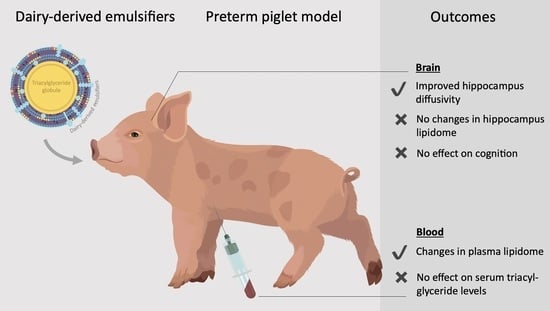
Dairy-Derived Emulsifiers in Infant Formula Show Marginal Effects on the Plasma Lipid Profile and Brain Structure in Preterm Piglets Relative to Soy Lecithin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Nutrition
2.3. Animal Housing and Treatment
2.4. In Vitro Lipolysis and Serum Triacyl-Glyceride Measurements
2.5. Magnetic Resonance Imaging
2.6. Mass Spectrometry-Based Lipidomics
2.7. Acquisition of Basic Motor Skills, Home Cage Activity and Short-Term Memory
2.8. Statistics
3. Results
3.1. Clinical Outcomes, Growth and Organ Weights
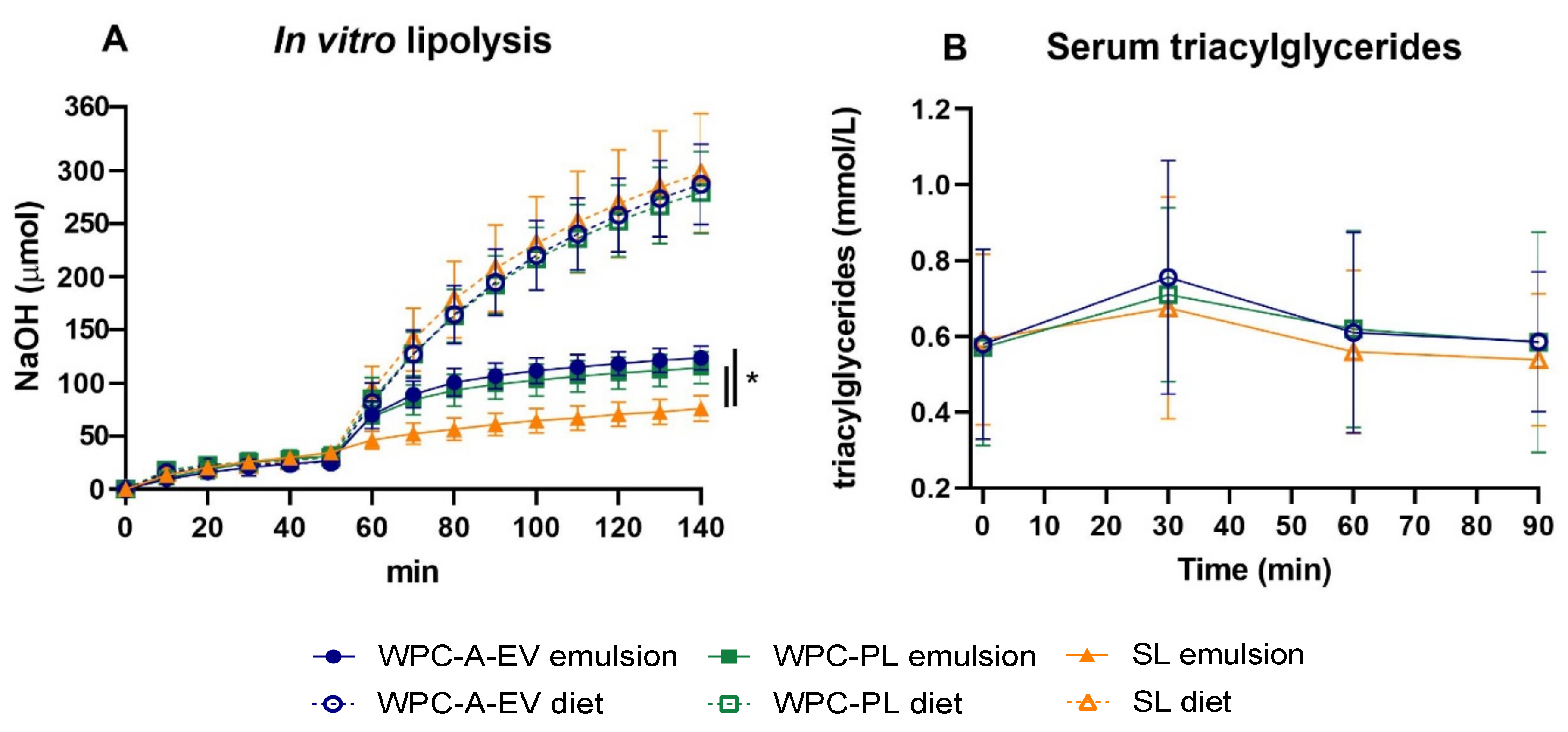
3.2. In Vitro Lipolysis and Serum Triacyl-Glyceride Measurements
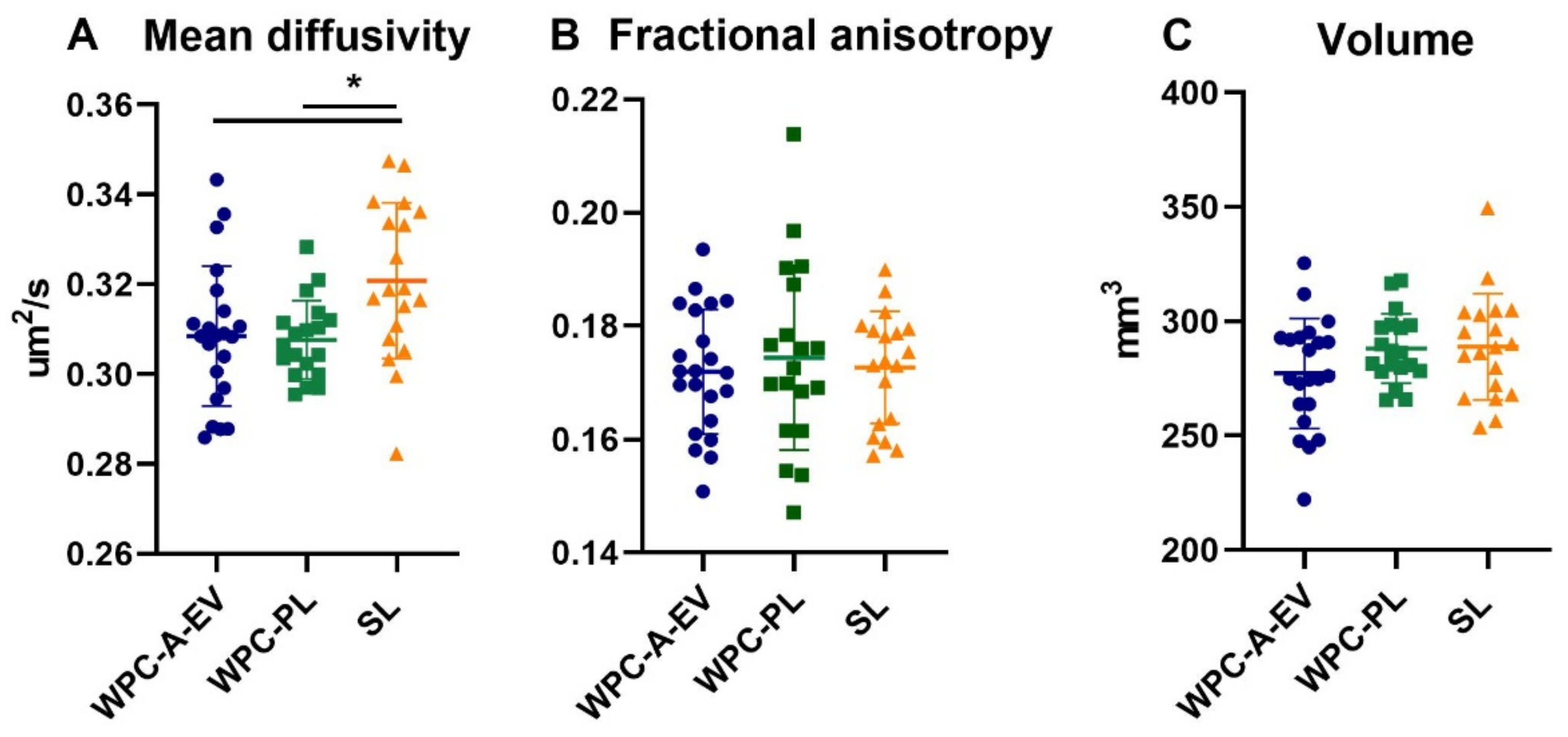
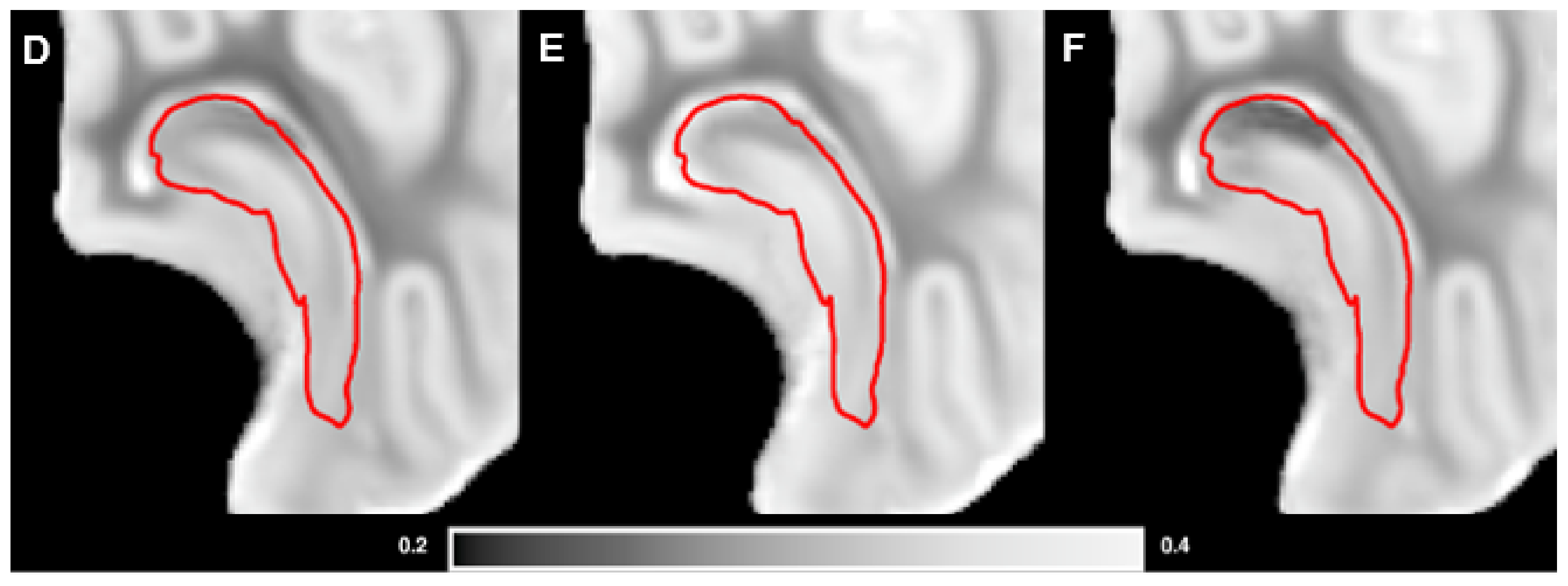
3.3. Brain Weights, Water Content and MRI Analysis
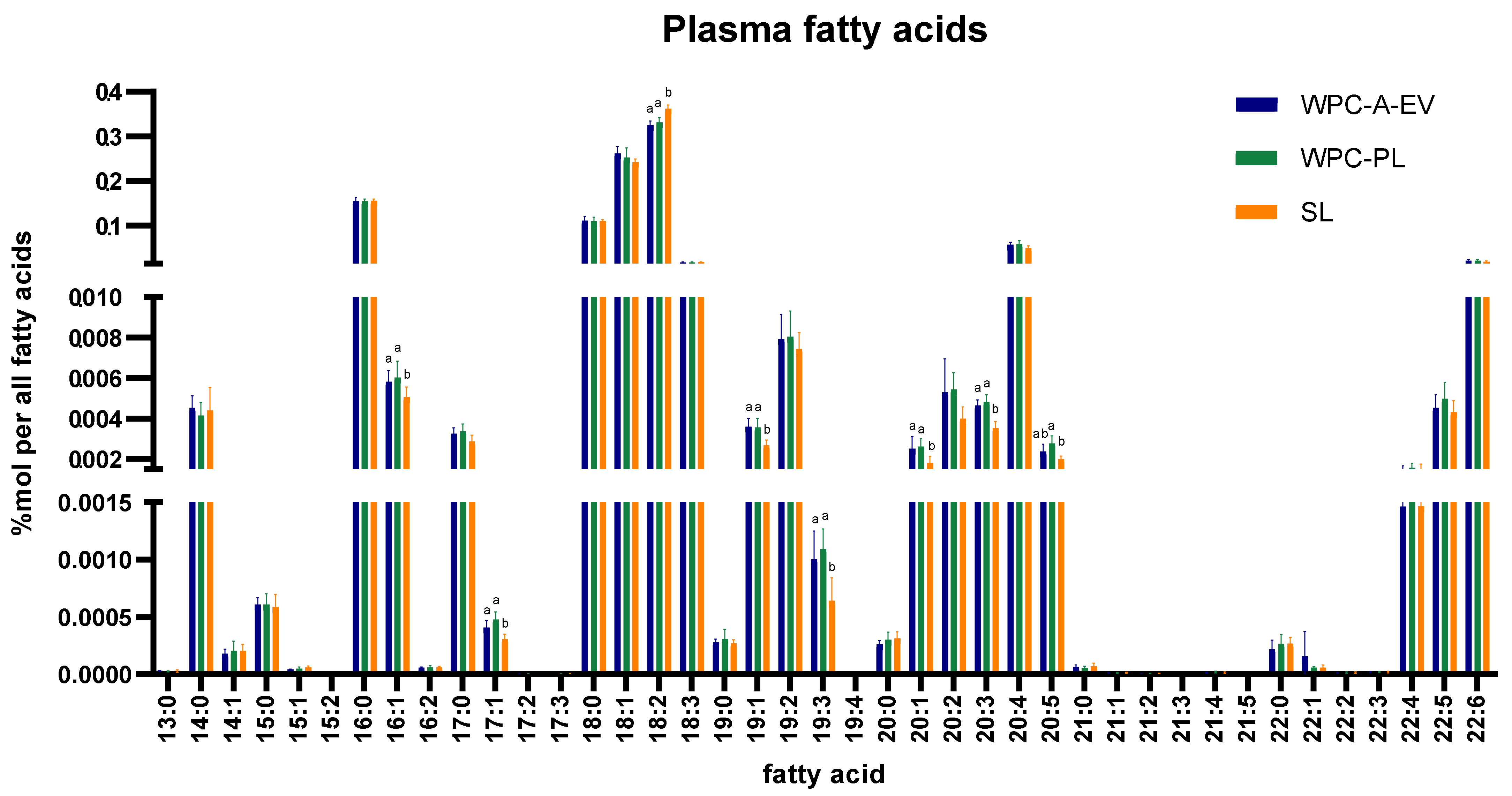
3.4. In-Depth Lipidomics
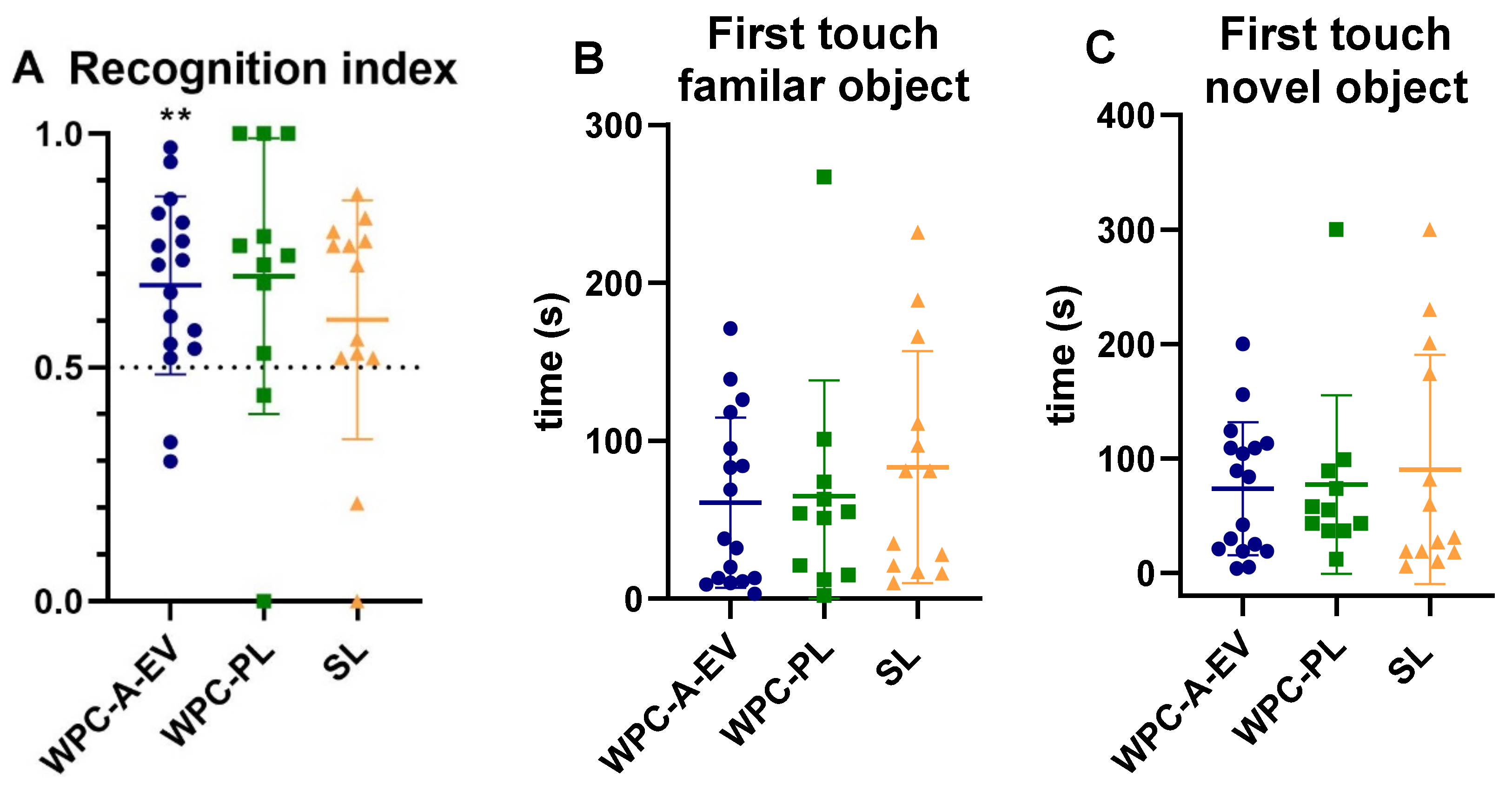
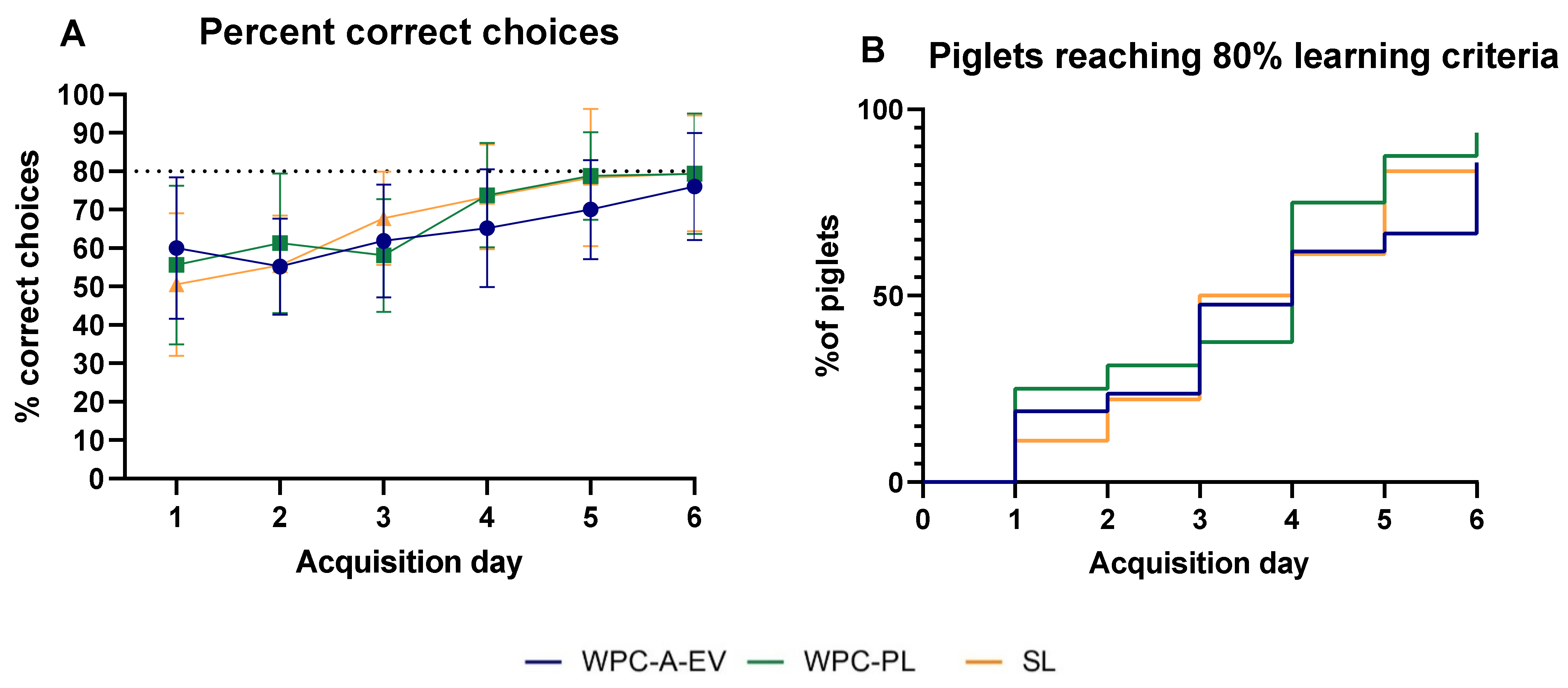
3.5. Acquisition of Basic Motor Skills, Home Cage Activity and Short-Term Memory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twilhaar, E.S.; Wade, R.M.; De Kieviet, J.F.; Van Goudoever, J.B.; Van Elburg, R.M.; Oosterlaan, J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: A meta-analysis and meta-regression. JAMA Pediatrics 2018, 172, 361–367. [Google Scholar] [CrossRef]
- Aarnoudse-Moens, C.S.H.; Weisglas-Kuperus, N.; Van Goudoever, J.B.; Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009, 124, 717–728. [Google Scholar] [CrossRef]
- De Kieviet, J.F.; Piek, J.P.; Aarnoudse-Moens, C.S.; Oosterlaan, J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: A meta-analysis. JAMA J. Am. Med. Assoc. 2009, 302, 2235–2242. [Google Scholar] [CrossRef]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Loret De Mola, C.; Victora, C.G. Breastfeeding and intelligence: A systematic review and meta-analysis. Acta Paediatr. Int. J. Paediatr. 2015, 104, 14–19. [Google Scholar] [CrossRef]
- Joffre, C.; Nadjar, A.; Lebbadi, M.; Calon, F.; Laye, S. N-3 LCPUFA improves cognition: The young, the old and the sick. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 1–20. [Google Scholar] [CrossRef]
