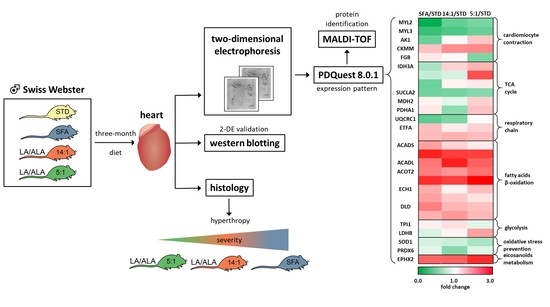
Effects of Three-Month Feeding High Fat Diets with Different Fatty Acid Composition on Myocardial Proteome in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, Housing Conditions, and Experiment Termination
2.2. Plasma Biochemistry
2.3. Histological Analyses
2.3.1. Hematoxylin and Eosin Staining (H&E)
2.3.2. Mallory Trichrome Staining
2.3.3. Quantitative Analysis of Mallory’s Trichrome Staining and Morphological Parameters
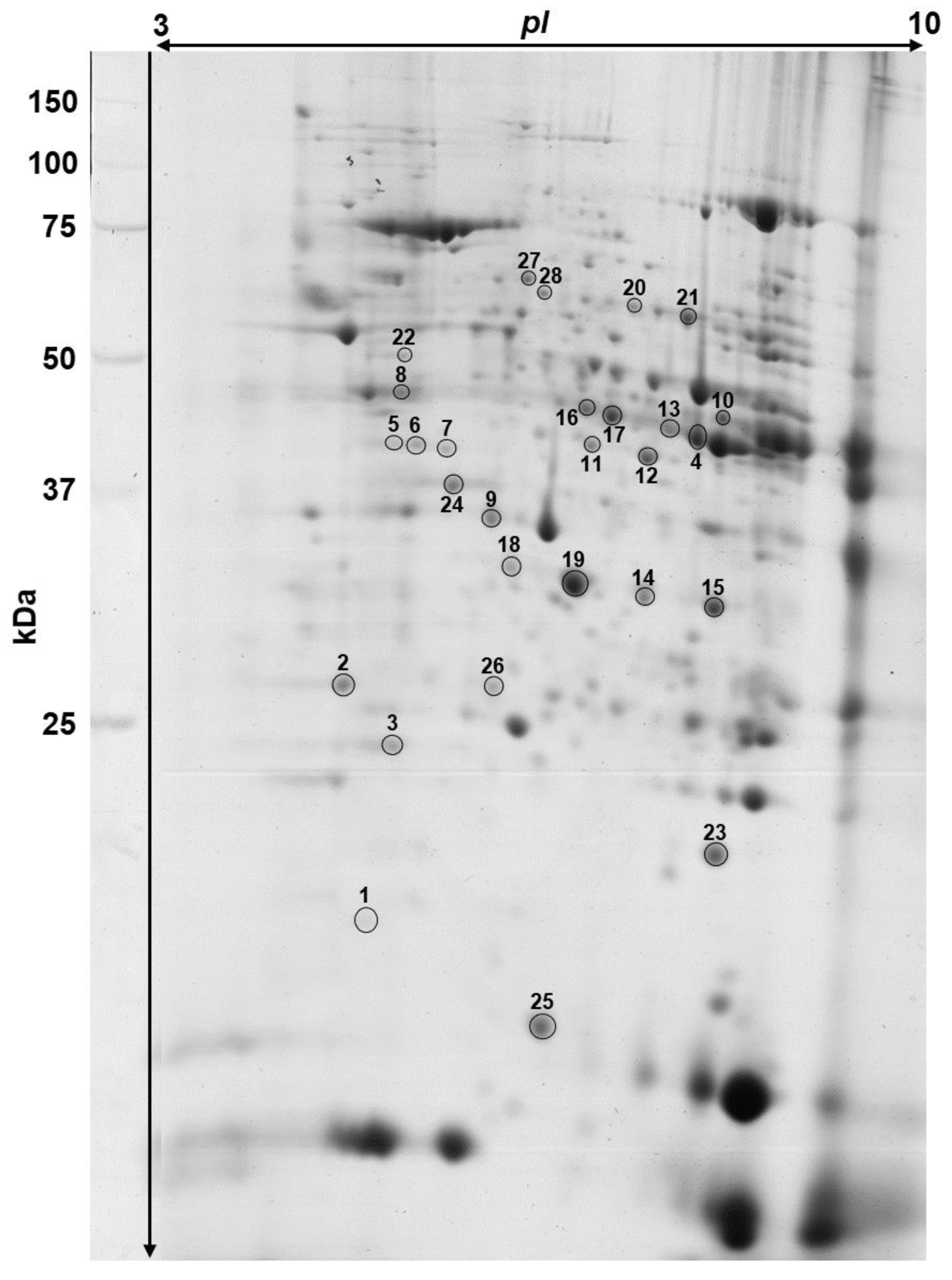
2.4. Two-Dimensional Electrophoresis
2.4.1. Homogenization
2.4.2. Isoelectrofocusing (IEF)
2.4.3. Second Dimension—SDS-PAGE
2.4.4. Image Staining and Analysis
2.4.5. Matrix-Assisted Laser Desorption Ionization—Time of Flight Mass Spectrometry (MALDI-ToF MS)
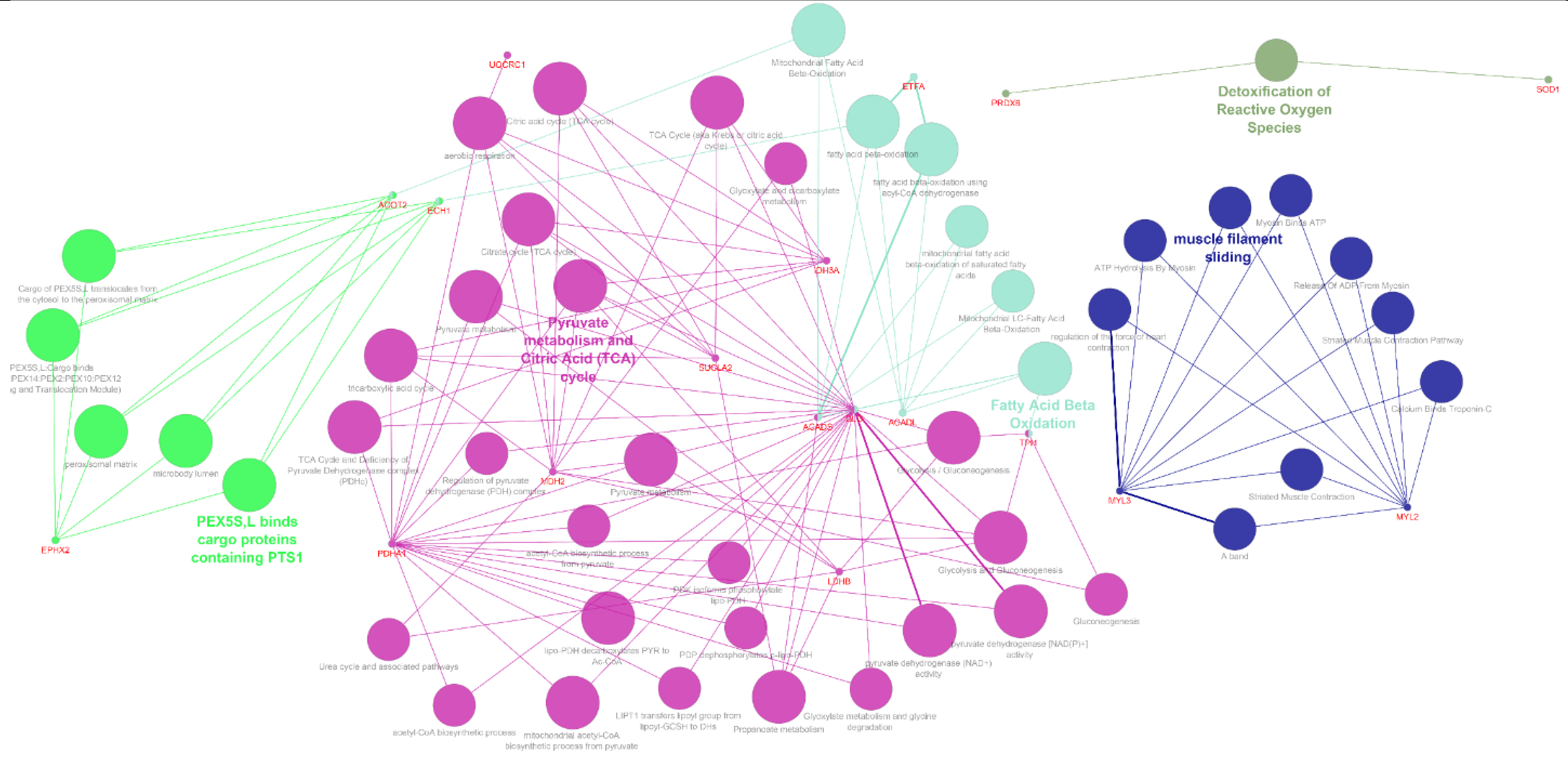
2.4.6. Geneo Ontology Analyses
2.5. Western Blot
2.6. Statistic Analysis
3. Results
3.1. Morphometric and Histological Heart Parameters and Blood Plasma Biochemistry
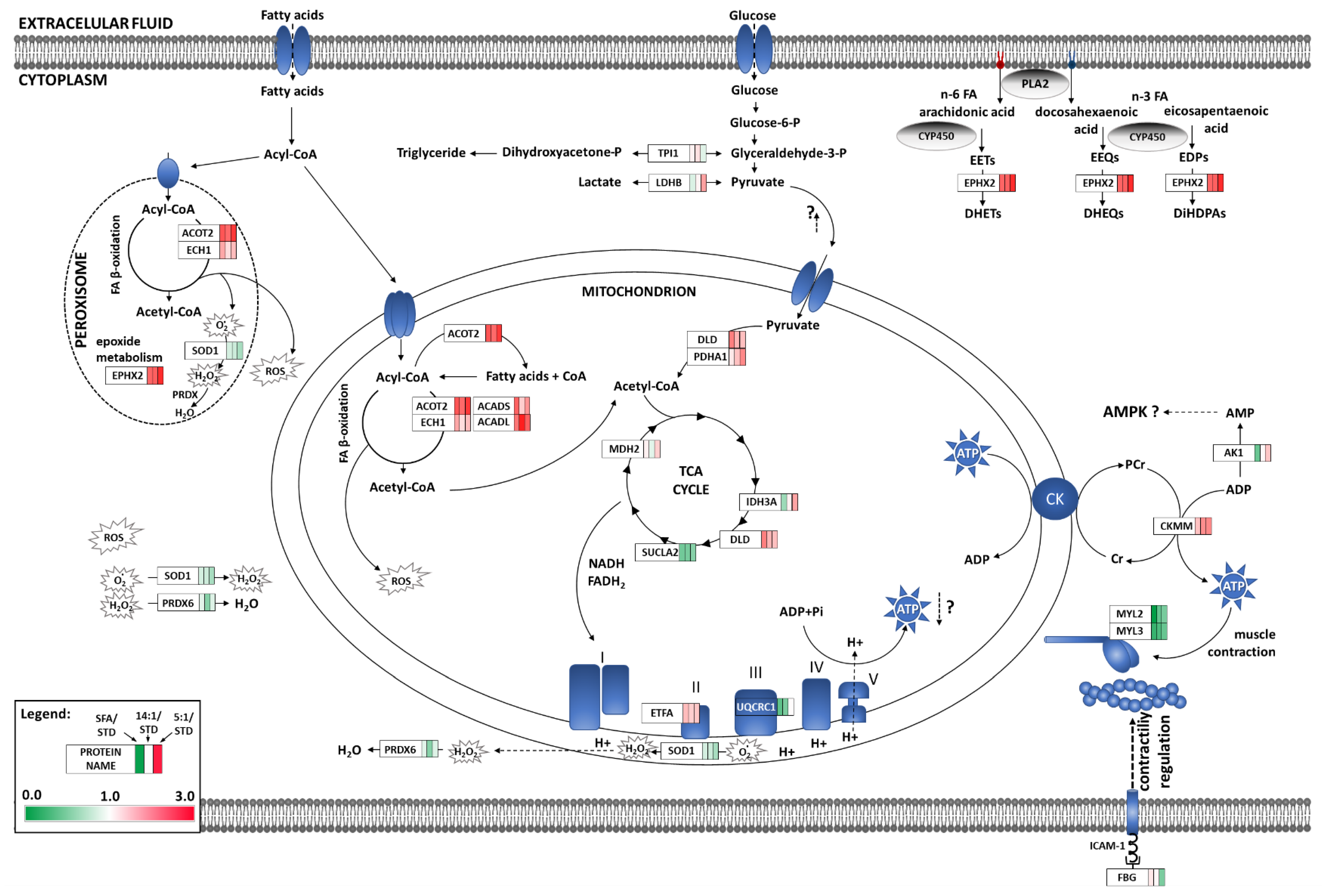
3.2. Analysis of Heart Proteome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krzysztoszek, J.; Laudańska-Krzemińska, I.; Bronikowski, M. Assessment of epidemiological obesity among adults in EU countries. Ann. Agric. Environ. Med. 2019, 26, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Tourlouki, E.; Matalas, A.-L.; Panagiotakos, D.B. Dietary habits and cardiovascular disease risk in middle-aged and elderly populations: A review of evidence. Clin. Interv. Aging 2009, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary Aspects of the Dietary Omega-6/Omega-3 Fatty Acid Ratio: Medical Implications. In Evolutionary Thinking in Medicine; Springer International Publishing: Cham, Swizterland, 2016; pp. 119–134. [Google Scholar]
- Di Pasquale, M.G. The essentials of essential fatty acids. J. Diet. Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Fats and fatty acids in human nutrition. Available online: https://www.who.int/nutrition/publications/nutrientrequirements/fatsandfattyacids_humannutrition/en/ (accessed on 30 December 2020).
- Simopoulos, A.P.; Leaf, A.; Salem, N. Workshop Statement on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 119–121. [Google Scholar] [CrossRef]
- Lands, B. Consequences of Essential Fatty Acids. Nutrients 2012, 4, 1338–1357. [Google Scholar] [CrossRef]
- Gillingham, B.L. The metabolic fate of alpha linolenic acid (ALA). IHP Mag. 2013, 1, 72–79. [Google Scholar]
