Abstract
Altered circulating levels of free fatty acids (FFAs), namely short chain fatty acids (SCFAs), medium chain fatty acids (MCFAs), and long chain fatty acids (LCFAs), are associated with metabolic, gastrointestinal, and malignant diseases. Hence, we compared the serum FFA profile of patients with celiac disease (CD), adenomatous polyposis (AP), and colorectal cancer (CRC) to healthy controls (HC). We enrolled 44 patients (19 CRC, 9 AP, 16 CD) and 16 HC. We performed a quantitative FFA evaluation with the gas chromatography–mass spectrometry method (GC–MS), and we performed Dirichlet-multinomial regression in order to highlight disease-specific FFA signature. HC showed a different composition of FFAs than CRC, AP, and CD patients. Furthermore, the partial least squares discriminant analysis (PLS-DA) confirmed perfect overlap between the CRC and AP patients and separation of HC from the diseased groups. The Dirichlet-multinomial regression identified only strong positive association between CD and butyric acid. Moreover, CD patients showed significant interactions with age, BMI, and gender. In addition, among patients with the same age and BMI, being male compared to being female implies a decrease of the CD effect on the (log) prevalence of butyric acid in FFA composition. Our data support GC–MS as a suitable method for the concurrent analysis of circulating SCFAs, MCFAs, and LCFAs in different gastrointestinal diseases. Furthermore, and notably, we suggest for the first time that butyric acid could represent a potential biomarker for CD screening.
1. Introduction
Fatty acids are carboxylic acids classified by the length of their aliphatic chain, the presence or absence of double bonds, and the location of double bonds. Short chain fatty acids (SCFAs) have five carbon atoms or fewer, medium chain fatty acids (MCFAs) have from six to 12 carbon atoms, while long chain fatty acids (LCFAs) have 13–20 carbon atoms [1].
Although fatty acids are usually considered a simple energy source, in recent decades they have emerged as fundamental intracellular and extracellular signaling molecules. They: (i) modulate the activation of gene transcription, (ii) regulate the post-transcriptional modification of proteins, and (iii) act as coactivators of enzymes [2,3].
A main source of lipids is plasmatic free fatty acids (FFAs), namely SCFAs, MCFAs, and LCFAs, which are hydrolyzed from adipose tissue stores and carried by circulating albumin to provide energy for tissues during fasting [4].
In particular, SCFAs are the main metabolites produced by the bacterial anerobic fermentation of indigestible polysaccharides and proteins in the large intestine. They are absorbed by colonocytes or transported into the portal circulation to be metabolized by hepatocytes, while the remaining SCFAs enter systemic circulation [5,6].
In general, they contribute to regulate the glucose and cholesterol metabolisms to maintain the intestinal barrier integrity and to modulate the differentiation of T cells [7,8].
The adequate gut microbiota production of SCFAs is critical to maintaining the host’s normal gut physiology and metabolic functions and dietary fiber [9]. Probiotic supplementation [10] can increase the capacity to produce larger quantities of SCFAs, with benefits for the host health.
Differently from SCFAs, MCFAs and LCFAs are generally encountered in the diet, especially from milk and dairy products, and are important regulators of energy metabolism, gene expression, ion channels and pump activities, membrane trafficking, and immune processes [11,12,13].
It is well known that higher FFA concentration causes an increase of oxidative stress and exerts pro-inflammatory effects. In particular, altered levels of FFAs have been associated with Crohn’s disease, autoimmune disorders, and various types of cancer [14,15,16,17].
Hence, as FFAs play important roles for the host, and their imbalance is linked to multiple metabolic, gastrointestinal, and malignant diseases, investigation into different biological samples is becoming noteworthy with the aim of researching new metabolic biomarkers [18,19,20]. However, it is important to underline that altered lipid profiles can be caused by dysregulated bacterial fermentation, an unbalanced diet, and de novo synthesis in cancer tissues [21].
Finally, concerning the serum profiles of FFAs, a quantitative determination with gas chromatography–mass spectrometry (GC–MS) was recently proposed, with advantages of high sensitivity, peak resolution, and reproducibility.
Starting from these premises and using Dirichlet-multinomial regression, in this explorative study we compared the serum FFA profile of patients with different gastrointestinal diseases, including celiac disease (CD), adenomatous polyposis (AP), and colorectal cancer (CRC), to healthy controls (HC) in order to highlight disease-specific FFA signature.
2. Materials and Methods
2.1. Study Design and Patient Enrolment
We used a dedicated previously described study protocol [22]. Briefly, 44 patients affected by different gut diseases (19 CRC, 9 AP, 16 CD) and 16 healthy controls (HC) were enrolled in different studies between January 2016 and February 2019 at the Azienda Ospedaliera Universitaria Careggi, Italy.