- Lien, E.L.; Richard, C.; Hoffman, D.R. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 26–40. [Google Scholar] [CrossRef]
- Jamieson, E.C.; Farquharson, J.; Logan, R.W.; Howatson, A.G.; Patrick, W.J.; Weaver, L.T.; Cockburn, F. Infant cerebellar gray and white matter fatty acids in relation to age and diet. Lipids 1999, 34, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, J.; Jamieson, E.C.; Abbasi, K.A.; Patrick, W.J.A.; Logan, R.W.; Cockbum, F. Effect of diet on the fatty acid composition of the major phospholipids of infant cerebral cortex. Arch. Dis. Child. 1995, 72, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Verfuerden, M.L.; Dib, S.; Jerrim, J.; Fewtrell, M.; Gilbert, R.E. Effect of long-chain polyunsaturated fatty acids in infant formula on long-term cognitive function in childhood: A systematic review and meta-analysis of randomised controlled trials. PLoS ONE 2020, 15, e0241800. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Rao, S.C.; Schulzke, S.M.; Patole, S.K.; Simmer, K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst. Rev. 2016, 12, CD000375. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Hamosh, M.; Mehta, N.R.; Angelus, P.A.; Philpott, J.R.; Henderson, T.R.; Dwyer, N.K.; Lairon, D.; Hamosh, P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatrics Res. 1996, 40, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.; Hernell, O. Lipid digestion and absorption in early life: An update. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Ménard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces 2011, 83, 29–41. [Google Scholar] [CrossRef]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Breakefield, X.O.; Abels, E.R. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; Nonnecke, B.J.; Sacco, R.E. Bovine milk exosome proteome. J. Proteom. 2012, 75, 1486–1492. [Google Scholar] [CrossRef]
- Mathiassen, J.H.; Nejrup, R.G.; Frøkiær, H.; Nilsson, Å.; Ohlsson, L.; Hellgren, L.I. Emulsifying triglycerides with dairy phospholipids instead of soy lecithin modulates gut lipase activity. Eur. J. Lipid Sci. Technol. 2015, 117, 1522–1539. [Google Scholar] [CrossRef]
