Differential Deleterious Impact of Highly Saturated Versus Monounsaturated Fat Intake on Vascular Function, Structure, and Mechanics in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Assessment of Biochemical Parameters
2.3. Functional Studies in the Thoracic Aorta Artery
2.4. Structural and Mechanical Properties in Mesenteric Resistance Arteries
2.5. Elastin Content and Organization in Mesenteric Resistance Arteries
2.6. Collagen Content in Superior Mesenteric Arteries
2.7. Statistical Analysis
3. Results
3.1. SOLF, UOLF, and HF Diets Increase Body Weight and Glucose Levels But Differently Affect NEFA Concentrations
3.2. SOLF, UOLF, and HF Diets Induce Endothelial Dysfunction and Reduce Contractile Responses to Phenylephrine in the Thoracic Aorta
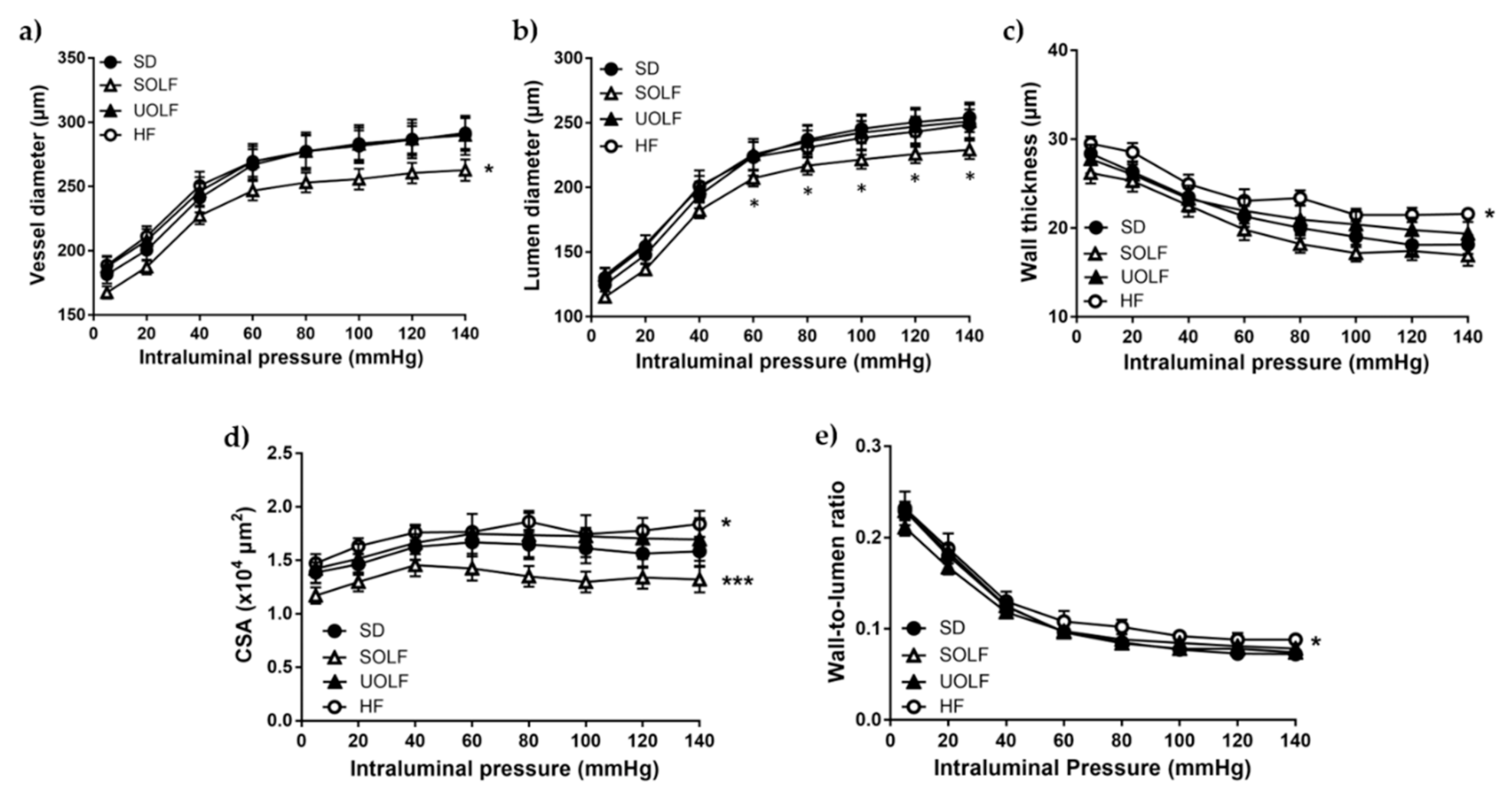
3.3. SOLF but Not UOLF or HF Diets Induced Hypotrophic Inward Remodeling in Mesenteric Resistance Arteries
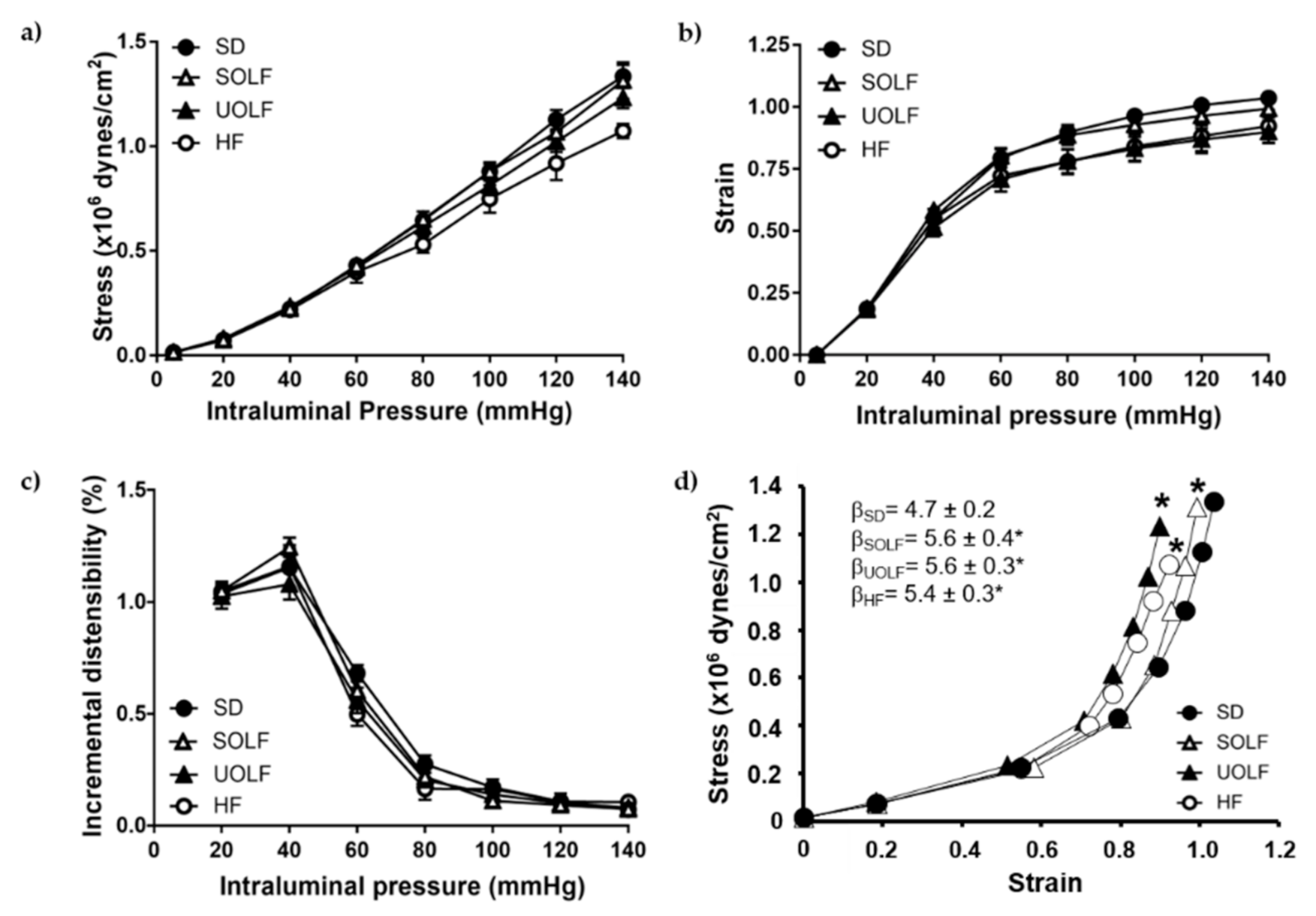
