Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies
Abstract
1. Introduction
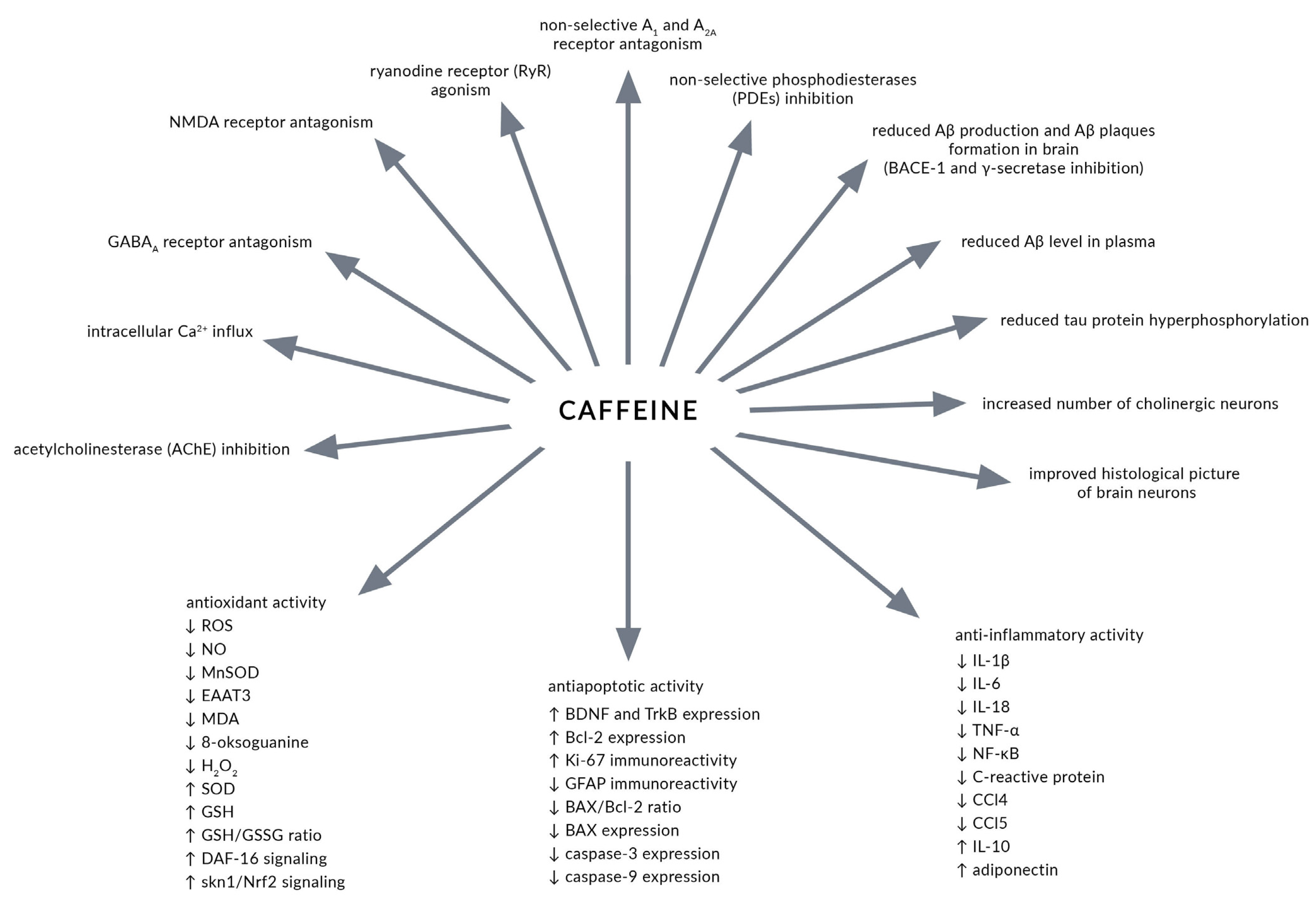
2. Caffeine—Main Mechanisms of Action
3. Alzheimer’s Disease (AD)
4. Caffeine in Alzheimer’s Disease
5. Experimental Models of AD
5.1. Effects of Caffeine in Experimental Animal Models of AD
5.1.1. Transgenic Rodent Models
5.1.2. Non-Transgenic Rodent Models
5.1.3. Rabbit Cholesterol-Induced Model
5.1.4. Nematode Models
5.2. In Vitro Studies
5.3. In Silico Studies
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lashley, T.; Schott, J.M.; Weston, P.; Murray, C.E.; Wellington, H.; Keshavan, A.; Foti, S.C.; Foiani, M.; Toombs, J.; Rohrer, J.D.; et al. Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis. Model. Mech. 2018, 11, dmm031781. [Google Scholar] [CrossRef]
- Wightman, E.L. Potential benefits of phytochemicals against Alzheimer’s disease. Proc. Nutr. Soc. 2017, 76, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; do Carmo Pereira, M. Natural compounds for Alzheimer’s disease therapy: A systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef] [PubMed]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia—Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Habtemariam, S. Natural products in Alzheimer’s disease therapy: Would old therapeutic approaches fix the broken promise of modern medicines? Molecules 2019, 24, 1519. [Google Scholar] [CrossRef]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Ashihara, H.; Kato, M.; Crozier, A. Distribution, biosynthesis and catabolism of methylxanthines in plants. Handb. Exp. Pharmacol. 2011, 200, 11–31. [Google Scholar] [CrossRef]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the diet: Country-level consumption and guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef]
- Verster, J.C.; Koenig, J. Caffeine intake and its sources: A review of national representative studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1250–1259. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimers Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef]
- Maia, L.; de Mendonça, A. Does caffeine intake protect from Alzheimer’s disease? Eur. J. Neurol. 2002, 9, 377–382. [Google Scholar] [CrossRef]
- Hussain, A.; Tabrez, E.S.; Mavrych, V.; Bolgova, O.; Peela, J.R. Caffeine: A potential protective agent against cognitive decline in Alzheimer’s disease. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 67–72. [Google Scholar] [CrossRef]
- Chen, J.-F.; Chern, Y. Impacts of methylxanthines and adenosine receptors on neurodegeneration: Human and experimental studies. Handb. Exp. Pharmacol. 2011, 200, 267–310. [Google Scholar] [CrossRef]
- Alasmari, F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm. J. 2020, 28, 445–451. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Isokawa, M. Caffeine-induced suppression of GABAergic inhibition and calcium-independent metaplasticity. Neural Plast. 2016, 2016, 1239629. [Google Scholar] [CrossRef]
- Simonin, C.; Duru, C.; Salleron, J.; Hincker, P.; Charles, P.; Delval, A.; Youssov, K.; Burnouf, S.; Azulay, J.-P.; Verny, C.; et al. Association between caffeine intake and age at onset in Huntington’s disease. Neurobiol. Dis. 2013, 58, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Sebastião, A.M. Caffeine and adenosine. J. Alzheimers Dis. 2010, 20, S3–S15. [Google Scholar] [CrossRef]
- Yang, J.Y.; Yang, G.; Ren, J.; Zhao, J.; Li, S. Caffeine suppresses GABA receptor-mediated current in rat primary sensory neurons via inhibition of intracellular phosphodiesterase. Neurophysiology 2015, 47, 108–114. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Folwarczna, J.; Sedlak, L.; Zych, M.; Wojnar, W.; Szumińska, I.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E. Effect of caffeine on biomarkers of oxidative stress in lenses of rats with streptozotocin-induced diabetes. Arch. Med. Sci. 2019, 15, 1073–1080. [Google Scholar] [CrossRef]
- Paiva, C.; Beserra, B.; Reis, C.; Dorea, J.G.; Da Costa, T.; Amato, A.A. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 652–663. [Google Scholar] [CrossRef]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C. Associations between coffee consumption and inflammatory markers in healthy persons: The ATTICA study. Am. J. Clin. Nutr. 2004, 80, 862–867. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Soto-Rojas, L.O.; de la Cruz-López, F.; Torres, M.A.O.; Viramontes-Pintos, A.; del Cárdenas-Aguayo, M.C.; Meraz-Ríos, M.A.; Salinas-Lara, C.; Florán-Garduño, B.; Luna-Muñoz, J. Neuroinflammation and alteration of the blood-brain barrier in Alzheimer’s disease. In Alzheimer’s Disease—Challenges for the Future; IntechOpen: London, UK, 2015. [Google Scholar]
- Pini, L.; Pievani, M.; Bocchetta, M.; Altomare, D.; Bosco, P.; Cavedo, E.; Galluzzi, S.; Marizzoni, M.; Frisoni, G.B. Brain atrophy in Alzheimer’s disease and aging. Aging Res. Rev. 2016, 30, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and psychological symptoms of dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Mak, M.S.; Lu, J.; Wu, Z.; Chen, Q.; Han, Y.; Li, Y.; Pi, R. Advance of sporadic Alzheimer’s disease animal models. Med. Res. Rev. 2020, 40, 431–458. [Google Scholar] [CrossRef]
- Atri, A. Current and future treatments in Alzheimer’s disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Morley, J.E.; Farr, S.A.; Nguyen, A.D. Alzheimer disease. Clin. Geriatr. Med. 2018, 34, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, S.E.; Pippin, J.J.; Barnard, N.D. Animal models of Alzheimer disease: Historical pitfalls and a path forward. ALTEX 2014, 31, 279–302. [Google Scholar] [CrossRef]
- Shi, Y.; Holtzman, D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018, 18, 759–772. [Google Scholar] [CrossRef]
- Riphagen, J.M.; Ramakers, I.H.G.M.; Freeze, W.M.; Pagen, L.H.G.; Hanseeuw, B.J.; Verbeek, M.M.; Verhey, F.R.J.; Jacobs, H.I.L. Neurobiology of aging linking APOE-ε4, blood-brain barrier dysfunction, and inflammation to Alzheimer’s pathology. Neurobiol. Aging 2020, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural insights and links to Alzheimer disease pathogenesis. Neuron 2020, 109, 205–221. [Google Scholar] [CrossRef]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef]
- Hickman, R.A.; Faustin, A.; Wisniewski, T. Alzheimer disease and its growing epidemic: Risk factors, biomarkers, and the urgent need for therapeutics. Neurol. Clin. 2016, 34, 941–953. [Google Scholar] [CrossRef]