- Li, Y.; Kang, J.X.; Leaf, A. Differential Effects of Various Eicosanoids on the Production or Prevention of Arrhythmias in Cultured Neonatal Rat Cardiac Myocytes. Prostaglandins 1997, 54, 511–530. [Google Scholar] [CrossRef]
- Schmilinsky-Fluri, G.; Valiunas, V.; Willi, M.; Weingart, R. Modulation of cardiac gap junctions: The mode of action of arachidonic acid. J. Mol. Cell. Cardiol. 1997, 29, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Fluri, G.S.; Rüdisüli, A.; Willi, M.; Rohr, S.; Weingart, R. Effects of arachidonic acid on the gap junctions of neonatal rat heart cells. Pflügers Arch. Eur. J. Physiol. 1990, 417, 149–156. [Google Scholar] [CrossRef]
- Ghosh, S.; Novak, E.M.; Innis, S.M. Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 α-linolenic acid ratios in normal, fat-fed pigs. Am. J. Physiol. Circ. Physiol. 2007, 293, H2919–H2927. [Google Scholar] [CrossRef] [PubMed]
- Beam, J.; Botta, A.; Ye, J.; Soliman, H.; Matier, B.J.; Forrest, M.; MacLeod, K.M.; Ghosh, S. Excess Linoleic Acid Increases Collagen I/III Ratio and “Stiffens” the Heart Muscle Following High Fat Diets. J. Biol. Chem. 2015, 290, 23371–23384. [Google Scholar] [CrossRef]
- Gamble, M. The Hematoxylins and Eosin. In Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008; pp. 121–134. [Google Scholar]
- Ozgo, M.; Lepczynski, A.; Robak, P.; Herosimczyk, A.; Marynowska, M. The current proteomic landscape of the porcine liver. J. Physiol. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Leopoldo, A.S.; Sugizaki, M.M.; Lima-Leopoldo, A.P.; do Nascimento, A.F.; Luvizotto, R.D.A.M.; de Campos, D.H.S.; Okoshi, K.; Pai-Silva, M.D.; Padovani, C.R.; Cicogna, A.C. Cardiac remodeling in a rat model of diet-induced obesity. Can. J. Cardiol. 2010, 26, 423–429. [Google Scholar] [CrossRef]
- Sahraoui, A.; Dewachter, C.; de Medina, G.; Naeije, R.; Aouichat Bouguerra, S.; Dewachter, L. Myocardial Structural and Biological Anomalies Induced by High Fat Diet in Psammomys obesus Gerbils. PLoS ONE 2016, 11, e0148117. [Google Scholar] [CrossRef]
- Sahraoui, A.; Dewachter, C.; Vegh, G.; Mc Entee, K.; Naeije, R.; Bouguerra, S.A.; Dewachter, L. High fat diet altered cardiac metabolic gene profile in Psammomys obesus gerbils. Lipids Health Dis. 2020, 19, 123. [Google Scholar] [CrossRef]