3.4. SOLF, UOLF, and HF Diets Induced Arterial Stiffness in Mesenteric Resistance Arteries
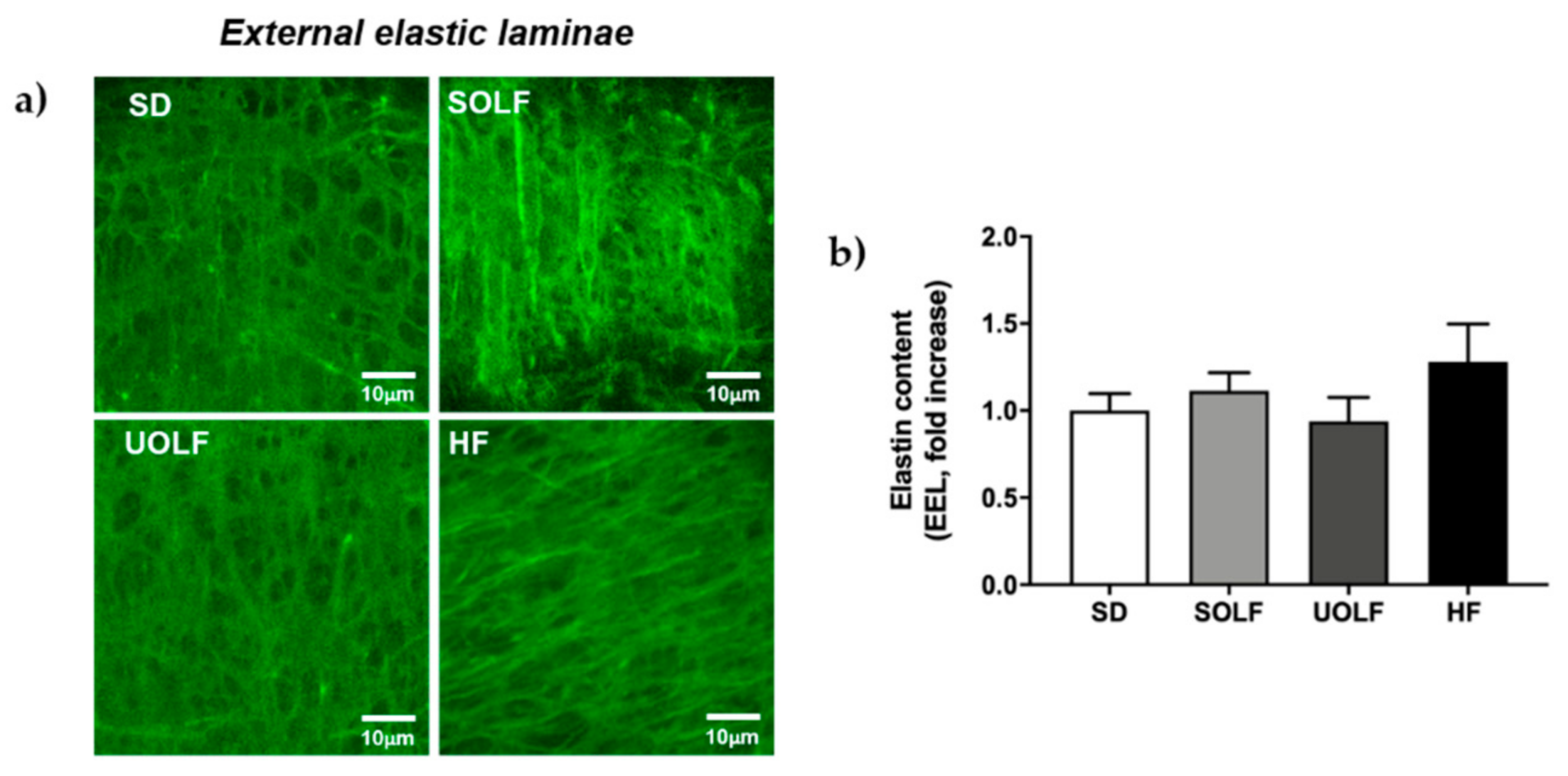
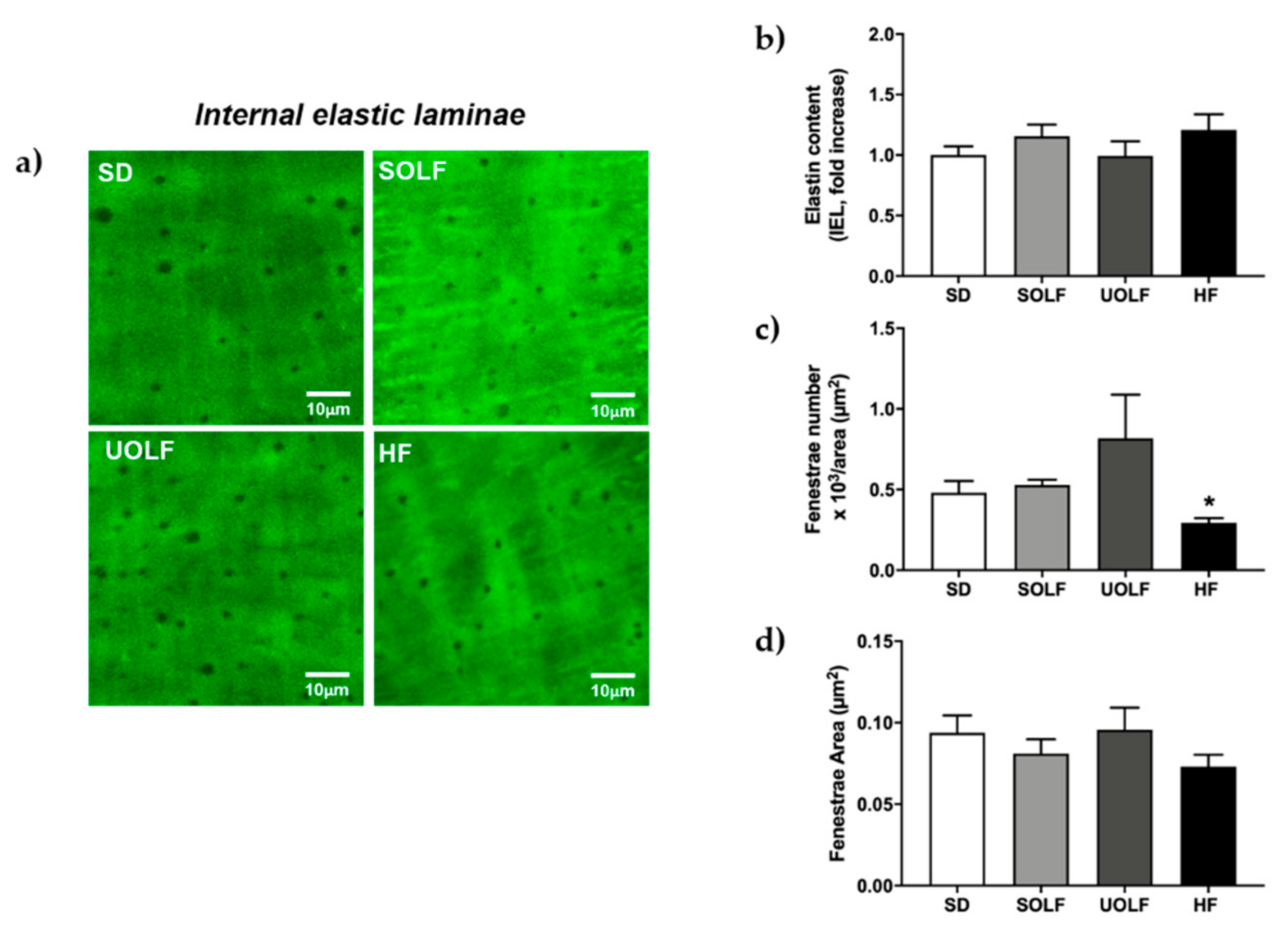
3.5. SOLF, UOLF, and HF Diets Induced An Increase in Collagen Deposition but No Major Changes in Elastin Content or Organization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 December 2020).