- Esteves, I.M.; Lopes-Aguiar, C.; Rossignoli, M.T.; Ruggiero, R.N.; Broggini, A.C.S.; Bueno-Junior, L.S.; Kandratavicius, L.; Monteiro, M.R.; Romcy-Pereira, R.N.; Leite, J.P. Chronic nicotine attenuates behavioral and synaptic plasticity impairments in a streptozotocin model of Alzheimer’s disease. Neuroscience 2017, 353, 87–97. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Tong, M.; Wands, J.R. The 20-year voyage aboard the Journal of Alzheimer’s Disease: Docking at “type 3 diabetes”, environmental/exposure factors, pathogenic mechanisms, and potential treatments. J. Alzheimers Dis. 2018, 62, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Lester-Coll, N.; Rivera, E.J.; Soscia, S.J.; Doiron, K.; Wands, J.R.; de la Monte, S.M. Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 13–33. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Patience, A.A.; Sharma, N.; Khurana, N. The present and future of pharmacotherapy of Alzheimer’s disease: A comprehensive review. Eur. J. Pharmacol. 2017, 815, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.-S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Cellai, L.; Carvalho, K.; Faivre, E.; Deleau, A.; Vieau, D.; Buée, L.; Blum, D.; Mériaux, C.; Gomez-Murcia, V. The adenosinergic signaling: A complex but promising therapeutic target for Alzheimer’s disease. Front. Neurosci. 2018, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. The role of adenosine in Alzheimer’s disease. Curr. Neuropharmacol. 2009, 7, 207–216. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine—Searching for the connections. Br. J. Pharmacol. 2012, 167, 324–352. [Google Scholar] [CrossRef]
- Takahashi, R.N.; Pamplona, F.A.; Prediger, R.D.S. Adenosine receptor antagonists for cognitive dysfunction: A review of animal studies. Front. Biosci. 2008, 13, 2614–2632. [Google Scholar] [CrossRef]
- Cieślak, M.; Wojtczak, A. Role of purinergic receptors in the Alzheimer’s disease. Purinergic Signal. 2018, 14, 331–344. [Google Scholar] [CrossRef]
- Liu, K.Y.; Stringer, A.E.; Reeves, S.J.; Howard, R.J. The neurochemistry of agitation in Alzheimer’s disease: A systematic review. Aging Res. Rev. 2018, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J. Alzheimers Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Dimech, A.S.; Chadha, A.S.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef]
- Mazure, C.M.; Swendsen, J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016, 15, 451–452. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, F1000 Faculty Rev-1161. [Google Scholar] [CrossRef] [PubMed]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Caporaso, R.; Basagni, F.; Catanzaro, M.; Abu, I.F.; Fagiani, F.; Fusco, F.; Masuzzo, S.; Albani, D.; et al. Merging memantine and ferulic acid to probe connections between NMDA receptors, oxidative stress and amyloid-β peptide in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. Biofactors 2019, 45, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M.; Sedaghat, M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 16953–16965. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Z.-Y.; Zheng, X.; Li, D.-L.; Zhou, R.-P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Giacomazza, D.; di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Meng, X.; Ma, X.; Zhang, G.; Hu, X.; Jin, A.; Zhao, Y.; Liu, X. Application of ferulic acid for Alzheimer’s disease: Combination of text mining and experimental validation. Front. Neuroinform. 2018, 12, 31. [Google Scholar] [CrossRef]
- He, M.; Zhao, L.; Wei, M.-J.; Yao, W.-F.; Zhao, H.-S.; Chen, F.-J. Neuroprotective effects of (-)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol. Pharm. Bull. 2009, 32, 55–60. [Google Scholar] [CrossRef]
- Polito, C.A.; Cai, Z.-Y.; Shi, Y.-L.; Li, X.-M.; Yang, R.; Shi, M.; Li, Q.-S.; Ma, S.-C.; Xiang, L.-P.; Wang, K.-R.; et al. Association of tea consumption with risk of Alzheimer’s disease and anti-beta-amyloid effects of tea. Nutrients 2018, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S.; Muzio, M.R.; Schiavone, V.; Cuomo, A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: An overview of pre-clinical studies and translational perspectives in clinical practice. Infect. Agent. Cancer 2017, 12, 36. [Google Scholar] [CrossRef]
- Oñatibia-Astibia, A.; Franco, R.; Martínez-Pinilla, E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017, 61, 1600670. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Flaten, V.; Laurent, C.; Coelho, J.E.; Sandau, U.; Batalha, V.L.; Burnouf, S.; Hamdane, M.; Humez, S.; Boison, D.; Lopes, L.V.; et al. From epidemiology to pathophysiology: What about caffeine in Alzheimer’s disease? Biochem. Soc. Trans. 2014, 42, 587–592. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kwak, S.M.; Myung, S.-K. Caffeine intake from coffee or tea and cognitive disorders: A meta-analysis of observational studies. Neuroepidemiology 2015, 44, 51–63. [Google Scholar] [CrossRef]
- Wierzejska, R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch. Med. Sci. 2017, 13, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Watanabe, H.; Murayama, N. Green tea intake and risks for dementia, Alzheimer’s disease, mild cognitive impairment, and cognitive impairment: A systematic review. Nutrients 2019, 11, 1165. [Google Scholar] [CrossRef]
- Ma, Q.-P.; Huang, C.; Cui, Q.-Y.; Yang, D.-J.; Sun, K.; Chen, X.; Li, X.-H. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS ONE 2016, 11, e0165861. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; He, Y. Coffee intake and the incident risk of cognitive disorders: A dose–response meta-analysis of nine prospective cohort studies. Clin. Nutr. 2017, 36, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-P.; Wu, Y.-F.; Cheng, H.-Y.; Xia, T.; Ding, H.; Wang, H.; Wang, Z.-M.; Xu, Y. Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta-analysis of prospective cohort studies. Nutrition 2016, 32, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N. Coffee consumption and risk of dementia and Alzheimer’s disease: A dose-response meta-analysis of prospective studies. Nutrients 2018, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Taylor, A.E.; Karhunen, V.; Zhan, Y.; Rovio, S.P.; Lahti, J.; Sjögren, P.; Byberg, L.; Lyall, D.M.; Auvinen, J.; et al. Habitual coffee consumption and cognitive function: A Mendelian randomization meta-analysis in up to 415530 participants. Sci. Rep. 2018, 8, 7526. [Google Scholar] [CrossRef]
- Larsson, S.C.; Traylor, M.; Malik, R.; Dichgans, M.; Burgess, S.; Markus, H.S.; CoSTREAM Consortium, on behalf of the International Genomics of Alzheimer’s Project. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ 2017, 359, j5375. [Google Scholar] [CrossRef]
- Schuster, J.; Mitchell, E.S. More than just caffeine: Psychopharmacology of methylxanthine interactions with plant-derived phytochemicals. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Papetti, A. An outlook on the role of decaffeinated coffee in neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2020, 60, 760–779. [Google Scholar] [CrossRef]
- Dong, X.; Li, S.; Sun, J.; Li, Y.; Zhang, D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients 2020, 12, 840. [Google Scholar] [CrossRef]
- Newman, M.; Kretzschmar, D.; Khan, I.; Chen, M.; Verdile, G.; Lardelli, M. Animal models of Alzheimer’s disease. In Animal Models for the Study of Human Disease, 2nd ed.; Michael Conn, P., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 1031–1085. [Google Scholar]
- Do Carmo, S.; Cuello, A.C. Modeling Alzheimer’s disease in transgenic rats. Mol. Neurodegener. 2013, 8, 37. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse models of Alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef]