- Oliveira Junior, S.A.; Padovani, C.R.; Rodrigues, S.A.; Silva, N.R.; Martinez, P.F.; Campos, D.H.S.; Okoshi, M.P.; Okoshi, K.; Dal-Pai, M.; Cicogna, A.C. Extensive impact of saturated fatty acids on metabolic and cardiovascular profile in rats with diet-induced obesity: A canonical analysis. Cardiovasc. Diabetol. 2013, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Shao, D.; Tomasi, L.C.; Braun, A.; de Mattos, A.B.M.; Choi, Y.S.; Villet, O.; Roe, N.; Halterman, C.R.; Tian, R.; et al. The effects of fatty acid composition on cardiac hypertrophy and function in mouse models of diet-induced obesity. J. Nutr. Biochem. 2017, 46, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Vileigas, D.F.; Harman, V.M.; Freire, P.P.; Marciano, C.L.C.; Sant’Ana, P.G.; de Souza, S.L.B.; Mota, G.A.F.; da Silva, V.L.; Campos, D.H.S.; Padovani, C.R.; et al. Landscape of heart proteome changes in a diet-induced obesity model. Sci. Rep. 2019, 9, 18050. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.C.; Zanella, F.; Omens, J.H.; Sheikh, F. Mechanotransduction in Cardiac Hypertrophy and Failure. Circ. Res. 2015, 116, 1462–1476. [Google Scholar] [CrossRef]
- Rayment, I.; Holden, H.; Whittaker, M.; Yohn, C.; Lorenz, M.; Holmes, K.; Milligan, R. Structure of the actin-myosin complex and its implications for muscle contraction. Science 1993, 261, 58–65. [Google Scholar] [CrossRef]
- Sheikh, F.; Lyon, R.C.; Chen, J. Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease. Gene 2015, 569, 14–20. [Google Scholar] [CrossRef]
- Ding, P.; Huang, J.; Battiprolu, P.K.; Hill, J.A.; Kamm, K.E.; Stull, J.T. Cardiac Myosin Light Chain Kinase Is Necessary for Myosin Regulatory Light Chain Phosphorylation and Cardiac Performance In Vivo. J. Biol. Chem. 2010, 285, 40819–40829. [Google Scholar] [CrossRef]
- Chowdhury, D.; Tangutur, A.D.; Khatua, T.N.; Saxena, P.; Banerjee, S.K.; Bhadra, M.P. A proteomic view of isoproterenol induced cardiac hypertrophy: Prohibitin identified as a potential biomarker in rats. J. Transl. Med. 2013, 11, 130. [Google Scholar] [CrossRef]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; von Schacky, C.; Luft, F.C.; Muller, D.N.; et al. Arachidonic Acid-metabolizing Cytochrome P450 Enzymes Are Targets of ω-3 Fatty Acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar] [CrossRef]
- Jamieson, K.L.; Endo, T.; Darwesh, A.M.; Samokhvalov, V.; Seubert, J.M. Cytochrome P450-derived eicosanoids and heart function. Pharmacol. Ther. 2017, 179, 47–83. [Google Scholar] [CrossRef]
- Li, P.L.; Campbell, W.B. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ. Res. 1997. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, Y.; Wen, Z.; Chen, C.; Xu, X.; Zhu, Y.; Wang, Y.; Wang, D.W. CYP2J2 metabolites, epoxyeicosatrienoic acids, attenuate Ang II-induced cardiac fibrotic response by targeting Gα 12/13. J. Lipid Res. 2017, 58, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, T.; Zhang, K.; Liu, Y.; Huang, F.; Zhu, X.; Liu, Y.; Wang, M.H.; Tang, W.; Wang, J.; et al. Deletion of soluble epoxide hydrolase attenuates cardiac hypertrophy via down-regulation of cardiac fibroblasts-derived fibroblast growth factor-2. Crit. Care Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Pang, W.; Li, N.; Xu, M.; Jones, P.D.; Yang, J.; Zhang, Y.; Chiamvimonvat, N.; Shyy, J.Y.J.; Hammock, B.D.; et al. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2009, 106, 564–569. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, D.; Oltman, C.; Dellsperger, K.; Lee, H.-C.; VanRollins, M. Cytochrome P-450 Epoxygenase Metabolites of Docosahexaenoate Potently Dilate Coronary Arterioles by Activating Large-Conductance Calcium-Activated Potassium Channels. J. Pharmacol. Exp. Ther. 2002, 303, 768–776. [Google Scholar] [CrossRef]
- Pakiet, A.; Jakubiak, A.; Mierzejewska, P.; Zwara, A.; Liakh, I.; Sledzinski, T.; Mika, A. The Effect of a High-Fat Diet on the Fatty Acid Composition in the Hearts of Mice. Nutrients 2020, 12, 824. [Google Scholar] [CrossRef]
- Boyd, J.H.; Chau, E.; Tokunanga, C.; Bateman, R.M.; Haljan, G.; Davani, E.Y.; Wang, Y.; Walley, K.R. Fibrinogen decreases cardiomyocyte contractility through an ICAM-1 dependent mechanism. Crit. Care 2008, 12, R2. [Google Scholar] [CrossRef]
- Niessen, H. Upregulation of ICAM-1 on cardiomyocytes in jeopardized human myocardium during infarction. Cardiovasc. Res. 1999, 41, 603–610. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshida, M.; Nakano, Y.; Suganami, T.; Satoh, N.; Mita, T.; Azuma, K.; Itoh, M.; Yamamoto, Y.; Kamei, Y.; et al. In Vivo and In Vitro Inhibition of Monocyte Adhesion to Endothelial Cells and Endothelial Adhesion Molecules by Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2173–2179. [Google Scholar] [CrossRef]
- Dzeja, P.; Terzic, A. Adenylate kinase and AMP signaling networks: Metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 2009, 10, 1729–1772. [Google Scholar]
- Rayner, J.J.; Peterzan, M.A.; Watson, W.D.; Clarke, W.T.; Neubauer, S.; Rodgers, C.T.; Rider, O.J. Myocardial Energetics in Obesity. Circulation 2020, 141, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Maguire, M.L.; McAndrew, D.J.; Lake, H.A.; Neubauer, S.; Zervou, S.; Schneider, J.E.; Lygate, C.A. Overexpression of mitochondrial creatine kinase preserves cardiac energetics without ameliorating murine chronic heart failure. Basic Res. Cardiol. 2020, 115, 12. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Vitkevicius, K.T.; Redfield, M.M.; Burnett, J.C.; Terzic, A. Adenylate Kinase–Catalyzed Phosphotransfer in the Myocardium. Circ. Res. 1999, 84, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, T.A.; Dyck, J.R.B.; Lopaschuk, G.D. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem. Soc. Trans. 2003, 31, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Lu, Q.; Ren, D.; Sun, X.; Rousselle, T.; Tan, Y.; Li, J. AMPK: A therapeutic target of heart failure—not only metabolism regulation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Sikder, K.; Shukla, S.K.; Patel, N.; Singh, H.; Rafiq, K. High Fat Diet Upregulates Fatty Acid Oxidation and Ketogenesis via Intervention of PPAR-γ. Cell. Physiol. Biochem. 2018. [Google Scholar] [CrossRef]
- Finck, B.N.; Lehman, J.J.; Leone, T.C.; Welch, M.J.; Bennett, M.J.; Kovacs, A.; Han, X.; Gross, R.W.; Kozak, R.; Lopaschuk, G.D.; et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J. Clin. Investig. 2002, 109, 121–130. [Google Scholar] [CrossRef]
- Dai, C.; Li, Q.; May, H.I.; Li, C.; Zhang, G.; Sharma, G.; Sherry, A.D.; Malloy, C.R.; Khemtong, C.; Zhang, Y.; et al. Lactate Dehydrogenase A Governs Cardiac Hypertrophic Growth in Response to Hemodynamic Stress. Cell Rep. 2020, 32, 108087. [Google Scholar] [CrossRef]
- Luptak, I.; Sverdlov, A.L.; Panagia, M.; Qin, F.; Pimentel, D.R.; Croteau, D.; Siwik, D.A.; Ingwall, J.S.; Bachschmid, M.M.; Balschi, J.A.; et al. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J. Mol. Cell. Cardiol. 2018, 116, 106–114. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; List, E.O.; Okada, S.; Kelder, B.; Kopchick, J.J. Proteomic changes in the heart of diet-induced pre-diabetic mice. J. Proteomics 2011, 74, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Dabkowski, E.R.; Baseler, W.A.; Williamson, C.L.; Powell, M.; Razunguzwa, T.T.; Frisbee, J.C.; Hollander, J.M. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am. J. Physiol. Circ. Physiol. 2010, 299, H529–H540. [Google Scholar] [CrossRef] [PubMed]
- Abdurrachim, D.; Nabben, M.; Hoerr, V.; Kuhlmann, M.T.; Bovenkamp, P.; Ciapaite, J.; Geraets, I.M.E.; Coumans, W.; Luiken, J.J.F.P.; Glatz, J.F.C.; et al. Diabetic db/db mice do not develop heart failure upon pressure overload: A longitudinal in vivo PET, MRI, and MRS study on cardiac metabolic, structural, and functional adaptations. Cardiovasc. Res. 2017, 113, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Prévilon, M.; Le Gall, M.; Chafey, P.; Federeci, C.; Pezet, M.; Clary, G.; Broussard, C.; François, G.; Mercadier, J.-J.; Rouet-Benzineb, P. Comparative differential proteomic profiles of nonfailing and failing hearts after in vivo thoracic aortic constriction in mice overexpressing FKBP12.6. Physiol. Rep. 2013, 1. [Google Scholar] [CrossRef]