- Li, H.; Bao, Y.; Zhang, X.; Yu, Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-kappaB pathway in rat aorta. Int. J. Cardiol. 2011, 152, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortega, M.; Condezo-Hoyos, L.A.; García-Prieto, C.F.; Arribas, S.M.; González, M.C.; Aránguez, I.I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PLoS ONE 2014, 9, e95312. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Crijns, F.R.L.; Wolffenbuttel, B.H.R.; De Mey, J.G.R.; Boudier, H.A.J.S. Mechanical properties of mesenteric arteries in diabetic rats: Consequences of outward remodeling. Am. J. Physiol. Circ. Physiol. 1999, 276 Pt 2, H1672–H1677. [Google Scholar] [CrossRef]
- Sloboda, N.; Fève, B.; Thornton, S.N.; Nzietchueng, R.; Regnault, V.; Simon, G.; Labat, C.; Louis, H.; Max, J.-P.; Muscat, A.; et al. Fatty acids impair endothelium-dependent vasorelaxation: A link between obesity and arterial stiffness in very old Zucker rats. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 927–938. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Martín-Ramos, M.; Arribas, S.M.; González, M.C.; Aránguez, I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. Arterial stiffness is associated with adipokine dysregulation in non-hypertensive obese mice. Vasc. Pharmacol. 2016, 77, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, R.M.; Shiang, T.; Al Sayah, L.; Fry, J.L.; Bajpai, S.; Reinhart-King, C.A.; Lob, H.E.; Santhanam, L.; Mitchell, G.; Cohen, R.A.; et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 2013, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Warden, C.H.; Fisler, J.S. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008, 7, 277. [Google Scholar] [CrossRef]
- González-Blázquez, R.; Alcalá, M.; Fernández-Alfonso, M.S.; Villa-Valverde, P.; Viana, M.; Gil-Ortega, M.; Somoza, B. Relevance of control diet choice in metabolic studies: Impact in glucose homeostasis and vascular function. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Keogh, J.B.; Grieger, J.A.; Noakes, M.; Clifton, P.M. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Beach, V.; Sorkin, J.D.; Mangano, C.; Dobmeier, C.; Novacic, D.; Rhyne, J.; Vogel, R.A. Comparative effects of three popular diets on lipids, endothelial function, and C-reactive protein during weight maintenance. J. Am. Diet. Assoc. 2009, 109, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Newens, K.J.; Thompson, A.K.; Jackson, K.G.; Wright, J.; Williams, C.M. Acute effects of elevated NEFA on vascular function: A comparison of SFA and MUFA. Br. J. Nutr. 2010, 105, 1343–1351. [Google Scholar] [CrossRef]
- The RISCK Study Group; Sanders, T.A.B.; Lewis, F.J.; Goff, L.M.; Chowienczyk, P.J. SFAs do not impair endothelial function and arterial stiffness. Am. J. Clin. Nutr. 2013, 98, 677–683. [Google Scholar] [CrossRef]
- Herrera, M.D.; Péerez-Guerrero, C.; Marhuenda, E.; Ruiz-Gutiéerrez, V. Effects of dietary oleic-rich oils (virgin olive and high-oleic-acid sunflower) on vascular reactivity in Wistar-Kyoto and spontaneously hypertensive rats. Br. J. Nutr. 2001, 86, 349–357. [Google Scholar] [CrossRef]