- Thompson, A.K.; Hindmarsh, J.P.; Haisman, D.; Rades, T.; Singh, H. Comparison of the structure and properties of liposomes prepared from milk fat globule membrane and soy phospholipids. J. Agric. Food Chem. 2006, 54, 3704–3711. [Google Scholar] [CrossRef] [PubMed]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-proteinformula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial. J. Pediatrics 2019, 215, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Radlowski, E.C.; Conrad, M.S.; Li, Y.; Dilger, R.N.; Johnson, R.W. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J. Nutr. 2014, 144, 1903–1909. [Google Scholar] [CrossRef]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatrics Res. 2003, 53, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Alexander, L.S.; Berding, K.; Waworuntu, R.V.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary prebiotics, milk fat globule membrane, and lactoferrin affects structural neurodevelopment in the young piglet. Front. Pediatrics 2016, 4, 4. [Google Scholar] [CrossRef]
- Fil, J.E.; Fleming, S.A.; Chichlowski, M.; Gross, G.; Berg, B.M.; Dilger, R.N. Evaluation of Dietary Bovine Milk Fat Globule Membrane Supplementation on Growth, Serum Cholesterol and Lipoproteins, and Neurodevelopment in the Young Pig. Front. Pediatrics 2019, 7, 417. [Google Scholar] [CrossRef]
- Conrad, M.S.; Dilger, R.N.; Johnson, R.W. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: A longitudinal MRI study. Dev. Neurosci. 2012, 34, 291–298. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 311, 79–83. [Google Scholar] [CrossRef]
- Odle, J.; Lin, X.; Jacobi, S.K.; Kim, S.W.; Stahl, C.H. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu. Rev. Anim. Biosci. 2014, 2, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Sangild, P.T.; Munch, S.L.; van der Beek, E.M.; Renes, I.B.; van Ginneken, C.; Greisen, G.O.; Thymann, T. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R481–R492. [Google Scholar] [CrossRef]
- Obelitz-Ryom, K.; Bering, S.B.; Overgaard, S.H.; Eskildsen, S.F.; Ringgaard, S.; Olesen, J.L.; Skovgaard, K.; Pankratova, S.; Wang, B.; Brunse, A.; et al. Bovine milk oligosaccharides with sialyllactose improves cognition in preterm pigs. Nutrients 2019, 11, 1335. [Google Scholar] [CrossRef]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.B.K.; Heerup, C.; Jensen, T.R.S.; Geng, X.; Drachmann, N.; Nordby, P.; Jeppesen, P.B.; Ifaoui, I.; Müllertz, A.; Sangild, P.T.; et al. Milk-derived emulsifiers increase triglyceride absorption in newborn formula-fed pigs. Nutrients 2021, 13, 410. [Google Scholar] [CrossRef]