- Echeverria, V.; Ducatenzeiler, A.; Alhonen, L.; Janne, J.; Grant, S.M.; Wandosell, F.; Muro, A.; Baralle, F.; Li, H.; Duff, K.; et al. Rat transgenic models with a phenotype of intracellular Aβ accumulation in hippocampus and cortex. J. Alzheimers Dis. 2004, 6, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Gulisano, W.; Palmeri, A.; Arancio, O. Rodent models for Alzheimer’s disease drug discovery. Expert Opin. Drug Discov. 2015, 10, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, R.; Yang, G.; Shi, Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl. Acad. Sci. USA 2017, 114, E476–E485. [Google Scholar] [CrossRef] [PubMed]
- Andorfer, C.; Kress, Y.; Espinoza, M.; de Silva, R.; Tucker, K.L.; Barde, Y.-A.; Duff, K.; Davies, P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003, 86, 582–590. [Google Scholar] [CrossRef]
- Van der Jeugd, A.; Vermaercke, B.; Derisbourg, M.; Lo, A.C.; Hamdane, M.; Blum, D.; Buée, L.; D’Hooge, R. Progressive age-related cognitive decline in tau mice. J. Alzheimers Dis. 2013, 37, 777–788. [Google Scholar] [CrossRef]
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Müller, C.E.; Buée, L.; et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging 2014, 35, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Zappettini, S.; Faivre, E.; Ghestem, A.; Carrier, S.; Buée, L.; Blum, D.; Esclapez, M.; Bernard, C. Caffeine consumption during pregnancy accelerates the development of cognitive deficits in offspring in a model of tauopathy. Front. Cell. Neurosci. 2019, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 289, 29–38. [Google Scholar] [CrossRef]
- Smith, I.F.; Hitt, B.; Green, K.N.; Oddo, S.; LaFerla, F.M. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neurochem. 2005, 94, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Cohen, R.M.; Rezai-Zadeh, K.; Weitz, T.M.; Rentsendorj, A.; Gate, D.; Spivak, I.; Bholat, Y.; Vasilevko, V.; Glabe, C.G.; Breunig, J.J.; et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. J. Neurosci. 2013, 33, 6245–6256. [Google Scholar] [CrossRef]
- Smith, L.A.; McMahon, L.L. Deficits in synaptic function occur at medial perforant path-dentate granule cell synapses prior to Schaffer collateral-CA1 pyramidal cell synapses in the novel TgF344-Alzheimer’s disease rat model. Neurobiol. Dis. 2018, 110, 166–179. [Google Scholar] [CrossRef]
- Lopez, E.M.; Bell, K.F.S.; Ribeiro-da-Silva, A.; Cuello, A.C. Early changes in neurons of the hippocampus and neocortex in transgenic rats expressing intracellular human a-β. J. Alzheimers Dis. 2004, 6, 421–431, discussion 443–449. [Google Scholar] [CrossRef]
- Van Dam, D.; De Deyn, P.P. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br. J. Pharmacol. 2011, 164, 1285–1300. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, F.; Xiu, C.; Zhang, J.; Li, Y. Hypericum perforatum extract attenuates behavioral, biochemical, and neurochemical abnormalities in Aluminum chloride-induced Alzheimer’s disease rats. Biomed. Pharmacother. 2017, 91, 931–937. [Google Scholar] [CrossRef]
- McDonald, M.P.; Overmier, J.B. Present imperfect: A critical review of animal models of the mnemonic impairments in Alzheimer’s disease. Neurosci. Biobehav. Rev. 1998, 22, 99–120. [Google Scholar] [CrossRef]
- Botton, P.H.; Costa, M.S.; Ardais, A.P.; Mioranzza, S.; Souza, D.O.; da Rocha, J.B.T.; Porciúncula, L.O. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav. Brain Res. 2010, 214, 254–259. [Google Scholar] [CrossRef]
- Nazem, A.; Sankowski, R.; Bacher, M.; Al-Abed, Y. Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and adenosine A(2a) receptor antagonists prevent β-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Salkovic-Petrisic, M.; Knezovic, A.; Hoyer, S.; Riederer, P. What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J. Neural Transm. 2013, 120, 233–252. [Google Scholar] [CrossRef]
- Ullah, F.; Ali, T.; Ullah, N.; Kim, M.O. Caffeine prevents d-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochem. Int. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Cunha, G.M.A.; Canas, P.M.; Melo, C.S.; Hockemeyer, J.; Müller, C.E.; Oliveira, C.R.; Cunha, R.A. Adenosine A2A receptor blockade prevents memory dysfunction caused by β-amyloid peptides but not by scopolamine or MK-801. Exp. Neurol. 2008, 210, 776–781. [Google Scholar] [CrossRef]
- Gulyaeva, N.V.; Bobkova, N.V.; Kolosova, N.G.; Samokhin, A.N.; Stepanichev, M.Y.; Stefanova, N.A. Molecular and cellular mechanisms of sporadic Alzheimer’s disease: Studies on rodent models in vivo. Biochemistry 2017, 82, 1088–1102. [Google Scholar] [CrossRef]
- Brothers, H.M.; Marchalant, Y.; Wenk, G.L. Caffeine attenuates lipopolysaccharide-induced neuroinflammation. Neurosci. Lett. 2010, 480, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine may abrogate LPS-induced oxidative stress and neuroinflammation by regulating Nrf2/TLR4 in adult mouse brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, R.; Wan Yaacob, W.M.; Othman, Z.; Long, I.; Ahmad, A.H.; Al-Rahbi, B. Lipopolysaccharide-induced memory impairment in rats: A model of Alzheimer’s disease. Physiol. Res. 2017, 66, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Muraleva, N.A.; Korbolina, E.E.; Kiseleva, E.; Maksimova, K.Y.; Kolosova, N.G. Amyloid accumulation is a late event in sporadic Alzheimer’s disease-like pathology in nontransgenic rats. Oncotarget 2015, 6, 1396–1413. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Kozhevnikova, O.S.; Vitovtov, A.O.; Maksimova, K.Y.; Logvinov, S.V.; Rudnitskaya, E.A.; Korbolina, E.E.; Muraleva, N.A.; Kolosova, N.G. Senescence-accelerated OXYS rats: A model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease. Cell Cycle 2014, 13, 898–909. [Google Scholar] [CrossRef]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef]
- Zeitlin, R.; Patel, S.; Burgess, S.; Arendash, G.W.; Echeverria, V. Caffeine induces beneficial changes in PKA signaling and JNK and ERK activities in the striatum and cortex of Alzheimer’s transgenic mice. Brain Res. 2011, 1417, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine reverses cognitive impairment and decreases brain amyloid-β levels in aged Alzheimer’s disease mice. J. Alzheimers Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Chang, W.-H.; Black, R.M.; Liu, J.-R.; Sompol, P.; Chen, Y.; Wei, H.; Zhao, Q.; Cheng, I.H. Crude caffeine reduces memory impairment and amyloid β(1–42) levels in an Alzheimer’s mouse model. Food Chem. 2012, 135, 2095–2102. [Google Scholar] [CrossRef]
- Han, K.; Jia, N.; Li, J.; Yang, L.; Min, L.-Q. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2013, 8, 737–740. [Google Scholar] [CrossRef]
- Dragicevic, N.; Delic, V.; Cao, C.; Copes, N.; Lin, X.; Mamcarz, M.; Wang, L.; Arendash, G.W.; Bradshaw, P.C. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer’s mice and cells. Neuropharmacology 2012, 63, 1368–1379. [Google Scholar] [CrossRef]