- Huth, P.J.; Fulgoni, V.L., III; Larson, B.T. A systematic review of high-oleic vegetable oil substitutions for other fats and oils on cardiovascular disease risk factors: Implications for novel high-oleic soybean oils. Adv. Nutr. 2015, 6, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Antonazzi, M.; Blanco-Urgoiti, J.; Del Olmo, N.; Ruiz-Gayo, M. Potential Role of Leptin in Cardiac Steatosis Induced by Highly Saturated Fat Intake during Adolescence. Mol. Nutr. Food Res. 2019, 63, e1900110. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group NCRRGW. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; García-Prieto, C.F.; Ruiz-Hurtado, G.; Steireif, C.; González, M.C.; Schulz, A.; Kreutz, R.; Fernández-Alfonso, M.S.; Arribas, S.; Somoza, B. Genetic predisposition to albuminuria is associated with increased arterial stiffness: Role of elastin. Br. J. Pharmacol. 2015, 172, 4406–4418. [Google Scholar] [CrossRef]
- Briones, A.M.; González, J.M.; Somoza, B.; Giraldo, J.; Daly, C.J.; Vila, E.; González, M.C.; McGrath, J.C.; Arribas, S.M. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J. Physiol. 2003, 552 Pt 1, 185–195. [Google Scholar] [CrossRef]
- Dobrin, P.B. Mechanical properties of arterises. Physiol. Rev. 1978, 58, 397–460. [Google Scholar] [CrossRef]
- González, J.M.; Briones, A.M.; Starcher, B.; Conde, M.V.; Somoza, B.; Daly, C.; Vila, E.; McGrath, I.; González, M.C.; Arribas, S.M.; et al. Influence of elastin on rat small artery mechanical properties. Exp. Physiol. 2005, 90, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Arribas, S.M.; De Pablo, A.L.L.; González, M.C.; Abderrahim, F.; Condezo-Hoyos, L. A simple dot-blot–Sirius red-based assay for collagen quantification. Anal. Bioanal. Chem. 2013, 405, 6863–6871. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Radich, J.; González-Blázquez, R.; Alcalá, M.; Martín-Ramos, M.; Viana, M.; Arribas, S.; Delporte, C.; Fernández-Alfonso, M.S.; Somoza, B.; Gil-Ortega, M. Beneficial effects of murtilla extract and madecassic acid on insulin sensitivity and endothelial function in a model of diet-induced obesity. Sci. Rep. 2019, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, C.F.; Hernandez-Nuno, F.; Rio, D.D.; Ruiz-Hurtado, G.; Aranguez, I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS pathway. Mol. Nutr. Food Res. 2015, 59, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial Adipose Tissue Promotes Endothelial Dysfunction via Oxidative Stress in Diet-Induced Obese C57Bl/6 Mice. Circ. J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef]
- Virdis, A.; Colucci, R.; Bernardini, N.; Blandizzi, C.; Taddei, S.; Masi, S. Microvascular endothelial dysfunction in human obesity: Role of TNF-α. J. Clin. Endocrinol. Metab. 2019, 104, 341–348. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Colucci, R.; Chiriacò, M.; Uliana, M.; Puxeddu, I.; Bernardini, N.; Blandizzi, C.; Taddei, S. Microvascular endothelial dysfunction in patients with obesity. Curr. Hypertens. Rep. 2019, 21, 32. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Arvandi, M.; Pasha, E.; Haley, A.; Stanforth, P.; Tanaka, H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 495–502. [Google Scholar] [CrossRef]
- Manco, M.; Nobili, V.; Alisi, A.; Panera, N.; Handberg, A. Arterial stiffness, thickness and association to suitable novel markers of risk at the origin of cardiovascular disease in obese children. Int. J. Med. Sci. 2017, 14, 711–720. [Google Scholar] [CrossRef]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Kangas, P.; Tikkakoski, A.J.; Tahvanainen, A.M.; Leskinen, M.H.; Viitala, J.M.; Kähönen, M.; Kööbi, T.; Niemela, O.J.; Mustonen, J.T.; Pörsti, I.H. Metabolic syndrome may be associated with increased arterial stiffness even in the absence of hypertension: A study in 84 cases and 82 controls. Metabolism 2013, 62, 1114–1122. [Google Scholar] [CrossRef]