- Heerup, C.; Ebbesen, M.F.; Geng, X.; Madsen, S.F.; Berthelsen, R.; Müllertz, A. Effects of recombinant human gastric lipase and pancreatin during in vitro pediatric gastro-intestinal digestion. Food Funct. 2021. submitted. [Google Scholar]
- Williams, H.D.; Sassene, P.; Kleberg, K.; Bakala-N’Goma, J.C.; Calderone, M.; Jannin, V.; Igonin, A.; Partheil, A.; Marchaud, D.; Jule, E.; et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, Part 1: Method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J. Pharm. Sci. 2012, 101, 3360–3380. [Google Scholar] [CrossRef]
- Goncharova, K.; Pierzynowski, S.G.; Grujic, D.; Kirko, S.; Szwiec, K.; Wang, J.; Kovalenko, T.; Osadchenko, I.; Ushakova, G.; Shmigel, H.; et al. A piglet with surgically induced exocrine pancreatic insufficiency as an animal model of newborns to study fat digestion. Br. J. Nutr. 2014, 112, 2060–2067. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Veraart, J.; Sijbers, J.; Sunaert, S.; Leemans, A.; Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 2013, 81, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Avants, B.B.; Tustison, N.J. The ITK Image Registration Framework. Front. Neuroinform. 2014, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Pauling, J.K.; Sokol, E.; Hannibal-Bach, H.K.; Ejsing, C.S. Comprehensive lipidome analysis by shotgun lipidomics on a hybrid quadrupole-orbitrap-linear ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2015, 26, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.F.; Højlund, K.; Ejsing, C.S. Easy, Fast, and Reproducible Quantification of Cholesterol and Other Lipids in Human Plasma by Combined High Resolution MSX and FTMS Analysis. J. Am. Soc. Mass Spectrom. 2018, 29, 34–41. [Google Scholar] [CrossRef]