- Rich, H.S. Impact of High Fat Dietary Treatment and Nox2-Deficiency on Prooxidantand Antioxidant Enzyme Expression in the Heart; University of Zurich: Zurich, Swizterland, 2010. [Google Scholar]
- Liu, J.; Lloyd, S.G. High-fat, low-carbohydrate diet alters myocardial oxidative stress and impairs recovery of cardiac function after ischemia and reperfusion in obese rats. Nutr. Res. 2013, 33, 311–321. [Google Scholar] [CrossRef]

| Group | Components | (g) | LA/ALA | % SFA | % PUFA | % MUFA |
|---|---|---|---|---|---|---|
| SFA | Labofeed H | 790 | 1.41 | 76.87 | 11.04 | 12.09 |
| virgin coconut oil | 200 | |||||
| pumpkin seed oil | 10 | |||||
| 14:1 | Labofeed H | 790 | 13.76 | 1.68 | 82.21 | 16.10 |
| pumpkin seed oil | 210 | |||||
| 5:1 | Labofeed H | 790 | 5.00 | 9.91 | 79.69 | 10.40 |
| sunflower seed oil | 80 | |||||
| pumpkin seed oil | 65 | |||||
| avocado oil | 20 | |||||
| virgin coconut oil | 20 | |||||
| hemp seed oil | 15 | |||||
| corn oil | 10 |
| Parameter | STD | SFA | 14:1 | 5:1 |
|---|---|---|---|---|
| body weight (g) | 35.03 A | 50.83 B | 47.49 B | 43.78 B |
| visceral fat (g) | 0.201 a | 0.311 b | 0.317 b | 0.306 b |
| heart weight (g) | 0.1871 | 0.2671 | 0.2384 | 0.2281 |
| cardiomyocyte diameter (µm) * | 8.19 (1.37) A | 14.46 B (3.36) | 12.35 C (2.56) | 10.03 D (1.64) |
| cardiomyocyte diameter range (µm) | 4.95–13.21 | 8.01–19.81 | 7.35–16.98 | 6.13–16.84 |
| collagenous tissue (%) * | 5.42 a (1.48) | 7.64 c (3.45) | 7.32 bc (3.09) | 5.92 ab (2.22) |
| lactate (mmol/l) | 9.75 (2.48) | 12.19 (3.13) | 12.39 (3.42) | 9.78 (1.79) |
| LDH (U/l) | 1018.5 (451.9) | 897.5 (229.7) | 957.6 (444.8) | 1021.1 (810) |
| CK (U/l) | 233.7 (87.63) | 150 (61.57) | 183 (152) | 289.1 (171) |
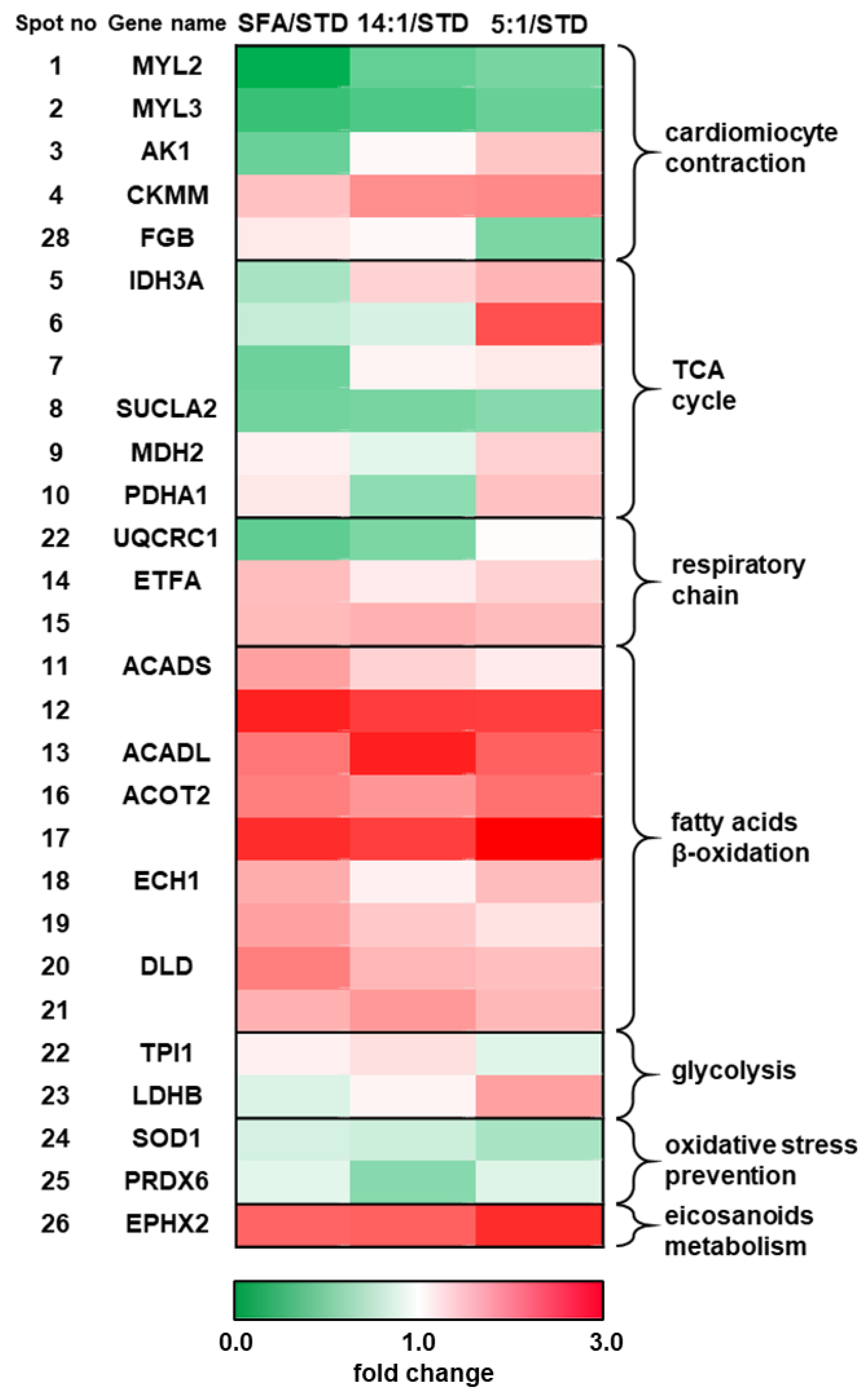
| Spot No | Protein Name | Gene Name | Seq. Cov. % | Mascot Score | STD | SFA | SFA/STD | 14:1 | 14:1/ STD | 5:1 | 5:1/ STD | Predicted pI/Mw | e\Estimated pI/Mw | SL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac Muscle Contraction | ||||||||||||||
| 1 | Myosin regulatory light chain 2, ventricular/cardiac muscle isoform | MYL2 | 58 | 90 | 754.4 | 68.3 ab | 0.09 | 339.5 a | 0.45 | 392.3 b | 0.52 | 4.86/18.9 | 4.5/16.6 | C |
| 2 | Myosin light chain 3 | MYL3 | 61 | 146 | 1585.6 | 460.3 | 0.29 | 588 | 0.37 | 734.3 | 0.46 | 5.03/22.5 | 4.4/25.9 | C |