- Cao, C.; Cirrito, J.R.; Lin, X.; Wang, L.; Verges, D.K.; Dickson, A.; Mamcarz, M.; Zhang, C.; Mori, T.; Arendash, G.W.; et al. Caffeine suppresses β-amyloid levels in plasma and brain of Alzheimer’s transgenic mice. J. Alzheimers Dis. 2009, 17, 681–697. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Johansson, B.; Giménez-Llort, L. Long-term treatment with low-dose caffeine worsens BPSD-like profile in 3xTg-AD mice model of Alzheimer’s disease and affects mice with normal aging. Front. Pharmacol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, L.; Lin, X.; Mamcarz, M.; Zhang, C.; Bai, G.; Nong, J.; Sussman, S.; Arendash, G. Caffeine synergizes with another coffee component to increase plasma GCSF: Linkage to cognitive benefits in Alzheimer’s mice. J. Alzheimers Dis. 2011, 25, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Streffer, J.R.; David, D.; Schild, A.; Hoerndli, F.; Pennanen, L.; Kurosinski, P.; Chen, F. Transgenic animal models of Alzheimer’s disease and related disorders: Histopathology, behavior and therapy. Mol. Psychiatry 2004, 9, 664–683. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-F.; Chen, Y.; Brown, P.H.; Lyle, B.J.; Black, R.M.; Cheng, I.H.; Ou, B.; Prior, R.L. Bioactivities of crude caffeine: Antioxidant activity, cyclooxygenase-2 inhibition, and enhanced glucose uptake. Food Chem. 2012, 131, 564–568. [Google Scholar] [CrossRef]
- Laurent, C.; Burnouf, S.; Ferry, B.; Batalha, V.L.; Coelho, J.E.; Baqi, Y.; Malik, E.; Mariciniak, E.; Parrot, S.; Van der Jeugd, A.; et al. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol. Psychiatry 2016, 21, 97–107. [Google Scholar] [CrossRef]
- Canas, P.M.; Porciúncula, L.O.; Cunha, G.M.A.; Silva, C.G.; Machado, N.J.; Oliveira, J.M.A.; Oliveira, C.R.; Cunha, R.A. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. J. Neurosci. 2009, 29, 14741–14751. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O. Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J. Alzheimers Dis. 2013, 34, 509–518. [Google Scholar] [CrossRef]
- Ghoneim, F.M.; Khalaf, H.A.; Elsamanoudy, A.Z.; Abo El-Khair, S.M.; Helaly, A.M.N.; Mahmoud, E.-H.M.; Elshafey, S.H. Protective effect of chronic caffeine intake on gene expression of brain derived neurotrophic factor signaling and the immunoreactivity of glial fibrillary acidic protein and Ki-67 in Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2015, 8, 7710–7728. [Google Scholar]
- Hosny, E.N.; Sawie, H.G.; Elhadidy, M.E.; Khadrawy, Y.A. Evaluation of antioxidant and anti-inflammatory efficacy of caffeine in rat model of neurotoxicity. Nutr. Neurosci. 2019, 22, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, K.G.; Rosário, B. dos A.; Camarini, R.; Hernandes, M.S.; Britto, L.R. Intracerebroventricular streptozotocin as a model of Alzheimer’s disease: Neurochemical and behavioral characterization in mice. Neurotox. Res. 2017, 31, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.M.N.; Carvalho, R.A.; Cunha, R.A.; Gruetter, R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J. Neurochem. 2009, 111, 368–379. [Google Scholar] [CrossRef]
- Al Marshad, R.; Al Khatib, R.; Amer, H.; Al Shammari, M.; Al Otaibi, A.; Al Otaibi, F.; Behbehani, N.; Al Sayed, A.; Al Hoty, N.; Hassan, Z.; et al. Streptozotocin-induced diabetes mellitus affects the NMDA receptors: Role of caffeine administration in enhancing learning, memory and locomotor deficits. Int. J. Health Sci. 2018, 12, 10–17. [Google Scholar]
- Song, J. Animal model of Aluminum-induced Alzheimer’s disease. Adv. Exp. Med. Biol. 2018, 1091, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Kumar, H.; Cho, D.-Y.; Yun, Y.-S.; Choi, D.-K. Toxin-induced experimental models of learning and memory impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef]
- Riedel, W.; Hogervorst, E.; Leboux, R.; Verhey, F.; van Praag, H.; Jolles, J. Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology 1995, 122, 158–168. [Google Scholar] [CrossRef]
- Lee, H.E.; Jeon, S.J.; Ryu, B.; Park, S.J.; Ko, S.Y.; Lee, Y.; Kim, E.; Lee, S.; Kim, H.; Jang, D.S.; et al. Swertisin, a C-glucosylflavone, ameliorates scopolamine-induced memory impairment in mice with its adenosine A1 receptor antagonistic property. Behav. Brain Res. 2016, 306, 137–145. [Google Scholar] [CrossRef]
- Pagnussat, N.; Almeida, A.S.; Marques, D.M.; Nunes, F.; Chenet, G.C.; Botton, P.H.S.; Mioranzza, S.; Loss, C.M.; Cunha, R.A.; Porciúncula, L.O. Adenosine A(2A) receptors are necessary and sufficient to trigger memory impairment in adult mice. Br. J. Pharmacol. 2015, 172, 3831–3845. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ghribi, O.; Geiger, J.D. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s disease. J. Alzheimers Dis. 2010, 20, S127–S141. [Google Scholar] [CrossRef]
- Chen, X.; Gawryluk, J.W.; Wagener, J.F.; Ghribi, O.; Geiger, J.D. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J. Neuroinflamm. 2008, 5, 12. [Google Scholar] [CrossRef]
- Prasanthi, J.R.P.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef]
- Nia, B.V.; Kang, C.; Tran, M.G.; Lee, D.; Murakami, S. Meta analysis of human AlzGene database: Benefits and limitations of using C. elegans for the study of Alzheimer’s disease and co-morbid conditions. Front. Genet. 2017, 8, 55. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; de Paula Oliveira, R. Guarana (Paullinia cupana) extract protects Caenorhabditis elegans models for Alzheimer disease and Huntington disease through activation of antioxidant and protein degradation pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9241308. [Google Scholar] [CrossRef]
- Griffin, E.F.; Caldwell, K.A.; Caldwell, G.A. Genetic and pharmacological discovery for Alzheimer’s disease using Caenorhabditis elegans. ACS Chem. Neurosci. 2017, 8, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Dostal, V.; Roberts, C.M.; Link, C.D. Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of β-amyloid peptide toxicity. Genetics 2010, 186, 857–866. [Google Scholar] [CrossRef]
- Machado, M.L.; Arantes, L.P.; da Silveira, T.L.; Zamberlan, D.C.; Cordeiro, L.M.; Obetine, F.B.B.; da Silva, A.F.; da Cruz, I.B.M.; Soares, F.A.A.; de Oliveira, R.P. Ilex paraguariensis extract provides increased resistance against oxidative stress and protection against Amyloid beta-induced toxicity compared to caffeine in Caenorhabditis elegans. Nutr. Neurosci. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhou, L.; Jiao, Y.; Bai, S.; Wang, L.; Ma, J.; Fu, X. Ingredients in Zijuan Pu’er tea extract alleviate β-amyloid peptide toxicity in a Caenorhabditis elegans model of Alzheimer’s disease likely through DAF-16. Molecules 2019, 24, 729. [Google Scholar] [CrossRef]
- Goussakov, I.; Miller, M.B.; Stutzmann, G.E. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J. Neurosci. 2010, 30, 12128–12137. [Google Scholar] [CrossRef]
- Giunta, S.; Andriolo, V.; Castorina, A. Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminum chloride. Int. J. Biochem. Cell Biol. 2014, 54, 122–136. [Google Scholar] [CrossRef]
- Keshavarz, M.; Farrokhi, M.R.; Amiri, A. Caffeine neuroprotective mechanism against β-amyloid neurotoxicity in SHSY5Y cell line: Involvement of adenosine, ryanodine, and N-methyl-D-aspartate receptors. Adv. Pharm. Bull. 2017, 7, 579–584. [Google Scholar] [CrossRef]
- Stoppelkamp, S.; Bell, H.S.; Palacios-Filardo, J.; Shewan, D.A.; Riedel, G.; Platt, B. In vitro modelling of Alzheimer’s disease: Degeneration and cell death induced by viral delivery of amyloid and tau. Exp. Neurol. 2011, 229, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dasmahapatra, A.K. Caffeine destabilizes preformed Aβ protofilaments: Insights from all atom molecular dynamics simulations. Phys. Chem. Chem. Phys. 2019, 21, 22067–22080. [Google Scholar] [CrossRef]