- Ellis, S.R.; Paine, M.R.L.; Eijkel, G.B.; Pauling, J.K.; Husen, P.; Jervelund, M.W.; Hermansson, M.; Ejsing, C.S.; Heeren, R.M.A. Automated, parallel mass spectrometry imaging and structural identification of lipids. Nat. Methods 2018, 15, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Ejsing, C.S.; Duchoslav, E.; Sampaio, J.; Simons, K.; Bonner, R.; Thiele, C.; Ekroos, K.; Shevchenko, A. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 2006, 78, 6202–6214. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Dilger, R.N.; Johnson, R.W. Place and direction learning in a spatial T-maze task by neonatal piglets. Anim. Cogn. 2012, 15, 667–676. [Google Scholar] [CrossRef]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; Van Der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf. B Biointerfaces 2015, 136, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.; van Dijk, G.; Broersen, L.M.; Loos, M.; Bartke, N.; Scheurink, A.J.W.; van der Beek, E.M. A postnatal diet containing phospholipids, processed to yield large, phospholipid-coated lipid droplets, affects specific cognitive behaviors in healthy male mice1-3. J. Nutr. 2016, 146, 1155–1161. [Google Scholar] [CrossRef]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Bouzerzour, K.; Ferret-Bernard, S.; Ménard, O.; Le Normand, L.; Perrier, C.; Le Bourgot, C.; Jardin, J.; Bourlieu, C.; Carton, T.; et al. A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets. Eur. J. Nutr. 2018, 57, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Conde, C.; Martinez, M.; Ballabriga, A. Some chemical aspects of human brain development. I. neutral glycosphingolipids, sulfatides, and sphingomyelin. Pediatr. Res. 1974, 89–92. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar] [CrossRef]
- Chung, W.L.; Chen, J.J.; Su, H.M. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 2008, 138, 1165–1171. [Google Scholar] [CrossRef]
- Moriguchi, T.; Greiner, R.S.; Salem, N. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000, 75, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res. Int. 2007, 40, 770–781. [Google Scholar] [CrossRef]
- Ishikawa, M.; Maekawa, K.; Saito, K.; Senoo, Y.; Urata, M.; Murayama, M.; Tajima, Y.; Kumagai, Y.; Saito, Y. Plasma and serum lipidomics of healthy white adults shows characteristic profiles by subjects’ gender and age. PLoS ONE 2014, 9, e91806. [Google Scholar] [CrossRef] [PubMed]