At enrollment, people with CRC, AP, and CD showed a median BMI < 25, all patients were on omnivorous diets, and none of them reported special dietary habits or dietary restrictions.
All CRC patients were affected by nonmetastatic colon cancer, and in particular 16 were at stage I-II, and three were at stage III.
Table 1 reports clinical characteristics of patients.

Table 1.
Features of enrolled patients. BMI = body mass index; IQR = interquartile range. HC = healthy controls; CD = celiac disease; AP = adenomatous polyposis; CRC = colorectal cancer.
Each patient’s whole blood was collected in a clinical tube containing 3.2% sodium citrate as anticoagulant and then centrifuged at 1500× g for 10 min, then the serum was collected and stored at −20 °C until analysis.
The study received approval from the local ethics committee—Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana, Sezione AREA VASTA CENTRO Institutional Review Board (CE: 11166_spe, 11/09/2018 and CE: 10443_oss, 14/02/2017)—and informed written consent was obtained from each participant.
2.2. FFAs Determination by GC–MS Analysis
The FFA analysis was performed using an Agilent GC–MS system composed of a 5971 single quadrupole mass spectrometer, a 5890 gas-chromatograph, and a 7673 autosampler. The chemicals, GC–MS conditions, and calibration parameters were reported in Section 1 of the Supplementary Material.
2.3. Sample Preparation
Just before the analysis, each sample was thawed. The FFAs were extracted as follows: an aliquot of 300 µL of plasma sample was added to 10 μL of ISTD mixture, 100 μL of tert-butyl methyl ether, and 20 µL of 6 M HCl + 0.5 M NaCl solution in 0.5 mL centrifuge tube. Afterwards, each tube was stirred in a vortex for 2 min, centrifuged at 10,000 rpm for 5 min, and finally the solvent layer was transferred in a vial with microvolume insert and analyzed (see Section 2 of the Supplementary Material).
2.4. Statistical Analysis
Statistical analysis of the FFA percentages was performed in R (R Core Team, version 3.5.3, Wien, Austria), and all the graphs were plotted with ggplot2 (R Core Team, version 3.1.1, Wien, Austria). Pairwise comparisons of FFA composition between patient groups were assessed using the Wilcoxon rank-sum test.
In order to highlight the separation between groups taking into account the diverse FFA percentage compositions, partial least squares discriminant analysis (PLS-DA) was performed with R package “DiscrMiner” (R Core Team, version 0.1-29, Wien, Austria).
p-values of less than 0.05 were considered statically significant, no multiplicity correction was applied, and findings were interpreted as hypothesis generating.
A Bayesian Dirichlet-multinomial regression model was implemented to explore the associations between clinical variables and serum FFA composition. Relevant associations were selected using our Bayesian variable selection (BVS) method [23]. This method detects both main effects and interactions between explanatory variables. This means that if the association of one clinical variable with serum FFA composition depends on the state of another clinical variable, the model effectively takes into account these relationships. The method’s output is a list of posterior probability of inclusion (PPI) and the posterior mean of the non-zero regression coefficients. PPI is the probability, between 0 and 1, that a given association is non-zero, accounting for the effect of all other clinical variables. The posterior mean is an estimate of the effect size of a given association.
3. Results
3.1. Descriptive Analysis of Serum FFA Distributions
Explorative descriptive analysis was firstly performed on the marginal distributions of each serum FFA percentage (Table 2).

Table 2.
Representation of the median (Q1–Q3) of each FFA percentage in CD, CRC, AP patients and healthy controls. FFA = free fatty acid; HC = healthy controls; CD = celiac disease; AP = adenomatous polyposis; CRC = colorectal cancer.
As reported in Figure 1, healthy controls showed an evidently different composition of serum FFAs than CRC, AP, and CD patients, with a high percentage of acetic acid and a reduction of other FFA levels such as isobutyric, isovaleric, 2-methylbutyric, heptanoic, dodecanoic, tetradecanoic, hexadecanoic, and octadecanoic acids.
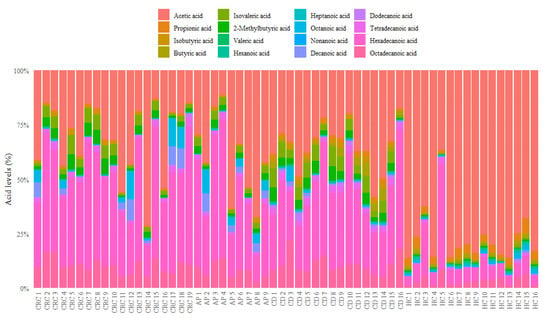
Figure 1.
Bar plot of relative abundances of each serum FFA of CRC, AP, CD patients and healthy controls. FFA = free fatty acid; HC = healthy controls; CD = celiac disease; AP = adenomatous polyposis; CRC = colorectal cancer.
