
| Parity (females, ages 12–49) b | |
| 0 children | 40.7 |
| 1 child | 16.1 |
| 2 or more | 43.2 |
| Pregnant (females, ages 12–49) b | 4.8 |
| Breastfeeding (females, ages 12–49) b | 2.6 |
| Smoking (ages 12–49) c | |
| Never [<100 lifetime cigarettes] | 57.3 |
| Former [not current smoker] | 17.6 |
| Smoker [<1 pack per day] | 14.8 |
| Heavy Smoker [≥1 pack per day] | 10.3 |
| Alcohol Use (ages 20–49) d | |
| Never [<12 lifetime drinks] | 12.0 |
| Former [0 drinks last 12 months] | 2.0 |
| Light Drinker [<1 drink per week] | 46.4 |
| Drinker [<7 drinks per week] | 36.6 |
| Heavy Drinker [≥7 drinks per week] | 3.0 |
| Dietary Intake | |
| Crude Dietary Fiber (g/day) | 13.3 (8.9, 19.4) |
| Energy Adjusted Fiber (g/day) | 13.8 (10.6, 18.3) |
| Energy Adjusted Fiber (g/day)/IUR | 1.8 (1.4, 2.4) |
| Total Energy Intake (kcal/day) | 2169 (1634, 2854) |
| Vitamin C (mg) | 60.6 (27.6, 124.0) |
| Vitamin E (mg) | 6.3 (4.2, 9.4) |
| Carotene (mcg RE) | 784 (335, 2332) |
| Protein (gm) | 77.6 (55.7, 104.8) |
| Selenium (mcg) | 99.1 (70.5, 138.0) |
| Zinc (mg) | 10.9 (7.5, 15.8) |
| Vitamin B6 (mg) | 1.7 (1.1, 2.4) |
| Folate (mcg) | 361.5 (248.0, 517.5) |
| Magnesium (mg) | 252.0 (179.9, 345.0) |
| Copper (mg) | 1.1 (0.8, 1.6) |
| Vitamin A (mcg) | 526.9 (294.0, 868.2) |
| Supplements | |
| Crude Supplement Fiber (g/day) | 0.0 (0.0, 0.0) |
| Vitamin C (mg) | 0.0 (0.0, 49.7) |
| Vitamin E (mg) | 0.0 (0.0, 7.2) |
| Carotene (mg) | 0.0 (0.0, 0.0) |
| Protein (gm) | 0.0 (0.0, 0.0) |
| Selenium (mcg) | 0.0 (0.0, 0.0) |
| Zinc (mg) | 0.0 (0.0, 0.5) |
| Vitamin B6 (mg) | 0.0 (0.0, 0.8) |
| Folate (mcg) | 0.0 (0.0, 66.7) |
| Magnesium (mg) | 0.0 (0.0, 0.0) |
| Copper (mg) | 0.0 (0.0, 0.0) |
| Vitamin A (mcg) | 0.0 (0.0, 197.9) |
| Met-Min/Month (ages 12–49) e | |
| <2000 | 27.9 |
| 2000–3999 | 17.8 |
| 4000–5999 | 11.3 |
| 6000–7999 | 9.0 |
| 8000+ | 33.9 |
| Antibody concentration (untransformed) | |
| Measles | 8.6 (4.2, 14.6) |
| Mumps | 2.6 (1.7, 3.7) f |
| Rubella | 46.8 (22.4, 85.5) |
| Varicella | 13.3 (7.9, 18.4) |
| Characteristic | Median or r (n = 13225) a |
|---|---|
| Age | |
| 6 to < 12 years | 13.7 (11.4, 16.4) |
| 12 to < 20 years | 12.7 (10.2, 16.3) |
| 20–49 years | 14.3 (10.6, 19.2) |
| Sex | |
| Female | 14.2 (11.3, 18.2) |
| Male | 13.4 (10.0, 18.4) |
| Race/Ethnicity | |
| Mexican American | 16.1 (12.3, 21.4) |
| Other Hispanic | 14.1 (11.3, 18.4) |
| Non-Hispanic White | 14.0 (10.6, 18.4) |
| Non-Hispanic Black | 12.2 (9.6, 15.4) |
| Other Race | 12.8 (9.8, 17.3) |
| Education | |
| <9th grade | 13.7 (11.2, 16.9) |
| Grades 9 to 11 | 12.5 (9.4, 16.6) |
| High School or GED (includes those in Grade 12) | 12.8 (9.5, 17.0) |
| Some College | 13.9 (10.6, 18.6) |
| College | 16.7 (12.7, 23.0) |
| Income-Poverty Ratio | |
| 1st Tertile | 13.2 (10.1, 17.2) |
| 2nd Tertile | 13.5 (10.3, 17.6) |
| 3rd Tertile | 14.8 (11.4, 19.9) |
| Survey Year | |
| 1999–2000 | 13.2 (9.9, 18.1) |
| 2001–2002 | 13.9 (10.6, 18.6) |
| 2003–2004 | 14.2 (11.3, 18.1) |
| BMI (kg/m2) | −0.05 |
| Parity (females, ages 12–49) b | |
| 0 children | 14.4 (11.4, 18.7) |
| 1 child | 13.9 (10.9, 18.3) |