- Sharma, B.; Paul, S. Action of caffeine as an amyloid inhibitor in the aggregation of Aβ16–22 peptides. J. Phys. Chem. B 2016, 120, 9019–9033. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mehta, V.; Raj, U.; Varadwaj, P.K.; Udayabanu, M.; Yennamalli, R.M.; Singh, T.R. Computational and in-vitro validation of natural molecules as potential acetylcholinesterase inhibitors and neuroprotective agents. Curr. Alzheimer Res. 2019, 16, 116–127. [Google Scholar] [CrossRef]
- Ali, B.; Jamal, Q.M.S.; Shams, S.; Al-Wabel, N.A.; Siddiqui, M.U.; Alzohairy, M.A.; Al Karaawi, M.A.; Kesari, K.K.; Mushtaq, G.; Kamal, M.A. In silico analysis of green tea polyphenols as inhibitors of AChE and BChE enzymes in Alzheimer’s disease treatment. Cns Neurol. Disord. Drug Targets 2016, 15, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Anastasiou, A.I.; Zachariou, V.; Pelidou, S.-H. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 2019, 7, 97. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Olonisola, O.E. Does caffeine influence the anticholinesterase and antioxidant properties of donepezil? Evidence from in vitro and in vivo studies. Metab. Brain Dis. 2017, 32, 629–639. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. Faseb J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Dall’lgna, O.P.; Porciúncula, L.O.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Neuroprotection by caffeine and adenosine A2A receptor blockade of β-amyloid neurotoxicity. Br. J. Pharmacol. 2003, 138, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Faivre, E.; Coelho, J.E.; Zornbach, K.; Malik, E.; Baqi, Y.; Schneider, M.; Cellai, L.; Carvalho, K.; Sebda, S.; Figeac, M.; et al. Beneficial effect of a selective adenosine A2A receptor antagonist in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Front. Mol. Neurosci. 2018, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.V.; Haberl, M.G.; Zhang, P.; Bethge, P.; Lemos, C.; Gonçalves, N.; Gorlewicz, A.; Malezieux, M.; Gonçalves, F.Q.; Grosjean, N.; et al. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat. Commun. 2016, 7, 11915. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Lemos, C.; Gonçalves, F.Q.; Pliássova, A.V.; Machado, N.J.; Silva, H.B.; Canas, P.M.; Cunha, R.A.; Lopes, J.P.; Agostinho, P. Blockade of adenosine A2A receptors recovers early deficits of memory and plasticity in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2018, 117, 72–81. [Google Scholar] [CrossRef]
- Cunha, R.A.; Agostinho, P.M. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimers Dis. 2010, 20, S95–S116. [Google Scholar] [CrossRef]
- Nabbi-Schroeter, D.; Elmenhorst, D.; Oskamp, A.; Laskowski, S.; Bauer, A.; Kroll, T. Effects of long-term caffeine consumption on the adenosine A1 receptor in the rat brain: An in vivo PET study with [18F]CPFPX. Mol. Imaging Biol. 2018, 20, 284–291. [Google Scholar] [CrossRef]
- Janitschke, D.; Nelke, C.; Lauer, A.A.; Regner, L.; Winkler, J.; Thiel, A.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Effect of caffeine and other methylxanthines on Aβ-homeostasis in SH-SY5Y cells. Biomolecules 2019, 9, 689. [Google Scholar] [CrossRef]
- Zhang, L.-F.; Zhou, Z.-W.; Wang, Z.-H.; Du, Y.-H.; He, Z.-X.; Cao, C.; Zhou, S.-F. Coffee and caffeine potentiate the antiamyloidogenic activity of melatonin via inhibition of Aβ oligomerization and modulation of the Tau-mediated pathway in N2a/APP cells. Drug Des. Devel. Ther. 2014, 9, 241–272. [Google Scholar] [CrossRef]
- Fukuyama, K.; Kakio, S.; Nakazawa, Y.; Kobata, K.; Funakoshi-Tago, M.; Suzuki, T.; Tamura, H. Roasted coffee reduces β-amyloid production by increasing proteasomal β-secretase degradation in human neuroblastoma SH-SY5Y cells. Mol. Nutr. Food Res. 2018, 62, e1800238. [Google Scholar] [CrossRef] [PubMed]
- Kakio, S.; Funakoshi-Tago, M.; Kobata, K.; Tamura, H. Coffee induces vascular endothelial growth factor (VEGF) expression in human neuroblastama SH-SY5Y cells. Nutr. Neurosci. 2017, 20, 336–342. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Ambrósio, A.F.; Santiago, A.R. Having a coffee break: The impact of caffeine consumption on microglia-mediated inflammation in neurodegenerative diseases. Mediat. Inflamm. 2017, 2017, 4761081. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Won, T.J.; Kim, H.R.; Lee, D.; Hwang, K.W.; Park, S.-Y. Protective effect of chlorogenic acid against Aβ-induced neurotoxicity. Biomol. Ther. 2011, 19, 181–186. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic acid alleviates Aβ25–35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des. Devel. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, J.; Hua, L.; Han, B.; Zhang, Y.; Yang, X.; Zeng, Z.; Bai, H.; Yin, H.; et al. Effects of caffeic acid on learning deficits in a model of Alzheimer’s disease. Int. J. Mol. Med. 2016, 38, 869–875. [Google Scholar] [CrossRef]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell. Mol. Life Sci. 2004, 61, 857–872. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Munafo, M.R. Mendelian randomization studies of coffee and caffeine consumption. Nutrients 2018, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, caffeine, and health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, F.; Muurlink, O.; Reid, N. Effects of caffeine on sleep quality and daytime functioning. Risk Manag. Healthc. Policy 2018, 11, 263–271. [Google Scholar] [CrossRef]
- Torres-Ugalde, Y.C.; Romero-Palencia, A.; Román-Gutiérrez, A.D.; Ojeda-Ramírez, D.; Guzmán-Saldaña, R.M.E. Caffeine consumption in children: Innocuous or deleterious? A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 2489. [Google Scholar] [CrossRef]
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 2017, 390, 2327–2329. [Google Scholar] [CrossRef]
- Palmqvist, S.; Insel, P.S.; Stomrud, E.; Janelidze, S.; Zetterberg, H.; Brix, B.; Eichenlaub, U.; Dage, J.L.; Chai, X.; Blennow, K.; et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol. Med. 2019, 11, e11170. [Google Scholar] [CrossRef]
- Iranpour, S.; Saadati, H.M.; Koohi, F.; Sabour, S. Association between caffeine intake and cognitive function in adults; effect modification by sex: Data from National Health and Nutrition Examination Survey (NHANES) 2013–2014. Clin. Nutr. 2020, 39, 2158–2168. [Google Scholar] [CrossRef]
- Ritchie, K.; Carrière, I.; de Mendonça, A.; Portet, F.; Dartigues, J.F.; Rouaud, O.; Barberger-Gateau, P.; Ancelin, M.L. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology 2007, 69, 536–545. [Google Scholar] [CrossRef]
- Johnson-Kozlow, M.; Kritz-Silverstein, D.; Barrett-Connor, E.; Morton, D. Coffee consumption and cognitive function among older adults. Am. J. Epidemiol. 2002, 156, 842–850. [Google Scholar] [CrossRef]
| Model | Caffeine Dose and Study Conditions | Aim | Effects | Reference |
|---|---|---|---|---|
| APPswe (K670N/ M671L) male and female mice | Caffeine: 0.3 g/L p.o. in drinking water for 5.5 months; start: 4-month-old mice, 8-month-old at the start of behavioral tests, 9.5-month-old at the end of behavioral tests | To investigate the possible neuroprotective effects of long-term dietary caffeine intake in APPswe transgenic mice. | Caffeine protected against cognitive impairment. It reduced Aβ levels in the hippocampus, restored brain adenosine levels, but did not affect the A1R and A2AR hippocampal density and expression in the cerebral cortex and hippocampus. | [129] |