In particular, the quantitative analysis of MCFAs was conducted taking into account the isovaleric acid ISTD, while the LCFA quantification was performed with a semiquantitative analysis using the nonanoic acid as reference. It is important to point out that abundance of valeric acid was often below the minimum detectable value; although these values do not have an impact on other abundances, all statistics and tests concerning valeric acid need to be carefully interpreted.
Figure 2 reports in detail each FFA level for CRC, CD, AP patients and HC, while the p-values of the pairwise comparisons conducted for FFA percentages are shown in Table 3.

Figure 2.
Boxplots representing each FFA percentage in CD, CRC, AP patients and HC.

Table 3.
p-values of the intergroup comparisons assessed with Wilcoxon tests conducted on FFA percentages. p-values less than 0.05 were considered statistically significant. FFA = free fatty acid; HC = healthy controls; CD = celiac disease; AP = adenomatous polyposis; CRC = colorectal cancer.
In detail, compared to CD patients, HC showed a significantly higher quantity of acetic, propionic, valeric, octanoic, and nonanoic acids and a significantly lower percentage of butyric, 2-methylbutyric, isobutyric, isovaleric, hexanoic, and decanoic acids and LCFAs.
CRC patients displayed a significantly lower percentage of acetic, propionic, butyric, valeric, and nonanoic acids and significantly higher percentages of 2-methylbutyric, isobutyric, isovaleric, hexanoic, and heptanoic acids and LCFAs compared to HC.
Compared to AP patients, HC displayed a significantly higher percentage of acetic, propionic, butyric, valeric, heptanoic, and nonanoic acids and a significant reduction of butyric, 2-methylbutyric, isovaleric, hexanoic, tetradecanoic, hexadecanoic, and octadecanoic acid abundances.
In addition, compared to CRC patients, CD patients showed significantly higher percentages of propionic, butyric, valeric, heptanoic nonanoic, dodecanoic, and tetradecanoic acids; however, they reported significantly reduced levels of isobutyric and isovaleric acids.
Otherwise, CD patients showed significantly higher percentages of propionic, isobutyric, butyric, valeric, heptanoic, nonanoic, dodecanoic, and tetradecanoic acids compared to AP patients, while lower levels of isovaleric and hexanoic acids are noted.
To conclude, CRC patients displayed a high percentage of only hexanoic acid in comparison to AP patients.
Furthermore, to characterize a state-specific FFA profile, we performed PLS-DA on the FFA percentage matrix. The classifier confirmed a perfect overlap between the CRC and AP patients and a separation of HC from the diseased groups (Figure 3).
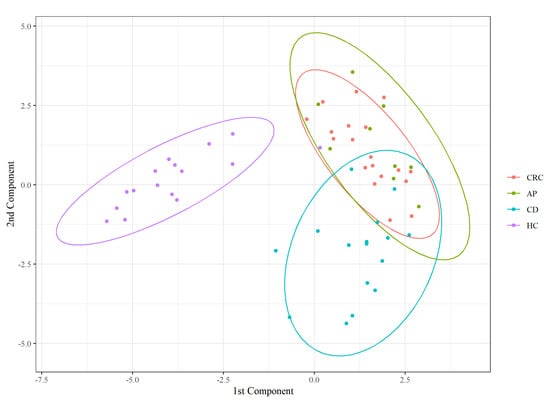
Figure 3.
Partial least squares discriminant analysis (PLS − DA) score plot.
As described in Table 4, AP patients are generally classified as CRC (8/9), most HC are correctly classified (15/16), and none of the misclassified subjects were assigned to this group. Most classification errors involved the CD state. Indeed, one AP and two CRC subjects were classified as CD, while two CD were assigned to CRC status. This suggests that CD subjects have an FFA composition closer to unhealthy subjects than controls.

Table 4.
Classification results of the PLS-DA model. PLS-DA = partial least squares discriminant analysis; HC = healthy controls; CD = celiac disease; AP = adenomatous polyposis; CRC = colorectal cancer.
3.2. Dirichlet-Multinomial Regression
Dirichlet-multinomial regression was performed to identify variables, and their interactions, that have an effect on the serum FFA composition.
The following clinical covariates were included in the analysis: age, BMI, gender, and group. Group is a categorical variable, and its categories are CD, AP, and CRC. The baseline category for gender is female, and in order to highlight any differences between inflammatory intestinal diseases, the baseline category for group is AP.
This approach identified a positive association between CD and butyric acid; this association is of a large magnitude (the posterior mean is equal to 3.8548), and it is strongly supported by the data (PPI = 1.0). All other associations were not included as they were not supported by the data. All other PPIs were less than 0.1. Among patients of the same age with the same BMI and same gender, those with CD, compared to those with AP, have on average a stronger prevalence of butyric acid in their FFA composition.