- Grip, T.; Dyrlund, T.S.; Ahonen, L.; Domellöf, M.; Hernell, O.; Hyötyläinen, T.; Knip, M.; Lönnerdal, B.; Orešič, M.; Timby, N. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatrics Res. 2018, 84, 726–732. [Google Scholar] [CrossRef]
- Moukarzel, S.; Dyer, R.A.; Garcia, C.; Wiedeman, A.M.; Boyce, G.; Weinberg, J.; Keller, B.O.; Elango, R.; Innis, S.M. Milk Fat Globule Membrane Supplementation in Formula-fed Rat Pups Improves Reflex Development and May Alter Brain Lipid Composition. Sci. Rep. 2018, 8, 15277. [Google Scholar] [CrossRef]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krägeloh, C.U.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef]
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome. Cell Rep. 2020, 32, 108132. [Google Scholar] [CrossRef]
- Liu, L.; Bartke, N.; Van Daele, H.; Lawrence, P.; Qin, X.; Park, H.G.; Kothapalli, K.; Windust, A.; Bindels, J.; Wang, Z.; et al. Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets. J. Lipid Res. 2014, 55, 531–539. [Google Scholar] [CrossRef]
- Wijendran, V.; Huang, M.C.; Diau, G.Y.; Boehm, G.; Nathanielsz, P.W.; Brenna, J.T. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatrics Res. 2002, 51, 265–272. [Google Scholar] [CrossRef]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Dubois, J.; Yu, Q.; Mukherjee, P.; Huang, H. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage 2019, 185, 836–850. [Google Scholar] [CrossRef]
- Chiapponi, C.; Piras, F.; Piras, F.; Fagioli, S.; Caltagirone, C.; Spalletta, G. Cortical Grey Matter and Subcortical White Matter Brain Microstructural Changes in Schizophrenia Are Localised and Age Independent: A Case-Control Diffusion Tensor Imaging Study. PLoS ONE 2013, 8, e75115. [Google Scholar] [CrossRef] [PubMed]
- Callow, D.D.; Canada, K.L.; Riggins, T. Microstructural Integrity of the Hippocampus During Childhood: Relations with Age and Source Memory. Front. Psychol. 2020, 11, 568953. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem. 2017, 24, 1–22. [Google Scholar] [CrossRef]
- Baptista, L.C.; Sun, Y.; Carter, C.S.; Buford, T.W. Crosstalk Between the Gut Microbiome and Bioactive Lipids: Therapeutic Targets in Cognitive Frailty. Front. Nutr. 2020, 7, 17. [Google Scholar] [CrossRef]