| APPswe (K670N/ M671L) mice | Caffeine: 1.5 mg p.o. for 2 weeks, every 12 h; start: 9.5-month-old mice | To investigate the effects of caffeine on the signal transduction pathways (PKA, CREB, JNK and ERK) in cognitively important areas of the brain. | Caffeine showed beneficial effects in the brain function and exerted neuroprotective and antiapoptotic effect by stimulating PKA activity, increasing the level of phosphorylated CREB and decreasing JNK and ERK phosphorylation. | [128] |
| APPswe (K670N/ M671L) mice | Caffeine: 0.6 mg/day in drinking water for 1 month; start: 11- to 12-month-old mice | To explain the protective mechanism of caffeine and melatonin administration against cognitive dysfunction in transgenic APPswe mice. | Caffeine and melatonin prevented cognitive impairment. Caffeine slightly increased mitochondrial functions, however it inhibited the enhancement of mitochondrial functions provided by melatonin. | [133] |
| APPswe (K670N/ M671L) and Indiana (V717F) mutation male mice | 0.0395% crude caffeine (95.95% caffeine, 1.1% moisture, 1.04% fat, 0.1% ash) in diet or 0.0375% pure caffeine in diet for 2 months; start: 3-month-old mice | To investigate the effects of consumption of crude and pure caffeine on the learning and memory processes in transgenic AD mice. | Crude and pure caffeine administered for two months partly prevented memory deficits (crude caffeine exerted greater effect). Crude caffeine (but not pure) reduced Aβ1-42 levels, suppressed Aβ accumulation and reduced the number of Aβ plaques in the hippocampus. Both prevented Aβ-induced neuronal cell death and exerted antiapoptotic activity suppressing caspase-3 activity. Antioxidant and anti-inflammatory effects of crude caffeine were also demonstrated in APPswe mice. | [131] |
| APPswe mice and APPswe/PS1 mice | Caffeine: 1.5 mg i.p., single administration, 3- to 4-month-old APPswe mice; caffeine: 1.5 mg i.p. or p.o., single administration, 14-month-old APPswe mice; caffeine: 1.5 mg i.p., single administration, 14-month-old APPswe/PS1 mice; caffeine: 1.5 mg p.o. twice-daily for 7 days, 15- to 20-month-old APPswe/PS1 mice; caffeine: 1.5 mg p.o., two administrations on one day every 4th day for 2 months, 20-month-old APPswe/PS1 mice; | To investigate the effects of acute and long-term caffeine administration on the cognitive performance and Aβ levels in APPswe and APPswe/PS1 transgenic mice. | Long-term caffeine intake improved cognitive functions in APPswe and APPswe/PS1 mice. Decreased Aβ levels in the plasma were observed after single administration of caffeine and long-term caffeine treatments in both transgenic mice models. Chronic caffeine treatment reduced soluble Aβ level in the cortex and hippocampus and insoluble Aβ level in the hippocampus in APPswe mice. Acute caffeine administration rapidly reduced the Aβ level also in the interstitial fluid in the hippocampus but did not affect Aβ elimination in APPswe mice. | [134] |
| APPswe/PS1 mice and APPswe mice, control non-transgenic mice | Caffeine: 0.3 mg (unconcentrated coffee) i.p., single administration or 1.5 mg (concentrated coffee) i.p., single administration or 0.06 mg (concentrated decaffeinated coffee) i.p., single administration, 6- to 8-month-old APPswe/PS1 transgenic mice; caffeine: 0.75 mg (concentrated coffee) p.o., twice weekly for 3 months or 0.03 mg (decaffeinated coffee) p.o., twice weekly for 3 months, 10-month-old APPswe transgenic mice | To investigate the effects of acute and long-term treatment with coffee or decaffeinated coffee (to compare the effect of caffeine to coffee) on the plasma cytokine and Aβ levels and behavior (only after long-term treatment) in transgenic mice models of AD. | Acute caffeine intake increased the level of G-CSF and IL-10 in plasma (concentrated and unconcentrated coffee) and IL-6 level in plasma (only concentrated coffee). Higher plasma caffeine concentrations were related to lower levels of Aβ in plasma. Single treatment with coffee (concentrated and unconcentrated) increased G-CSF, IL-6 and IL-10 plasma levels also in non-transgenic mice. Long-term treatment with concentrated coffee (but not decaffeinated coffee) favorably affected cognitive interference task and elevated level of G-CSF (but not other cytokines) in plasma in transgenic mice. Improvement of cognitive performance was associated with higher G-CSF levels suggesting that elevated G-CSF levels may be associated with possible beneficial effects of coffee against AD. | [136] |
| APPswe/PS1 mice | Caffeine: 0.75 mg/day or 1.5 mg/day p.o. for 8 weeks; 12-month-old mice | To investigate the effects of caffeine intake on the memory deficits, BDNF and TrkB expression in APPswe/PS1 double transgenic mice. | Caffeine at both doses used increased spatial learning ability and memory capability. Caffeine treatment increased the expression of hippocampal BDNF and TrkB. Caffeine exerted protective role against memory impairment in APPswe/PS1 mice. | [132] |
| THY-Tau22 male mice | Caffeine: 0.3 g/L p.o. in drinking water for 10 months; start: 2-month-old mice | To investigate the effects of chronic caffeine intake on the development of hippocampal tau protein pathologies and spatial memory disorders in THY-Tau22 transgenic mice. | Chronic caffeine intake prevented spatial memory deficits and improved memory performance. Caffeine effect was associated with a reduction of neuroinflammation and decrease in the hippocampal level of hyperphosphorylated tau protein. Caffeine treatment decreased oxidative stress (reduced expression of MnSOD and EAAT3) in THY-Tau22 mice. | [104] |
| THY-Tau22 female mice | Caffeine: 0.3 g/L p.o. in drinking water; start of caffeine administration: 2 weeks before mating; end of caffeine administration: 15th postnatal day; 8- or 12-month-old at the start of behavioral tests. | To investigate the effects of long-term caffeine exposure during pregnancy in offspring in THY-Tau22 transgenic mice. | The exposure to caffeine during pregnancy induced physiological disorders and accelerated cognitive disorders in THY-Tau22 transgenic mice model and may be a risk factor for early stages of AD. | [105] |
| 3xTg (APPswe, PS1/M146V and tau P301L) male mice, control non-transgenic mice | Caffeine: 0.3 mg/mL in drinking water p.o. for 7 months; start: 6-month-old mice; behavioral testing at 13 months of age. | To investigate the effects of long-term caffeine administration on the memory and learning in 3xTg-AD mice with behavioral and psychological symptoms of dementia (BPSD) profile. | Caffeine increased motor activity, total horizontal activity and emotionality in the behavioral tests in non-transgenic mice and reduced it in 3xTg-AD mice. Caffeine administration increased spontaneous motor activity (to a greater extent at night) only in 3xTg-AD mice. Results indicate that aggravation of BPSD-like behaviors, anxiety-related behaviors or neophobia adversely affected possible beneficial effects of caffeine treatment (improvement of memory and learning) in 3xTg-AD mice. | [135] |