We also evaluated possible interaction effects. Results are reported in Table 5: CD shows significant interactions with age (PPI = 0.6676), BMI (PPI = 0.8411), and gender (PPI = 0.7380).

Table 5.
Interaction effects evaluated with Dirichlet-multinomial regression between butyric acid, age, BMI, gender, CRC, and CD variables. Baseline category for gender is female and baseline category for state is AP. BMI = body mass index; HC = healthy controls; CD = celiac disease; AP = adenomatous polyposis; CRC = colorectal cancer.
The magnitude of these interactions is moderate, and it should be interpreted as “deviation” from the CD–butyric acid effect. That means, e.g., that on average among patients with the same age and same gender, every additional BMI point is associated with a 0.1161 increase of the CD effect on the (log) prevalence of butyric acid in FFA composition. Further interesting information given by the interaction is that on average among patients with the same age and same BMI, being male compared to being female implies a 0.2278 decrease of the effect of CD on the (log) prevalence of butyric acid in FFA composition. The 3D plots in Figure 4 illustrate the prevalence of butyric acid as a function of age and BMI in a female subject affected or not affected by CD.
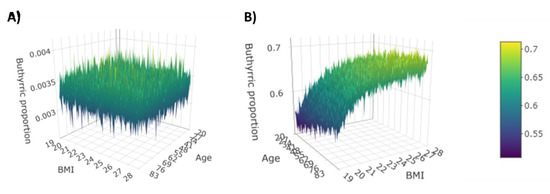
Figure 4.
Prevalence of butyric acid for female without CD (A) and with CD (B).
4. Discussion
It is well documented that circulating FFAs act as signaling molecules, are fundamental components of cellular structures, and are important sources of energy [24], and many studies demonstrated the association between altered FFA levels and different pathological conditions, such as inflammatory and cardiovascular diseases, neurological disorders, and cancer [25,26,27,28].
Thus, the quantitative and qualitative analysis of FFAs in various biological specimens, like stool, urine, saliva, and blood, has recently attracted attention in efforts to identify new potential biomarkers [29,30,31,32].
In recent years, different analytical technologies have been used to detect FFAs, including gas chromatography–mass spectrometry, gas chromatography with flame ionization detection (GC–FID), and liquid chromatography–mass spectrometry (LC-MS) [33,34,35]. However, GC–MS is currently the most frequently used method for FFA analysis because it provides higher selectivity, specificity, and accuracy compared to other detection methods [36].
In the present study, we therefore used for the first time a GC–MS method to perform a simultaneously qualitative and quantitative analysis of SCFAs, MCFAs, and LCFAs in serum samples of healthy controls and patients with different intestinal diseases, namely adenomatous polyposis, colorectal cancer, and celiac disease.
First, the PLS-DA model used for the FFA percentage matrix showed a clear separation between HC and the other three disease groups, a perfect overlap between CRC and AP patients, and a partial separation between CD patients and the two coincident groups of CRC and AP patients.
Recently, using the same GC–MS system, we analyzed the fecal SCFA profile of the same patients and found a definite overlap between HC and CD patients, while CRC and AP patients displayed a divergence from the other two classes and did not completely coincide [22].
So, taking into account only the SCFA abundances, we did not find a relationship between circulating and fecal concentration. In agreement with our results, Muller et al. reported that fecal acetic acid and butyric acid were not related to their respective plasmatic concentrations, and only propionic acid seems to be reflective of its respective fecal concentration [37].
Nevertheless, both CRC and AP patients showed similar profiles of serum FFAs and, as we previously demonstrated [22], of fecal SCFAs, so these results strengthen the established evidence that adenomas can evolve into cancers following the adenoma–carcinoma sequence.
Moreover, the healthy controls displayed different FFA profiles compared to AP, CRC, and CD patients. In particular, CRC and AP patients showed lower levels of SCFAs compared to HC, except for 2-methylbutyric acid, isobutyric acid, and isovaleric acid, which were more abundant in HC.
In accordance with our results, Yusuf et al. reported that CRC patients had lower plasma levels of acetic, propionic, and butyric acids than healthy subjects; on the contrary, Amiot et al. reported that acetate, propionate, and butyrate were increased in fecal samples of CRC patients [38,39].
SCFA-producing bacteria abundance is closely negatively related to the degree of malignancy, and it has recently emerged that the manipulation of intestinal SCFA levels could be a preventive/therapeutic strategy for CRC because of their anti-inflammatory and anti-tumorigenesis properties [40,41].
MCFAs are potent agonists of peroxisome proliferator-activated receptors and are involved in cell death and survival regulation [42,43]. CRC and AP patients showed elevated percentages of hexanoic, heptanoic, octanoic, decanoic, and dodecanoic acids compared to HC, while nonanoic acid was higher in HC.