- Lu, J.; Lu, L.; Yu, Y.; Cluette-Brown, J.; Martin, C.R.; Claud, E.C. Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Sci. Rep. 2018, 8, 5443. [Google Scholar] [CrossRef]
- Walfisch, A.; Sermer, C.; Cressman, A.; Koren, G. Breast milk and cognitive development-the role of confounders: A systematic review. BMJ Open 2013, 3, e003259. [Google Scholar] [CrossRef]
- Fleming, S.A.; Dilger, R.N. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav. Brain Res. 2017, 321, 50–60. [Google Scholar] [CrossRef]
- Holme Nielsen, C.; Bladt Brandt, A.; Thymann, T.; Obelitz-Ryom, K.; Jiang, P.; Vanden Hole, C.; Van Ginneken, C.; Pankratova, S.; Sangild, P.T. Rapid Postnatal Adaptation of Neurodevelopment in Pigs Born Late Preterm. Dev. Neurosci. 2018, 40, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Plomgaard, A.M.; Andersen, A.D.; Petersen, T.H.; van de Looij, Y.; Thymann, T.; Sangild, P.T.; Thomsen, C.; Sizonenko, S.V.; Greisen, G. Structural brain maturation differs between preterm and term piglets, whereas brain activity does not. Acta Paediatr. Int. J. Paediatr. 2019, 108, 637–644. [Google Scholar] [CrossRef]
| Constituents 1 | WPC-A-EV Diet | WPC-PL Diet | SL Diet | |
|---|---|---|---|---|
| Energy | kJ/L | 3462 | 3460 | 3382 |
| Protein | g/L | 50 | 49 | 46 |
| Carbohydrate | g/L | 43 | 43 | 43 |
| Fat | g/L | 51 | 51 | 51 |
| Docosahexaenoic acid | g/L | 0.1150 | 0.1150 | 0.1150 |
| Arachidonic acid | g/L | 0.0580 | 0.0580 | 0.0580 |
| Constituents (% of Sample) | WPC-A-EV | WPC-PL | SL |
|---|---|---|---|
| Dry matter | 96.7 | 95.7 | N/A |
| Ash | 4.9 | 1.9 | N/A |
| Lactose | 0.23 | 0.5 | N/A |
| Fat | 24.1 | 18.0 | N/A |
| Phospholipids | 11.04 | 6.87 | 43.29 |
| Phosphatidyl-choline | 2.99 | 1.88 | 13.49 |
| Lyso-phosphatidylcholine | 0.05 | 0.02 | 0.63 |
| Phosphatidylinositol | 0.60 | 0.45 | 11.34 |
| Lyso-phosphatidylinositol | N/A | N/A | ND |
| Phosphatidylserine | 1.06 | 0.73 | 0.26 |
| Lyso-phosphatidylserine | N/A | N/A | ND |
| Sphingomyelin | 2.94 | 1.69 | ND |
| Phosphatidylethanolamine | 3.28 | 2.06 | 7.20 |
| Lyso-phosphatidylethanolamine | 0.06 | 0.02 | 0.25 |
| N-acyl-phosphatidylethanolamine | N/A | N/A | 3.73 |
| Phosphatidylglycerol | N/A | N/A | 0.42 |