| Model | Caffeine Dose and Study Conditions | Aim | Effects | Reference |
|---|---|---|---|---|
| Adult CF1 male mice administered with Aβ25–35 i.c.v. (3 nmol; volume: 3 μL) | Caffeine: 1 mg/mL p.o. in drinking water (22 mg/kg/day) for 12 days, Aβ i.c.v. on 7th day; caffeine: 30 or 80 mg/kg i.p., single administration, 30 min before Aβ i.c.v. caffeine: 30 mg/kg for 4 days, Aβ i.c.v. after 2 days of caffeine intake; caffeine: 1 mg/mL p.o. in drinking water (for 12 days) and 30 mg/kg i.p., single administration, 30 min before Aβ i.c.v.; behavioral tests were performed 8–9 days after Aβ i.c.v. administration. | To investigate the effects of caffeine and a selective A2AR antagonist against cognitive impairment in AD induced by i.c.v. Aβ25–35 administration in mice. | Blockade of A2AR by caffeine or by a selective A2AR antagonist prevented cognitive impairment, neurodegeneration and brain destruction in Aβ-induced AD mice model. | [117] |
| Adult male Sprague-Dawley rats with accelerated aging induced by d-galactose administration (120 mg/kg i.p. for 60 days) | Caffeine: 3 mg/kg/day i.p. for 60 days (during d-galactose treatment period) | To investigate the effects of chronic caffeine intake on neurodegeneration induced by d-galactose-aging rat model. | Chronic caffeine intake reduced oxidative stress (decreased 8-oxoguanine level), neuroinflammation, neuronal cells apoptosis, neurodegeneration, synaptic dysfunction and memory deficits induced by d-galactose injections. | [119] |
| Adult male Wistar rats with AD induced by i.c.v. administration of STZ (3 mg/kg; single bilateral administration) | Caffeine: 1 g/L in drinking water for 2 weeks before and 4 weeks after STZ administration. Behavioral tests 4 weeks after STZ administration | To investigate the effects of caffeine intake on the expression and density of adenosine receptors and hippocampal neurodegeneration in STZ-induced rat model of AD. | Caffeine administration prevented the STZ-induced memory deficits, sporadic dementia, neurodegeneration and decreased expression and density of A2AR in the hippocampus. | [141] |
| Adult male Sprague-Dawley rats treated with AlCl3 (17 mg/kg p.o. for 4 weeks) | Caffeine 1.5 mg/kg p.o. for 4 weeks concurrently with AlCl3 or two weeks before the start and for 4 weeks during AlCl3 administration | To investigate the effects of caffeine on the histological picture of hippocampus, expression of BDNF, TrkB, and immunoreactivity of Ki-67 and GFAP in the hippocampus in rats with AlCl3-induced AD. | Caffeine intake exerted neuroprotective activity (improvement of histological hippocampus picture, stronger Ki-67 and weaker GFAP immunoreactivity, increased BDNF and TrkB gene expression). The effects were stronger in rats treated with caffeine also before the start of AlCl3 administration. | [142] |
| Adult male Wistar rats treated with AlCl3 (100 mg/kg p.o. for 30 days) | Caffeine: 20 mg/kg i.p. for 30 days, 1 h before AlCl3 p.o. intake | To evaluate an antioxidant, anti-inflammatory and anticholinesterase properties of caffeine against AlCl3-induced neurotoxicity in rats. | Caffeine exerted neuroprotective, antioxidant and anticholinesterase activity against AlCl3-induced neurotoxicity in rats. It reduced oxidative stress parameters (NO level), decreased AChE and Na+/K+-ATPase activity in the cerebral cortex and hippocampus (Na+/K+-ATPase activity also in the striatum). Caffeine revealed also anti-inflammatory properties by reducing the increased TNF-α levels in the hippocampus and striatum associated with AlCl3-induced neurotoxicity. | [143] |
| Fisher-344 young male rats (3-month-old) treated with LPS (0.250 µg/h i.c.v. by osmotic minipump for 4 weeks); Fisher-344 male aged rats (24-month-old) | Caffeine: 0.5, 5, 10, 20 or 40 mg/kg/day i.p. for 2 or 4 weeks to young rats; caffeine: 40 mg/kg/day i.p. for 2 weeks to aged rats | To investigate the effects of different caffeine doses in LPS-induced neuroinflammation in young rats and in age-related neuroinflammation in aged rats (with naturally increased level of microglia activation). | Caffeine exerted potential protective effect against LPS-induced neuroinflammation. It was demonstrated that caffeine may decrease neuroinflammation by a reduction in the number of activated microglial cells in the hippocampus and through regulation of glutamate neurotransmission. | [122] |
| C57BL/6N male mice treated with LPS (250 µg/kg i.p. for 2 weeks; 7 doses in 2 weeks) | Caffeine: 30 mg/kg/day i.p. for 6 weeks | To investigate the effect of caffeine administration against LPS-induced oxidative stress, neuroinflammation, apoptotic cell death, neurodegeneration and synaptic impairment in mice. | Caffeine reduced LPS-induced oxidative stress, neuroinflammation and synaptic dysfunctions (increased expression of Nrf2, HO-1 and Bcl-2, reduced expression of TLR-4, p-NF-κB, p-JNK, BAX, caspase-3, TNF-α, COX-2, NOS-2 and synaptic markers) in mouse brains. | [123] |
| CF1 male mice (3-4-month-old) with cholinergic blockade induced by a single scopolamine hydrobromide administration (2 mg/kg i.p.) | Caffeine: 10 mg/kg i.p. for 4 days before scopolamine hydrobromide administration; scopolamine administration: 15 min before or immediately after the inhibitory avoidance test, immediately after training session in the novel object recognition task, 90 min before the open field test; independent group of mice for each test | To investigate the effect of caffeine intake on short-term and long-term memory impairment induced by a single scopolamine administration, assessed in three behavioral tests. | Pretreatment with caffeine prevented scopolamine-induced impairment in the acquisition phase when short-term memory was assessed in the inhibitory avoidance task, and in the consolidation phase when short-term and long-term memory were assessed in the inhibitory avoidance task. Caffeine administration prevented scopolamine-induced short-term and long-term memory deficits (assessed in the novel object recognition task). Caffeine exerted beneficial effect in the cholinergic-induced memory disruption. There was no effect of caffeine treatment on the spontaneous locomotor activity assessed in the open field test. | [115] |
| Model | Caffeine Dose and Study Conditions | Aim | Effects | Reference |
|---|---|---|---|---|
| New Zealand white rabbits (1.5- to 2-years-old) fed a 2% cholesterol-enriched diet | Caffeine: 3 mg/day in 50 mL of drinking water for 12 weeks | To investigate the effects of caffeine on BBB leakage in rabbits fed a cholesterol-enriched diet as a model of sporadic AD. | Chronic caffeine administration prevented dysfunction of BBB, decreased activation of astrocytes and decreased density of microglia induced by high cholesterol diet in rabbits. | [155] |