However, contrary to a study conducted on 117 plasma samples of CRC patients (at different tumor stages) [44], which reported higher levels of hexanoic acid in high-grade dysplasia adenoma patients compared to CRC patients, we found that AP patients showed lower levels of hexanoic acid than people with CRC. These different findings could be explained by the fact that our enrolled AP patients showed low-grade dysplasia.
In according to our results, Crotti et al. found that both CRC and high-grade dysplasia adenoma patients showed significantly higher levels of octanoic and dodecanoic acids. Moreover, they also reported higher levels of decanoic acid and suggest its potential role as a specific CRC biomarker [44].
In addition, Iemoto et al. documented that the serum level of octanoic acid was significantly associated with disease progression, so it may serve as a useful predictor for CRC prognosis [45].
Compared to HC, CRC and AP patients showed higher abundances of LCFAs. Similar to MCFAs, LCFAs play key roles in cell homeostasis and the maintenance of cellular integrity and act as activators of peroxisome proliferator-activated receptor [46].
Different studies reported that an increased concentration of LCFAs and very long fatty acids was associated with an increased CRC risk, for example, linoleic acid and palmitic acid enhance colon carcinogenesis and metastasis [47,48,49,50].
Compared to HC, CD patients showed lower levels of acetic, propionic, and valeric acids but higher percentages of other SCFAs (butyric, 2-methylbutyric, isobutyric, and isovaleric acids) and MCFAs and LCFAs. On the contrary, Jakobsdottir et al. found that both CD patients and healthy subjects showed similar levels of serum SCFAs, while many papers reported that fecal SCFAs could be a signature for CD [51,52,53].
Since fecal SCFA production is related to the composition of gut microbiota and the availability of fermentable substrates, is well documented that CD patients with a reduction of nutrient absorption, caused by villous atrophy, show lower abundances of fecal SCFAs [54]. Instead, MCFAs have been identified as discriminatory metabolites for intestinal bowel disease (IBD), showing significantly decreased results in IBD patients compared to HC. However, in accordance with our data, Solakivi et al. found that CD patients displayed higher levels of serum LCFAs than HC [55,56].
We used a Dirichlet-multinomial regression model with the aim of evaluating which variables and their interactions had a significant effect on the serum FFA profiles.
Interestingly, we found a strong correlation between CD and butyric acid, and we reported interaction effects between the age, BMI, and gender variables and CD.
Butyric acid, one of the most abundant SCFAs in the human colon, is quickly consumed by the intestinal epithelium and used by colonocites primarily as an energy source.
Butyric acid is also an essential regulator of intestinal homeostasis and notably is involved in the processes of anti-inflammation and immunomodulation, enhancing the naïve T-cell polarization to Tregs or Th17 and Th1 effector cells [8,57].
Although systemic butyrate appears to limit the antitumor effect of the anti-CTLA-4 blockade [58], the importance of butyric acid, in particular for human colon health, has been documented in many studies conducted on patients with inflammatory gut diseases. For example, butyrate has been hypothesized to play a key role in reducing hypersensitivity to intestinal receptors, so its supplementation seems to be a promising therapy for irritable bowel syndrome [59].
Regarding CD, it is well established that besides environmental and genetic factors, an imbalance of the microbiota composition is related to its onset and consequent altered intestinal metabolic profile, including SCFA production [52,60]. Indeed, some authors reported that fecal SCFA amounts were higher in patients with untreated and treated CD than healthy adults [53,61]. Therefore, due to the altered gut homeostasis, microbial metabolites such as butyric acid could easily enters the systemic circulation of CD patients.
In addition, we found that only in female patients affected by CD did the prevalence of butyric acid rise with increasing BMI. While the higher CD prevalence in female compared to male individuals is well established, contrasting evidence has been found regarding the relationship between CD and BMI [62]. Many studies found a positive correlation between BMI and serum SCFAs, particularly propionic, butyric, and isovaleric acid in obese patients compared to lean individuals [63,64].
However, Males affected by CD are more commonly overweight than females [65], and CD females have significantly lower mean BMI than the general population [66].
Moreover, sex differences between males and females are responsible for a dissimilar flux of fatty acids, but in response to obesity, both men and women show increased fatty acid release into the bloodstream [67]. However, a different study reported contrasting data, documenting higher serum FFAs in women with respect to men with obesity [68].
Inflammation processes associated with both obesity and CD may lead to loss of intestinal integrity, known as “leaky gut syndrome”, which is responsible for an increased permeability of the intestinal mucosa that could allow microorganisms, small molecules, and bacterial metabolites like SCFAs to enter the bloodstream [69,70,71]. Finally, our different findings between males and females could be supported by a “hormonal hypothesis” because female reduced fertility, delayed menarche, amenorrhea, and early menopause often represent the initial clinical features that ultimately result in a CD diagnosis [72].
