Mediterranean Diet, Screen-Time-Based Sedentary Behavior and Their Interaction Effect on Adiposity in European Adolescents: The HELENA Study
Abstract
1. Introduction
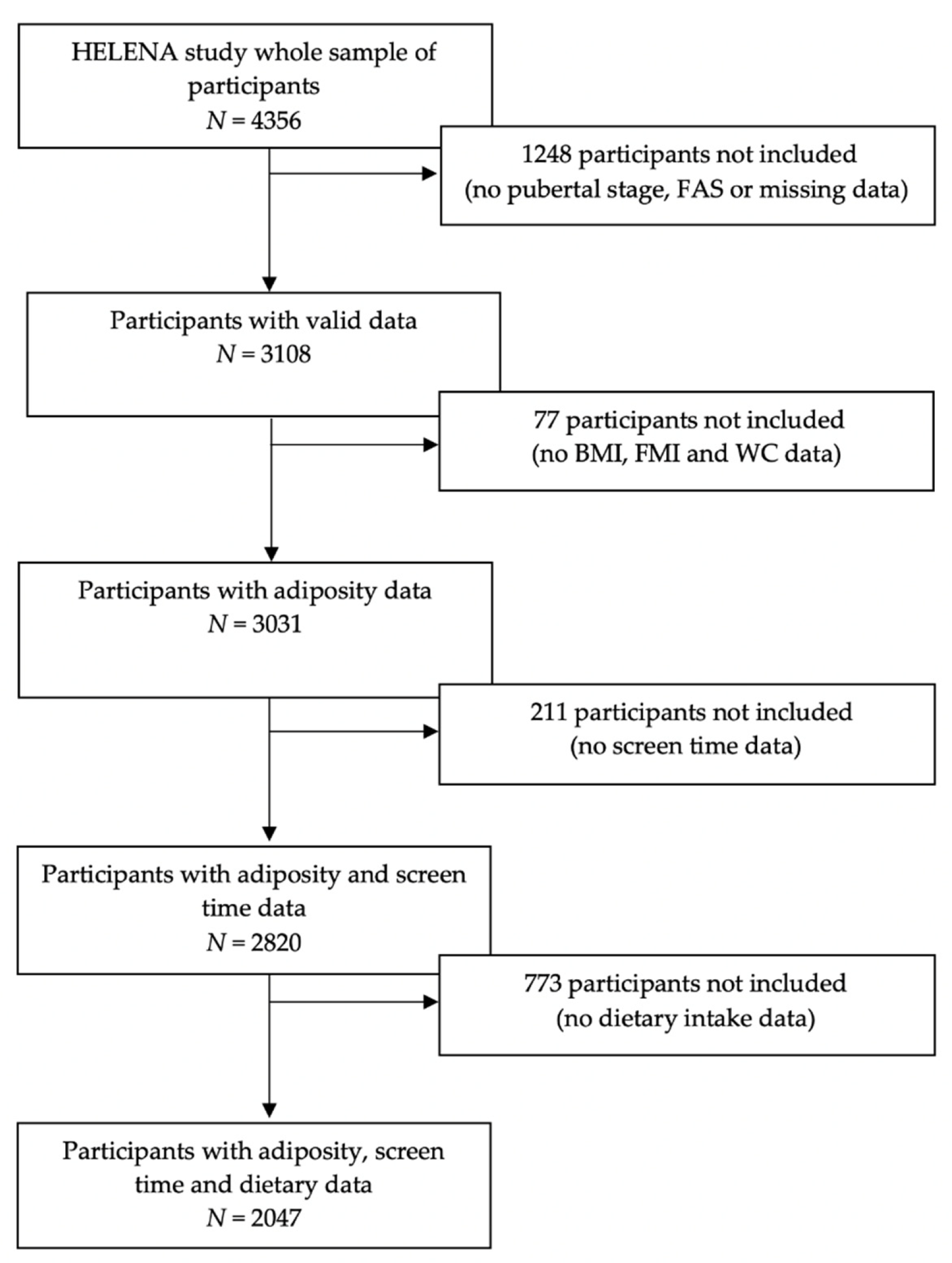
2. Materials and Methods
2.1. Study Design and Population
2.2. Physical Examination and Adiposity Measurements
2.3. Dietary Intake and Mediterranean Diet Score (MDS) Assessment
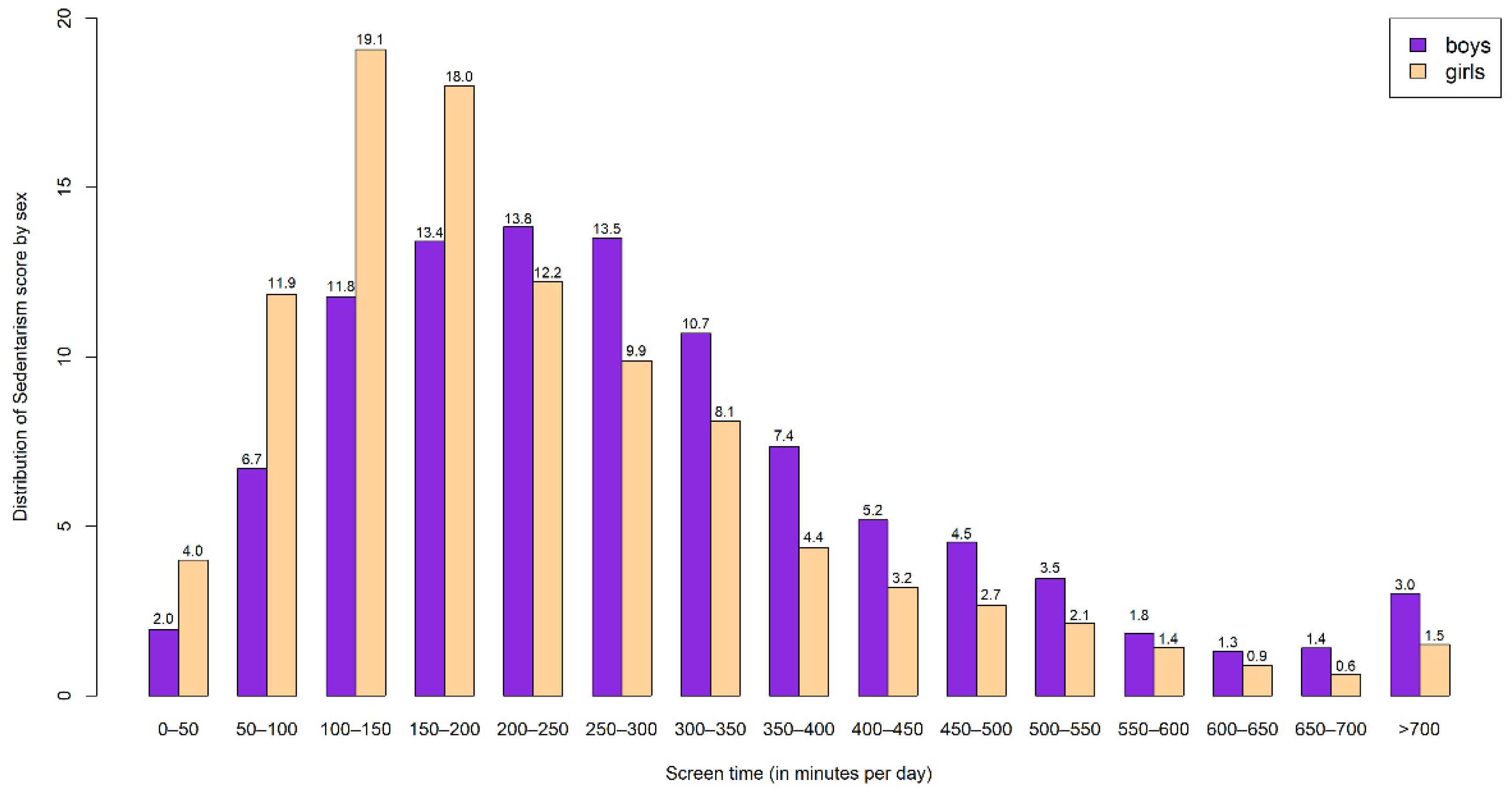
2.4. Screen-Time-Based Sedentary Behavior Assessment
2.5. Socioeconomic Status
2.6. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Study Sample
3.2. Association between MD Adherence and Screen-Time-Based Sedentary Behaviors
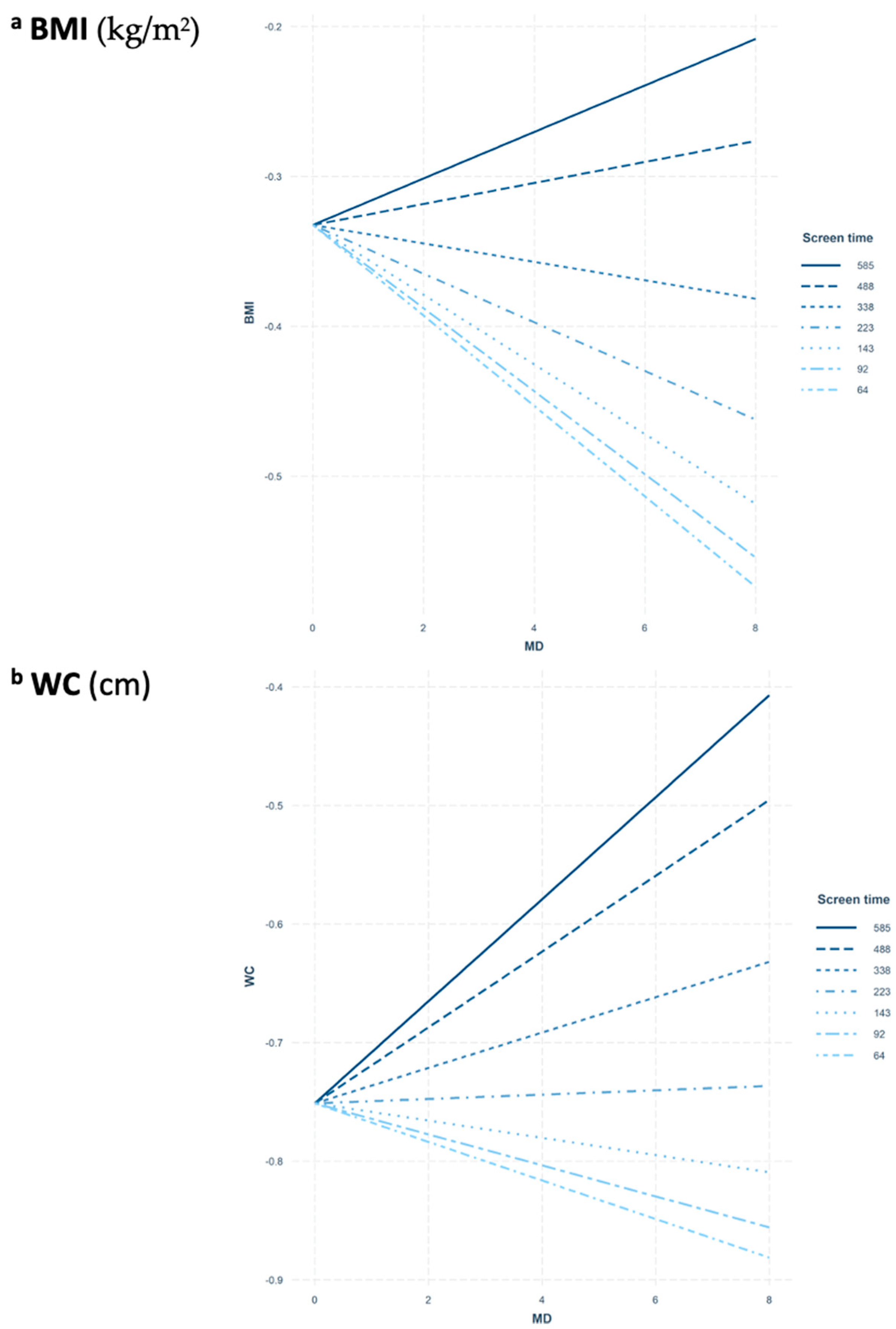
3.3. Interaction between MD Adherence and Screen-Time-Based Sedentary Behaviors on Adiposity