- Zebekakis, P.E.; Nawrot, T.; Thijs, L.; Balkestein, E.J.; van der Heijden-Spek, J.; Van Bortel, L.M.; Struijker-Boudier, H.A.; Safar, M.E.; Staessen, J.A. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 2005, 23, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr. Res. Rev. 2009, 22, 18–38. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Weech, M.; Sharma, V.; Yaqoob, P.; Todd, S.; Williams, C.M.; Jackson, K.G.; Lovegrove, J.A. A review of the evidence for the effects of total dietary fat, saturated, monounsaturated and n-6 polyunsaturated fatty acids on vascular function, endothelial progenitor cells and microparticles. Br. J. Nutr. 2012, 107, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Tysseling, K.A.; Rice, J.; Pham, M.; Haji, L.; Gallis, B.M.; Baas, A.S.; Paramsothy, P.; Giachelli, C.M.; Corson, M.A.; et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Greene, E.L.; Nagai, T.; Egan, B.M. Reactive oxygen species are critical in the oleic acid–mediated mitogenic signaling pathway in vascular smooth muscle cells. Hypertension 1998, 32, 1003–1010. [Google Scholar] [CrossRef]
- Juarez, E.; Tufiño, C.; Querejeta, E.; Bracho-Valdes, I.; Bobadilla-Lugo, R.A. Evidence of changes in alpha-1/AT1 receptor function generated by diet-induced obesity. Diabetes Vasc. Dis. Res. 2017, 14, 485–493. [Google Scholar] [CrossRef]
- Bouvet, C.; De Chantemeèle, E.B.; Guihot, A.-L.; Vessieères, E.; Bocquet, A.; Dumont, O.; Jardel, A.; Loufrani, L.; Moreau, P.; Henrion, D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension 2007, 50, 248–254. [Google Scholar] [CrossRef]
- Souza-Smith, F.M.; Katz, P.S.; Trask, A.J.; Stewart, J.A.; Lord, K.C.; Varner, K.J.; Vassallo, D.V.; Lucchesi, P.A. Mesenteric resistance arteries in type 2 diabetic db/db mice undergo outward remodeling. PLoS ONE 2011, 6, e23337. [Google Scholar] [CrossRef] [PubMed]
- de Chantemele, E.J.B.; Vessieres, E.; Guihot, A.L.; Toutain, B.; Maquignau, M.; Loufrani, L.; Henrion, D. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc. Res. 2009, 81, 788–796. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Scopelliti, F.; Dell’Oro, R.; Fattori, L.; Quarti-Trevano, F.; Brambilla, G.; Schiffrin, E.L.; Mancia, G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity 2010, 18, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Xavier, F.E.; Arribas, S.M.; González, M.C.; Rossoni, L.V.; Alonso, M.J.; Salaices, M. Alterations in structure and mechanics of resistance arteries from ouabain-induced hypertensive rats. Am. J. Physiol. Circ. Physiol. 2006, 291, H193–H201. [Google Scholar] [CrossRef]
- Dangardt, F.; Chen, Y.; Berggren, K.; Osika, W.; Friberg, P. Increased rate of arterial stiffening with obesity in adolescents: A five-year follow-up study. PLoS ONE 2013, 8, e57454. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Gavallér, H.; Csajbók, É.; Forster, T.; Csanády, M. Obesity is associated with aortic enlargement and increased stiffness: An echocardiographic study. Int. J. Cardiovasc. Imaging 2007, 24, 165–171. [Google Scholar] [CrossRef]