| New Zealand white male rabbits (1.5- to 2-years-old) fed a 2% cholesterol-enriched diet | Caffeine: 0.5 mg/day or 30 mg/day in drinking water for 12 weeks | To investigate the effects of caffeine treatment on molecular mechanisms of AD-like pathology in the cholesterol-fed rabbit model of AD. | Chronic caffeine intake decreased Aβ accumulation in the hippocampus and reduced hyperphosphorylated tau protein level in the hippocampus (reduced phosphorylated GSK-3β enzyme level). Caffeine prevented oxidative damage (reduced ROS generation and H2O2 production, increased GSH/GSSG ratio) and restored the level of A1R, reduced by cholesterol in rabbits. Caffeine did not affect the level of A2AR and RyRs and the cholesterol concentration in plasma. | [156] |
| Model | Caffeine Dose and Study Conditions | Aim | Effects | Reference |
|---|---|---|---|---|
| Caenorhabditis elegans strains: wild-type N2, CL2006 dvIs2 | Ilex paraguariensis hydroalcoholic extract (IPHE): 2 or 4 mg/mL (41 or 87 μM caffeine); caffeine: 200 or 400 μM. Treatment since first larval stage till required age for test. | To investigate the effects of IPHE and caffeine administration on the Aβ-induced toxicity in Caenorhabditis elegans. | IPHE and caffeine extended lifespan of worms, decreased AChE activity and reduced Aβ deposition and toxicity leading to worms’ paralysis. IPHE and caffeine reduced also Aβ mRNA levels and increased expression of hsp-16.2 (chaperone protein which overexpression causes suppression of Aβ-toxicity). After both treatments an antioxidant activity (reduced ROS levels) was observed. | [161] |
| Various Caenorhabditis elegans strains | 10% coffee extract (3.6 mM caffeine); | To investigate the effects of coffee extract treatment on the Aβ-induced toxicity in Caenorhabditis elegans. | Coffee extract prevented Aβ-induced toxicity in the transgenic models of AD in Caenorhabditis elegans. It induced also a delay in the paralysis progression in worms. No reduction in Aβ expression, Aβ aggregation and distribution was observed in coffee-treated group. The beneficial effect of coffee may result from skn-1/Nrf2 pathway induction. | [160] |
| Caenorhabditis elegans strains: wild-type N2, CL4176 dvls27, TJ356 zIs356, CF1553 muIs84 | 0.1, 0.2 and 0.4 mg/mL Zijuan Pu’er tea water extract (ZTWE) containing: (+)-catechins, caffeine, procyanidins. Mixture of three main constituents in ZTWE: (+)-catechins, caffeine, procyanidins—MCCP. | To investigate the effects of ZTWE and MCPP on the Aβ-induced toxicity in various Caenorhabditis elegans strains. | ZTWE and MCCP delayed Aβ-induced paralysis in worms. MCCP alleviated AD progression and pathologies related to AD due to reduced Aβ-induced toxicity (decreased Aβ aggregation), and increased antioxidant activity (activated DAF-16 signaling pathway associated with oxidative stress resistance; decreased ROS production). | [162] |
| Caenorhabditis elegans strains: wild-type N2, CL4176 dvls27, CL2006, AM141, HA759, rtIs11, TJ375, CL2166 | Guarana hydroalcoholic extract (GHE) containing: caffeine: 166.1 μg/mL, theobromine: 2.5 μg/mL, catechin: 34.6 μg/mL, epicatechin: 36.3 μg/mL. 10 or 50 mg/mL GHE | To investigate the effects of GHE treatment in Caenorhabditis elegans models of AD. | GHE prevented Aβ-induced toxicity in the transgenic models of AD in Caenorhabditis elegans. GHE delayed paralysis in nematodes, reduced ROS level and activated protein degradation. DAF-16 and skn-1 are responsible for the beneficial effect against Aβ-induced toxicity. | [158] |
| Model | Caffeine Dose and Study Conditions | Aim | Effects | Reference |
|---|---|---|---|---|
| Primary cortical neurons from 12- to 17-day-old cultures from 3xTg (APPswe, PS1/M146V KI and tau P301L) mouse embryos | Caffeine: 25 mM | To investigate the effects of caffeine treatment on disorders in Ca2+ homeostasis in the primary cortical neurons obtained from 3xTg-AD mice, assessed by microfluorimetric measurements of Ca2+ concentration. | Caffeine treatment increased Ca2+ content in the cortical neurons of 3xTg-AD mice. Caffeine increased release of Ca2+ from RyR-sensitive Ca2+ stores. Enhanced Ca2+ response to the caffeine was probably associated with an increased expression of RyRs in the cortical neurons. | [107] |
| Prefrontal cortex brain slices from 3xTg (APPswe, PS1/M146V KI, and tau P130L) mice (1- to 3-month-old) | Caffeine: 10 mM | To investigate the relationship between Ca2+ influx by NMDA receptors and RyR activation in 3xTg-AD mice. | Caffeine stimulated RyRs increasing synaptic excitability. RyR and NMDA receptor activation increased Ca2+ release in 3xTg-AD mice. | [163] |
| Human neuroblastoma SH-SY5Y cells treated with 2 μM Aβ25–35 or 10 μM AlCl3 or combined | Caffeine: 10 μM; caffeine: 1-100 μM in the cell viability test | To investigate the role of A1R and A2AR in the neuroprotective activity of caffeine in the AlCl3- and Aβ25–35-induced models of AD in human SH-SY5Y neuroblastoma cells. | Neuroprotective effect of caffeine observed in the combined AlCl3- and Aβ25–35 -induced model of neurotoxicity required a dual antagonism of A1R and A2AR (probably due to combined involvement in the restoration of Ca2+ homeostasis). Caffeine prevented neuronal cell death and exerted antioxidant activity (reduced ROS production, increased SOD activity and decreased MDA concentration). | [164] |
| Human neuroblastoma SH-SY5Y cells treated with 20 μM Aβ25–35 | Caffeine: 0.6 or 1 mM | To investigate the mechanism of neuroprotective activity of caffeine against Aβ-induced neurotoxicity in the human neuroblastoma SH-SY5Y cells. | Caffeine prevented Aβ-induced toxicity in neuronal cells, which probably resulted from the blockade of adenosine receptors (A1 and A2A), blockade of NMDA receptors and activation of RyRs. | [165] |
| Primary hippocampal neurons from 2- to 5-day-old Sprague-Dawley rats or dorsal root ganglion (DRG) from 1- to 4-day-old Sprague-Dawley rats; both transfected with human APP fused to EGFP or mutant h-tau protein fused to DsRed2 | Caffeine: 50 μM | To investigate the effects of caffeine and various drugs treatment after viral delivery of mutated APP and h-tau protein in the primary hippocampal cells and DRG cells on the degeneration and neuronal cell death. | Experiments demonstrated that delivering mutated APP and h-tau protein accelerated neuronal cell death and morphological damage. Caffeine administration ameliorated APP-induced and tau-induced neuronal damage. Caffeine treatment exerted neuroprotective effects in APP-induced and tau-induced models (prevented morphological damage in both models, increasing the number of healthy neurons). | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Londzin, P.; Zamora, M.; Kąkol, B.; Taborek, A.; Folwarczna, J. Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies. Nutrients 2021, 13, 537. https://doi.org/10.3390/nu13020537
Londzin P, Zamora M, Kąkol B, Taborek A, Folwarczna J. Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies. Nutrients. 2021; 13(2):537. https://doi.org/10.3390/nu13020537
Chicago/Turabian StyleLondzin, Piotr, Milena Zamora, Beata Kąkol, Aleksandra Taborek, and Joanna Folwarczna. 2021. "Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies" Nutrients 13, no. 2: 537. https://doi.org/10.3390/nu13020537
APA StyleLondzin, P., Zamora, M., Kąkol, B., Taborek, A., & Folwarczna, J. (2021). Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies. Nutrients, 13(2), 537. https://doi.org/10.3390/nu13020537