Thus, we propose that butyric acid could represent a potential biomarker for screening of CD, and more studies will be necessary in order to evaluate the serum levels of butyric acid in other intestinal pathologies. The serum FFA profile could potentially be used to discriminate CD patients from potential CD patients.
5. Conclusions
Despite our findings being exploratory for the limited sample size, we propose a GC–MS method for the concurrent analysis of SCFAs, MCFAs, and LCFAs in different gastrointestinal diseases. For the first time, we highlighted the existence of a serum SCFA signature in HC and in patients with CRC and AP, while the CD patients did not show a profile completely distinguishable from HC and CRC patients. However, we reported a strong interaction between butyric acid and CD. Of course, a better investigation of the circulating butyric acid in other intestinal diseases such as IBD is needed. Nevertheless, our results suggest that butyric acid could be a potential (and easy to assess) biomarker for celiac disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/3/742/s1, Table S1. Retention times (Rt) and ionic signals used for quali-quantitation of FFAs and relative ISTD used.
Author Contributions
Conceptualization, A.S.C., F.C.S., E.R., G.B., A.A.; Formal analysis, S.B., G.B., M.P. (Marco Pallecchi), G.P., M.M., M.P. (Matteo Pedone); Funding acquisition, A.S.C., A.A.; Investigation, S.B., G.B., M.P. (Marco Pallecchi), G.P., M.M, M.P. (Matteo Pedone); Resources, G.N., E.N., F.R. (Federica Ricci), D.R., F.R. (Francesca Romano); Supervision, A.A., G.B., F.C.S.; Writing—original draft, S.B., G.B., A.A.; Writing—review & editing, A.A., A.T., A.S.C., G.B., F.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research has been funded by Foundation “Ente Cassa di Risparmio di Fi renze”, Italian Society for Celiac Disease and Foundation for Celicac Disease, No. 007_FC_2016 and with a grant from the regional contribution of “The Programma Attuativo Regionale (Toscana)” funded by FAS (now FSC)—MICpROBIMM grant number 4042.16092014.066000029.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana, Sezione AREA VASTA CENTRO Institutional Review Board (CE: 11166_spe, 11/09/2018 and CE: 10443_oss, 14/02/2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shores, D.R.; Binion, D.G.; Freeman, B.A.; Baker, P.R. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2192–2204. [Google Scholar] [CrossRef]
- Papackova, Z.; Cahova, M. Fatty acid signaling: The new function of intracellular lipases. Int. J. Mol. Sci. 2015, 16, 3831–3855. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Sieber, J.; Jehle, A.W. Free Fatty acids and their metabolism affect function and survival of podocytes. Front. Endocrinol. (Lausanne) 2014, 5, 186. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.; Hanekamp, D.; Veldhoen, M.; et al. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells to Induce Mucosal Tolerogenic Dendritic Cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef]
- Kaur, H.; Golovko, S.; Golovko, M.Y.; Singh, S.; Darland, D.C.; Combs, C.K. Effects of Probiotic Supplementation on Short Chain Fatty Acids in the AppNL-G-F Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2020, 76, 1083–1102. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz, M.L.; Ma’ayan, A. GPR84: An immune response dial? Nat. Rev. Drug Discov. 2020, 19, 374. [Google Scholar] [CrossRef] [PubMed]
- Pinkosky, S.L.; Scott, J.W.; Desjardins, E.M.; Smith, B.K.; Day, E.A.; Ford, R.J.; Langendorf, C.G.; Ling, N.X.Y.; Nero, T.L.; Loh, K.; et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat. Metab. 2020, 2, 873–881. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Adams, D.W.; Motley, A.K.; Peyton, S.C.; Ferguson, S.L.; Horst, S.N.; Williams, C.S.; Beaulieu, D.B.; Schwartz, D.A.; et al. Serum Polyunsaturated Fatty Acids Correlate with Serum Cytokines and Clinical Disease Activity in Crohn’s Disease. Sci. Rep. 2019, 9, 2882. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragoulakis, V.; Sarandi, E.; Docea, A.O.; Papakonstaninou, E.; Tsilimidos, G.; Anamaterou, C.; Fragkiadaki, P.; Aschner, M.; Tsatsakis, A.; et al. Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases. Front. Mol. BioSci. 2019, 6, 120. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Rydén, M. Fatty Acids, Obesity and Insulin Resistance. Obes. Facts 2015, 8, 147–155. [Google Scholar] [CrossRef]