| 3 | Adenylate kinase isoenzyme 1 | AK1 | 52 | 108 | 45.4 | 21.2 ab | 0.47 | 48.3 a | 1.06 | 65.2 b | 1.44 | 5.67/21.6 | 4.6/24.8 | C |
| 4 | Creatine kinase M-type | CKMM | 36 | 101 | 264.5 | 390.5 | 1.48 | 494.6 | 1.87 | 506.2 | 1.91 | 6.58/43.2 | 7.6/41.60 | C /MT |
| 28 | Fibrinogen beta chain | FGB | 26 | 95 | 18.8 | 22 a | 1.17 | 20 b | 1.06 | 10 ab | 0.53 | 6.68/55.4 | 6.1/58.4 | EX |
| Glycolysis | ||||||||||||||
| 23 | Triosephosphate isomerase | TPI1 | 33 | 85 | 152 | 169.1 | 1.11 | 188.3 a | 1.24 | 134.7 a | 0.89 | 5.56/32.7 | 7.6/25.0 | C |
| 24 | L-lactate dehydrogenase B chain | LDHB | 37 | 108 | 161.3 | 139.9 a | 0.87 | 176.7 b | 1.09 | 278.1 ab | 1.72 | 5.70/36.8 | 5.3/36.3 | C |
| TCA Cycle | ||||||||||||||
| 5 | Isocitrate dehydrogenase (NAD) subunit alpha, mitochondrial | IDH3A | 28 | 94 | 69.3 | 48.1 ab | 0.69 | 93.1 a | 1.34 | 108.9 b | 1.57 | 5.86/35.0 | 4.8/39.4 | MT |
| 6 | 39 | 121 | 31.4 | 24.7 a | 0.79 | 27.1 b | 0.86 | 73.7 ab | 2.35 | 5.0/39.6 | ||||
| 7 | 28 | 84 | 67 | 32 ab | 0.48 | 73.3 a | 1.09 | 78.5 b | 1.17 | 5.3/39.3 | ||||
| 8 | Succinate-CoA ligase (ADP-forming) subunit beta, mitochondrial | SUCLA2 | 34 | 87 | 303.8 | 151.5 | 0.5 | 158.9 | 0.52 | 172 | 0.57 | 4.94/36.3 | 4.8/44.7 | MT |
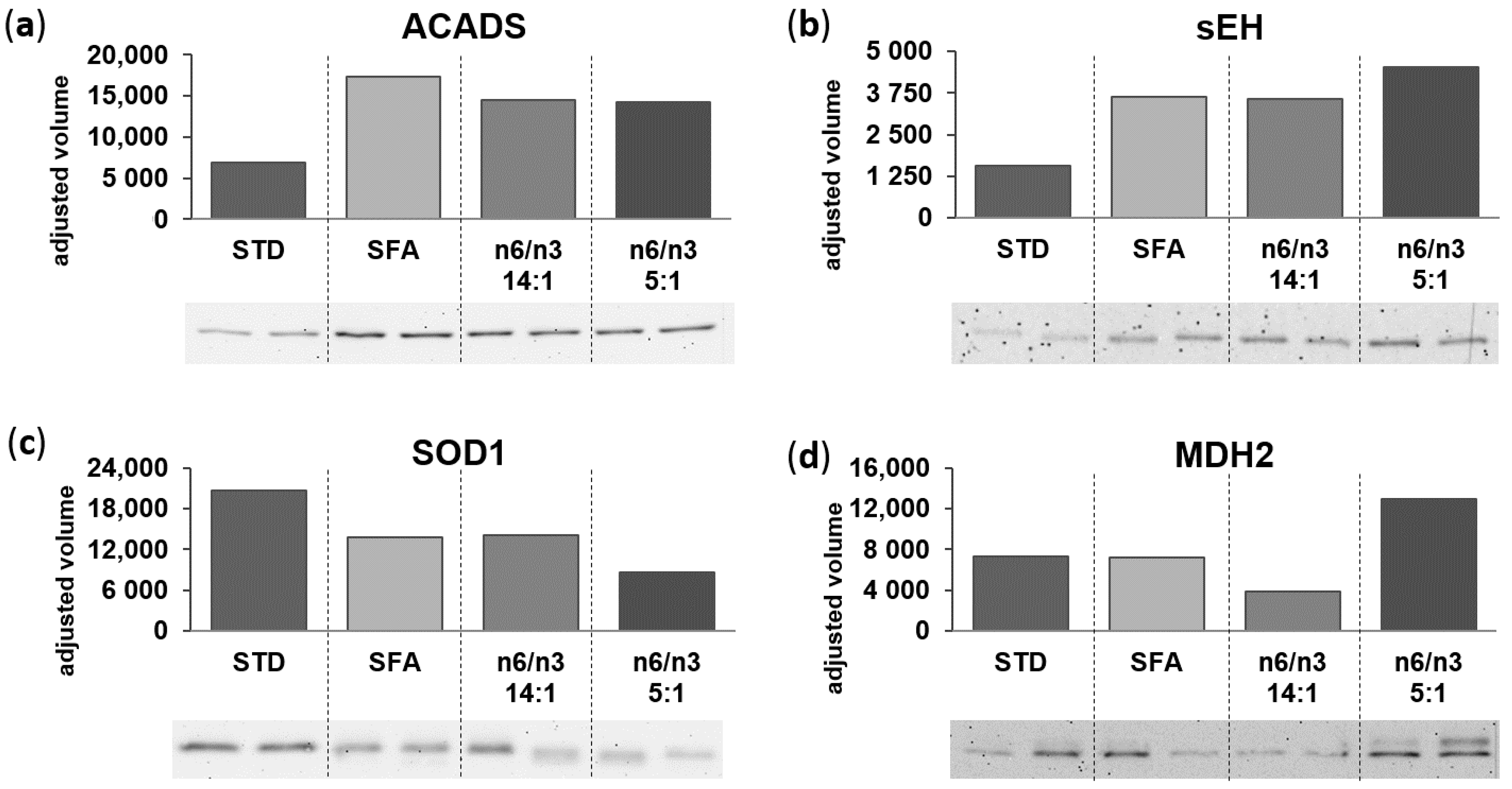
| 9 | Malate dehydrogenase, mitochondrial | MDH2 | 30 | 63 | 106.1 | 117.7 | 1.11 | 95.9 a | 0.9 | 144.8 a | 1.36 | 6.16/36.7 | 5.7/34.4 | MT |
| 10 | Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial | PDHA1 | 22 | 81 | 156.8 | 184.5 a | 1.18 | 94.7 ab | 0.6 | 232.1 b | 1.48 | 8.49/43.9 | 7.4/42.2 | MT |
| Respiratory Chain | ||||||||||||||
| 14 | Electron transfer flavoprotein subunit alpha, mitochondrial | ETFA | 40 | 96 | 92.8 | 140.4 | 1.51 | 107.9 | 1.16 | 125.4 | 1.35 | 8.62/35.3 | 7.2/30.6 | MT |
| 15 | 42 | 144 | 197 | 302.1 | 1.53 | 314.4 | 1.6 | 300 | 1.52 | 7.8/30.4 | ||||
| 22 | Cytochrome b-c1 complex subunit 1, mitochondrial | UQCRC1 | 35 | 90 | 56.8 | 24.4 a | 0.43 | 30.1 b | 0.53 | 58.5 ab | 1.03 | 5.81/53.4 | 4.9/48.5 | MT |
| Fatty Acid Metabolism and β-oxidation | ||||||||||||||
| 11 | Short-chain specific acyl-CoA dehydrogenase, mitochondrial | ACADS | 39 | 88 | 34.1 | 58.7 | 1.72 | 45.5 | 1.34 | 39.6 | 1.16 | 8.68/45.1 | 6.6/40.6 | MT |
| 12 | 49 | 156 | 53.7 | 145.6 | 2.71 | 134 | 2.49 | 133.2 | 2.48 | 7.2/39.7 | ||||
| 13 | Long-chain specific acyl-CoA dehydrogenase, mitochondrial | ACADL | 32 | 115 | 112.6 | 231.2 | 2.05 | 306.7 | 2.72 | 249.6 | 2.22 | 8.53/48.3 | 7.8/40.9 | MT |