| Di-phosphatidylglycerol | N/A | N/A | 0.44 |
| Phosphatidic acid | 0 | 0.01 | 4.34 |
| Lysophosphatidic acid | N/A | N/A | 0.16 |
| Other phospholipids | 0.07 | 0.01 | 0.96 |
| Ganglioside D3 (mg/kg) | 2942 | 1702 | N/A |
| Cholesterol | 1.43 | 0.68 | N/A |
| Protein | 65.7 | 73.0 | N/A |
| Native α-lactalbumin | 4.68 | 0.88 | N/A |
| Native β-lactoglobulin | 22.4 | 19.1 | N/A |
| Native cGMP | 0 | 2.87 | N/A |
| Total α-lactalbumin | 5.41 | 3.49 | N/A |
| Total β-lactoglobulin | 25.8 | 28.1 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksen, N.L.; Aasmul-Olsen, K.; Venkatasubramanian, R.; Nygaard, M.K.E.; Sprenger, R.R.; Heckmann, A.B.; Ostenfeld, M.S.; Ejsing, C.S.; Eskildsen, S.F.; Müllertz, A.; et al. Dairy-Derived Emulsifiers in Infant Formula Show Marginal Effects on the Plasma Lipid Profile and Brain Structure in Preterm Piglets Relative to Soy Lecithin. Nutrients 2021, 13, 718. https://doi.org/10.3390/nu13030718
Henriksen NL, Aasmul-Olsen K, Venkatasubramanian R, Nygaard MKE, Sprenger RR, Heckmann AB, Ostenfeld MS, Ejsing CS, Eskildsen SF, Müllertz A, et al. Dairy-Derived Emulsifiers in Infant Formula Show Marginal Effects on the Plasma Lipid Profile and Brain Structure in Preterm Piglets Relative to Soy Lecithin. Nutrients. 2021; 13(3):718. https://doi.org/10.3390/nu13030718
Chicago/Turabian StyleHenriksen, Nicole L., Karoline Aasmul-Olsen, Ramakrishnan Venkatasubramanian, Mikkel K. E. Nygaard, Richard R. Sprenger, Anne B. Heckmann, Marie S. Ostenfeld, Christer S. Ejsing, Simon F. Eskildsen, Anette Müllertz, and et al. 2021. "Dairy-Derived Emulsifiers in Infant Formula Show Marginal Effects on the Plasma Lipid Profile and Brain Structure in Preterm Piglets Relative to Soy Lecithin" Nutrients 13, no. 3: 718. https://doi.org/10.3390/nu13030718
APA StyleHenriksen, N. L., Aasmul-Olsen, K., Venkatasubramanian, R., Nygaard, M. K. E., Sprenger, R. R., Heckmann, A. B., Ostenfeld, M. S., Ejsing, C. S., Eskildsen, S. F., Müllertz, A., Sangild, P. T., Bering, S. B., & Thymann, T. (2021). Dairy-Derived Emulsifiers in Infant Formula Show Marginal Effects on the Plasma Lipid Profile and Brain Structure in Preterm Piglets Relative to Soy Lecithin. Nutrients, 13(3), 718. https://doi.org/10.3390/nu13030718