- Tian, Z.; Zhuang, X.; Luo, M.; Yin, W.; Xiong, L. The propionic acid and butyric acid in serum but not in feces are increased in patients with diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2020, 20, 73. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, Y.; Zhang, T.; He, J.; Luo, X.; Bian, X.; Wu, J.; Zou, C.; Wang, Y.; Fu, L. Identifying potential serum biomarkers of breast cancer through targeted free fatty acid profiles screening based on a GC-MS platform. Biomed. Chromatogr. 2020, 34, e4922. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Benson, J.; Ma, K.N.; Schaffer, D.; Potter, J.D. Trans-fatty acids and colon cancer. Nutr. Cancer 2001, 39, 170. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef]
- Pedone, M.; Stingo, F.C. Subject-Specific Bayesian Hierarchical Model for Compositional Data Analysis. In Book of Short Papers SIS 2020; Pearson Italia: Milano, Italy, 2020. [Google Scholar]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef]
- Djoussé, L.; Benkeser, D.; Arnold, A.; Kizer, J.R.; Zieman, S.J.; Lemaitre, R.N.; Tracy, R.P.; Gottdiener, J.S.; Mozaffarian, D.; Siscovick, D.S.; et al. Plasma free fatty acids and risk of heart failure: The Cardiovascular Health Study. Circ. Heart Fail. 2013, 6, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; He, J.; Lv, J.; Pan, R.; Lv, T. A high serum-free fatty acid level is associated with cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 705–710. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Ukolov, A.I.; Orlova, T.I.; Savel’Eva, E.I.; Radilov, A.S. Chromatographic–mass spectrometric determination of free fatty acids in blood plasma and urine using extractive alkylation. J. Anal. Chem. 2015, 70, 1123–1130. [Google Scholar] [CrossRef]
- Neyraud, E.; Cabaret, S.; Brignot, H.; Chabanet, C.; Labouré, H.; Guichard, E.; Berdeaux, O. The basal free fatty acid concentration in human saliva is related to salivary lipolytic activity. Sci. Rep. 2017, 7, 5969. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.F.; Hermansson, M.; Liebisch, G.; Hodson, L.; Ejsing, C.S. Total Fatty Acid Analysis of Human Blood Samples in One Minute by High-Resolution Mass Spectrometry. Biomolecules 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Serafim, V.; Tiugan, D.-A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC–MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, N.S.; Dias, D.A. A Robust GC-MS Method for the Quantitation of Fatty Acids in Biological Systems. Adv. Struct. Saf. Stud. 2013, 1055, 39–56. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Kuo, C.-H. Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; Van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Yusuf, F.; Adewiah, S.; Fatchiyah, F. The Level Short Chain Fatty Acids and HSP 70 in Colorectal Cancer and Non-Colorectal Cancer. Acta Inform. Med. 2018, 26, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Dona, A.C.; Wijeyesekera, A.; Tournigand, C.; Baumgaertner, I.; Lebaleur, Y.; Sobhani, I.; Holmes, E. 1H NMR Spectroscopy of Fecal Extracts Enables Detection of Advanced Colorectal Neoplasia. J. Proteome Res. 2015, 14, 3871–3881. [Google Scholar] [CrossRef]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.R.; Barreto, J.; Pereira, H.; Chaves, S.R.; Rodrigues, L.R.; Casal, M.; Côrte-Real, M.; et al. The Role of Diet Related Short-Chain Fatty Acids in Colorectal Cancer Metabolism and Survival: Prevention and Therapeutic Implications. Curr. Med. Chem. 2020, 27, 4087–4108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Yu, E.; Wang, N.; Cai, Q.; Shuai, Q.; Yan, F.; Jiang, L.; Wang, H.; Liu, J.; et al. Changes in gut microbiota and plasma inflammatory factors across the stages of colorectal tumorigenesis: A case-control study. BMC Microbiol. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef]
- Hara, T.; Kimura, I.; Inoue, D.; Ichimura, A.; Hirasawa, A. Free Fatty Acid Receptors and Their Role in Regulation of Energy Metabolism. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 77–116. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Agnoletto, E.; Cancemi, G.; Di Marco, V.; Traldi, P.; Pucciarelli, S.; Nitti, D.; Agostini, M. Altered plasma levels of decanoic acid in colorectal cancer as a new diagnostic biomarker. Anal. Bioanal. Chem. 2016, 408, 6321–6328. [Google Scholar] [CrossRef] [PubMed]
- Iemoto, T.; Nishiumi, S.; Kobayashi, T.; Fujigaki, S.; Hamaguchi, T.; Kato, K.; Shoji, H.; Matsumura, Y.; Honda, K.; Yoshida, M. Serum level of octanoic acid predicts the efficacy of chemotherapy for colorectal cancer. Oncol. Lett. 2018, 17, 831–842. [Google Scholar] [CrossRef]
- Vockley, J. Long-chain fatty acid oxidation disorders and current management strategies. Am. J. Manag. Care 2020, 26, S147–S154. [Google Scholar] [PubMed]