| 16 | Acyl-coenzyme A thioesterase 2, mitochondrial | ACOT2 | 40 | 142 | 87.2 | 173.2 | 1.99 | 157.7 | 1.81 | 182.9 | 2.1 | 6.88/49.9 | 6.6/43.7 | MT |
| 17 | 40 | 128 | 101.1 | 264.8 | 2.62 | 250.9 | 2.48 | 298.7 | 2.95 | 6.8/43.3 | ||||
| 18 | Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase, mitochondrial | ECH1 | 34 | 74 | 21.6 | 35.3 | 1.64 | 23.9 | 1.11 | 32.9 | 1.52 | 7.6/36.4 | 5.9/31.7 | MT/P |
| 19 | 49 | 101 | 388.1 | 666.6 | 1.72 | 552.6 | 1.42 | 473 | 1.22 | 6.4/31.1 | ||||
| 20 | Dihydrolipoyl dehydrogenase, mitochondrial | DLD | 25 | 63 | 32.1 | 63.4 | 1.98 | 50.1 | 1.56 | 47.8 | 1.49 | 7.99/54.7 | 7.0/53.7 | MT |
| 21 | 28 | 90 | 104.4 | 166.9 | 1.6 | 186.4 | 1.79 | 161.9 | 1.55 | 7.6/56.3 | ||||
| Oxidative Stress Prevention | ||||||||||||||
| 25 | Superoxide dismutase (Cu-Zn) | SOD1 | 31 | 74 | 283.9 | 239.9 | 0.85 | 233 | 0.82 | 196.2 | 0.69 | 6.02/16.1 | 6.2/14.0 | C/P |
| 26/29 | Peroxiredoxin-6 | PRDX6 | 62 | 136 | 29.9 | 26.8 | 0.9 | 17.2 | 0.57 | 26.2 | 0.88 | 5.98/24.9 | 5.8/25.9 | C |
| 27/35 | Bifunctional epoxide hydrolase 2 | EPHX2 | 39 | 121 | 19.9 | 43.5 | 2.18 | 44.1 | 2.22 | 52.2 | 2.62 | 5.85/63.0 | 6.0/61.1 | C/P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepczyński, A.; Ożgo, M.; Michałek, K.; Dratwa-Chałupnik, A.; Grabowska, M.; Herosimczyk, A.; Liput, K.P.; Poławska, E.; Kram, A.; Pierzchała, M. Effects of Three-Month Feeding High Fat Diets with Different Fatty Acid Composition on Myocardial Proteome in Mice. Nutrients 2021, 13, 330. https://doi.org/10.3390/nu13020330
Lepczyński A, Ożgo M, Michałek K, Dratwa-Chałupnik A, Grabowska M, Herosimczyk A, Liput KP, Poławska E, Kram A, Pierzchała M. Effects of Three-Month Feeding High Fat Diets with Different Fatty Acid Composition on Myocardial Proteome in Mice. Nutrients. 2021; 13(2):330. https://doi.org/10.3390/nu13020330
Chicago/Turabian StyleLepczyński, Adam, Małgorzata Ożgo, Katarzyna Michałek, Alicja Dratwa-Chałupnik, Marta Grabowska, Agnieszka Herosimczyk, Kamila P. Liput, Ewa Poławska, Andrzej Kram, and Mariusz Pierzchała. 2021. "Effects of Three-Month Feeding High Fat Diets with Different Fatty Acid Composition on Myocardial Proteome in Mice" Nutrients 13, no. 2: 330. https://doi.org/10.3390/nu13020330
APA StyleLepczyński, A., Ożgo, M., Michałek, K., Dratwa-Chałupnik, A., Grabowska, M., Herosimczyk, A., Liput, K. P., Poławska, E., Kram, A., & Pierzchała, M. (2021). Effects of Three-Month Feeding High Fat Diets with Different Fatty Acid Composition on Myocardial Proteome in Mice. Nutrients, 13(2), 330. https://doi.org/10.3390/nu13020330