| 2 or more | 14.2 (11.2, 18.8) |
| Pregnant (females, ages 12–49) b | |
| No | 14.2 (11.2, 18.5) |
| Yes | 15.0 (11.6, 19.7) |
| Breastfeeding (females, ages 12–49) b | |
| No | 14.2 (11.2, 18.5) |
| Yes | 15.6 (12.1, 21.6) |
| Smoking (ages 12–49) c | |
| Never [<100 lifetime cigarettes] | 14.9 (11.2, 19.7) |
| Former [not current smoker] | 15.0 (11.6, 20.7) |
| Smoker [< 1 pack per day] | 12.0 (9.0, 15.8) |
| Heavy Smoker [≥ 1 pack per day] | 10.8 (8.0, 14.4) |
| Alcohol Use (ages 20–49) d | |
| Never [<12 lifetime drinks] | 15.4 (11.9, 20.8) |
| Former [0 drinks last 12 months] | 15.6 (9.9, 20.0) |
| Light Drinker [<1 drink per week] | 14.2 (10.7, 19.2) |
| Drinker [<7 drinks per week] | 13.9 (9.9, 19.1) |
| Heavy Drinker [≥7 drinks per week] | 13.5 (10.3, 19.8) |
| Dietary Intake | |
| Vitamin C (mg) | 0.22 |
| Vitamin E (mg) | 0.27 |
| Carotene (mcg RE) | 0.26 |
| Protein (gm) | 0.08 |
| Selenium (mcg) | 0.07 |
| Zinc (mg) | 0.15 |
| Vitamin B6 (mg) | 0.29 |
| Folate (mcg) | 0.44 |
| Magnesium (mg) | 0.67 |
| Copper (mg) | 0.36 |
| Vitamin A (mcg) | 0.18 |
| Supplements | |
| Crude Supplement Fiber (g/day) | 0.01 |
| Vitamin C (mg) | 0.01 |
| Vitamin E (mg) | −0.00 |
| Carotene (mg) | 0.04 |
| Protein (gm) | 0.05 |
| Selenium (mcg) | 0.05 |
| Zinc (mg) | 0.07 |
| Vitamin B6 (mg) | 0.04 |
| Folate (mcg) | 0.08 |
| Magnesium (mg) | 0.06 |
| Copper (mg) | 0.07 |
| Vitamin A (mcg) | 0.00 |
| Met-Min/Month (ages 12–49) e | |
| <2000 | 13.6 (10.3, 18.5) |
| 2000–3999 | 15.0 (11.0, 19.3) |
| 4000–5999 | 14.5 (11.4, 19.9) |
| 6000–7999 | 14.3 (10.8, 19.3) |
| 8000+ | 14.4 (10.7, 19.3) |
| Minimally-Adjusted | Full Model | |||
|---|---|---|---|---|
| Antibody Type | %∆ | (95% Cl) | %∆ | (95% Cl) |
| Measles | 2.42 | (−1.59, 6.59) | 0.47 | (−4.34, 5.51) |
| Mumps * | 5.36 | (2.17, 8.64) | 6.34 | (3.10, 9.68) |
| Rubella | 4.04 | (0.60, 7.61) | 0.98 | (−4.19, 6.43) |
| Varicella | −2.98 | (−5.97, 0.11) | −2.64 | (−5.99, 0.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Landingham, C.B.; Keast, D.R.; Longnecker, M.P. Serum Concentration of Antibodies to Mumps, but Not Measles, Rubella, or Varicella, Is Associated with Intake of Dietary Fiber in the NHANES, 1999–2004. Nutrients 2021, 13, 813. https://doi.org/10.3390/nu13030813
Van Landingham CB, Keast DR, Longnecker MP. Serum Concentration of Antibodies to Mumps, but Not Measles, Rubella, or Varicella, Is Associated with Intake of Dietary Fiber in the NHANES, 1999–2004. Nutrients. 2021; 13(3):813. https://doi.org/10.3390/nu13030813
Chicago/Turabian StyleVan Landingham, Cynthia B., Debra R. Keast, and Matthew P. Longnecker. 2021. "Serum Concentration of Antibodies to Mumps, but Not Measles, Rubella, or Varicella, Is Associated with Intake of Dietary Fiber in the NHANES, 1999–2004" Nutrients 13, no. 3: 813. https://doi.org/10.3390/nu13030813
APA StyleVan Landingham, C. B., Keast, D. R., & Longnecker, M. P. (2021). Serum Concentration of Antibodies to Mumps, but Not Measles, Rubella, or Varicella, Is Associated with Intake of Dietary Fiber in the NHANES, 1999–2004. Nutrients, 13(3), 813. https://doi.org/10.3390/nu13030813