- Cottet, V.; Vaysse, C.; Scherrer, M.-L.; Ortega-Deballon, P.; Lakkis, Z.; Delhorme, J.-B.; Deguelte-Lardière, S.; Combe, N.; Bonithon-Kopp, C. Fatty acid composition of adipose tissue and colorectal cancer: A case-control study. Am. J. Clin. Nutr. 2014, 101, 192–201. [Google Scholar] [CrossRef]
- Shen, S.; Yang, L.; Li, L.; Bai, Y.; Cai, C.; Liu, H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B 2017, 41–48. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Huang, C.; Zhang, W.; Cai, J.; Huang, M.; Gong, R.-H.; Chen, M.; Ho, A.H.M.; Su, T.; et al. High-fat diet feeding and palmitic acid increase CRC growth in β2AR-dependent manner. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Shimomoto, T.; Luo, Y.; Ohmori, H.; Chihara, Y.; Fujii, K.; Sasahira, T.; Denda, A.; Kuniyasu, H. Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J. Gastroenterol. 2012, 47, 1073–1083. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Bjerregaard, J.H.; Skovbjerg, H.; Nyman, M. Fasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: No difference compared with controls, but between genders. Scand. J. Gastroenterol. 2013, 48, 696–701. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef]
- Nistal, E.; Caminero, A.; Vivas, S.; Ruiz de Morales, J.M.; Sáenz de Miera, L.E.; Rodríguez-Aparicio, L.B.; Casqueiro, J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Tjellström, B.; Högberg, L.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.-E.; Norin, E.; Sundqvist, T.; Midtvedt, T. Faecal short-chain fatty acid pattern in childhood coeliac disease is normalised after more than one year’s gluten-free diet. Microb. Ecol. Heal. Dis. 2013, 24, 10. [Google Scholar] [CrossRef]
- De Preter, V.; Machiels, K.; Joossens, M.; Arijs, I.; Matthys, C.; Vermeire, S.; Rutgeerts, P.; Verbeke, K. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 2015, 64, 447–458. [Google Scholar] [CrossRef]
- Solakivi, T.; Kaukinen, K.; Kunnas, T.; Lehtimäki, T.; Mäki, M.; Nikkari, S.T. Serum fatty acid profile in celiac disease patients before and after a gluten-free diet. Scand. J. Gastroenterol. 2009, 44, 826–830. [Google Scholar] [CrossRef]
- Zeng, H.; Taussig, D.P.; Cheng, W.-H.; Johnson, L.K.; Hakkak, R. Butyrate Inhibits Cancerous HCT116 Colon Cell Proliferation but to a Lesser Extent in Noncancerous NCM460 Colon Cells. Nutrients. 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.-M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Załęski, A.; Banaszkiewicz, A.; Walkowiak, J. Butyric acid in irritable bowel syndrome. Gastroenterol. Rev. 2013, 8, 350–353. [Google Scholar] [CrossRef]
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrition 2015, 7, 6900–6923. [Google Scholar] [CrossRef]
- Caminero, A.; Nistal, E.; Herrán, A.R.; Pérez-Andrés, J.; Ferrero, M.A.; Ayala, L.V.; Vivas, S.; De Morales, J.M.G.R.; Albillos, S.M.; Casqueiro, F.J. Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. Br. J. Nutr. 2015, 114, 1157–1167. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef]
- Barengolts, E.; Green, S.J.; Chlipala, G.E.; Layden, B.T.; Eisenberg, Y.; Priyadarshini, M.; Dugas, L.R. Predictors of Obesity among Gut Microbiota Biomarkers in African American Men with and without Diabetes. Microorganisms 2019, 7, 320. [Google Scholar] [CrossRef]
- Irving, B.A.; Wood, G.C.; Bennotti, P.N.; Babu, E.; Deshpande, A.; Lent, M.R.; Petrick, A.; Gabrielsen, J.; Strodel, W.; Gerhard, G.S. Nutrient Transporter Expression in the Jejunum in Relation to Body Mass Index in Patients Undergoing Bariatric Surgery. Nutrients 2016, 8, 683. [Google Scholar] [CrossRef]
- Van Der Pals, M.; Myléus, A.; Norström, F.; Hammarroth, S.; Högberg, L.; Rosén, A.; Ivarsson, A.; Carlsson, A. Body mass index is not a reliable tool in predicting celiac disease in children. BMC Pediatr. 2014, 14, 165. [Google Scholar] [CrossRef]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H.R. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Magkos, F.; Fabbrini, E.; Mohammed, B.; Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009, 17, 1872–1877. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty Acids, Obesity, and Insulin Resistance: Time for a Reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef]
- Niccolai, E.; Boem, F.; Russo, E.; Amedei, A. The Gut⁻Brain Axis in the Neuropsychological Disease Model of Obesity: A Classical Movie Revised by the Emerging Director “Microbiome”. Nutrients 2019, 11, 156. [Google Scholar] [CrossRef]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Freeman, H.J. Reproductive changes associated with celiac disease. World J. Gastroenterol. 2010, 16, 5810–5814. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).