- Wildman, R.P.; Mackey, R.H.; Bostom, A.; Thompson, T.; Sutton-Tyrrell, K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 2003, 42, 468–473. [Google Scholar] [CrossRef]
- Sista, A.K.; O’Connell, M.K.; Hinohara, T.; Oommen, S.S.; Fenster, B.E.; Glassford, A.J.; Schwartz, E.A.; Taylor, C.A.; Reaven, G.M.; Tsao, P.S. Increased aortic stiffness in the insulin-resistant Zucker fa/fa rat. Am. J. Physiol. Circ. Physiol. 2005, 289, H845–H851. [Google Scholar] [CrossRef][Green Version]
- Chen, J.-Y.; Tsai, P.-J.; Tai, H.-C.; Tsai, R.-L.; Chang, Y.-T.; Wang, M.-C.; Chiou, Y.-W.; Yeh, M.-L.; Tang, M.-J.; Lam, C.-F.; et al. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 839–846. [Google Scholar] [CrossRef]
- Briones, A.M.; Arribas, S.M.; Salaices, M. Role of extracellular matrix in vascular remodeling of hypertension. Curr. Opin. Nephrol. Hypertens. 2010, 19, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Tziakas, D.N.; Chalikias, G.K.; Mitrousi, K.; Tsigalou, C.; Boudoulas, H. Associations between collagen synthesis and degradation and aortic function in arterial hypertension. Am. J. Hypertens. 2010, 23, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.G.; De Carvalho, M.H.C.; Akamine, E. Obesity induces artery-specific alterations: Evaluation of vascular function and inflammatory and smooth muscle phenotypic markers. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Rodriguez, C.; Galan, M.; Miana, M.; Jurado-Lopez, R.; Bartolome, M.V.; Luaces, M.; Islas, F.; Martínez-González, J.; López-Andrés, N.; et al. The lysyl oxidase inhibitor (beta-aminopropionitrile) reduces leptin profibrotic effects and ameliorates cardiovascular remodeling in diet-induced obesity in rats. J. Mol. Cell. Cardiol. 2016, 92, 96–104. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Martín, E.; Gil-Ortega, M.; González-Blázquez, R.; Benedito, S.; Fernández-Felipe, J.; Ruiz-Gayo, M.; del Olmo, N.; Chowen, J.A.; Frago, L.M.; Somoza, B.; et al. Differential Deleterious Impact of Highly Saturated Versus Monounsaturated Fat Intake on Vascular Function, Structure, and Mechanics in Mice. Nutrients 2021, 13, 1003. https://doi.org/10.3390/nu13031003
Vega-Martín E, Gil-Ortega M, González-Blázquez R, Benedito S, Fernández-Felipe J, Ruiz-Gayo M, del Olmo N, Chowen JA, Frago LM, Somoza B, et al. Differential Deleterious Impact of Highly Saturated Versus Monounsaturated Fat Intake on Vascular Function, Structure, and Mechanics in Mice. Nutrients. 2021; 13(3):1003. https://doi.org/10.3390/nu13031003
Chicago/Turabian StyleVega-Martín, Elena, Marta Gil-Ortega, Raquel González-Blázquez, Sara Benedito, Jesús Fernández-Felipe, Mariano Ruiz-Gayo, Nuria del Olmo, Julie A. Chowen, Laura M. Frago, Beatriz Somoza, and et al. 2021. "Differential Deleterious Impact of Highly Saturated Versus Monounsaturated Fat Intake on Vascular Function, Structure, and Mechanics in Mice" Nutrients 13, no. 3: 1003. https://doi.org/10.3390/nu13031003
APA StyleVega-Martín, E., Gil-Ortega, M., González-Blázquez, R., Benedito, S., Fernández-Felipe, J., Ruiz-Gayo, M., del Olmo, N., Chowen, J. A., Frago, L. M., Somoza, B., & Fernández-Alfonso, M. S. (2021). Differential Deleterious Impact of Highly Saturated Versus Monounsaturated Fat Intake on Vascular Function, Structure, and Mechanics in Mice. Nutrients, 13(3), 1003. https://doi.org/10.3390/nu13031003