3.4. Sensitivity Analysis for Screen-Time-Based Sedentary Behaviors Plausible Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Co-ordinator: Luis A. Moreno.
- Core Group members: Luis A. Moreno, Fréderic Gottrand, Stefaan De Henauw, Marcela González-Gross, Chantal Gilbert.
- Steering Committee: Anthony Kafatos (President), Luis A. Moreno, Christian Libersa, Stefaan De Henauw, Sara Castelló, Fréderic Gottrand, Mathilde Kersting, Michael Sjöstrom, Dénes Molnár, Marcela González-Gross, Jean Dallongeville, Chantal Gilbert, Gunnar Hall, Lea Maes, Luca Scalfi.
- Project Manager: Pilar Meléndez.
- Universidad de Zaragoza (Spain)Luis A. Moreno, Jose A. Casajús, Jesús Fleta, Gerardo Rodríguez, Concepción Tomás, María I. Mesana, Germán Vicente-Rodríguez, Adoración Villarroya, Carlos M. Gil, Ignacio Ara, Juan Fernández Alvira, Gloria Bueno, Olga Bueno, Juan F. León, Jesús Mª Garagorri, Idoia Labayen, Iris Iglesia, Silvia Bel, Luis A. Gracia Marco, Theodora Mouratidou, Alba Santaliestra-Pasías, Iris Iglesia, Esther González-Gil, Pilar De Miguel-Etayo, Cristina Julián, Mary Miguel-Berges, Isabel Iguacel, Azahara Ruperez and Miguel Seral-Cortes.
- Consejo Superior de Investigaciones Científicas (Spain)Ascensión Marcos, Julia Wärnberg, Esther Nova, Sonia Gómez, Ligia Esperanza Díaz, Javier Romeo, Ana Veses, Belén Zapatera, Tamara Pozo, David Martínez.
- Université de Lille 2 (France).Laurent Beghin, Christian Libersa, Frédéric Gottrand, Catalina Iliescu, Juliana Von Berlepsch.
- Research Institute of Child Nutrition Dortmund, Rheinische Friedrich-Wilhelms-Universität Bonn (Germany)Mathilde Kersting, Wolfgang Sichert-Hellert, Ellen Koeppen.
- Pécsi Tudományegyetem (University of Pécs) (Hungary) Dénes Molnar, Eva Erhardt, Katalin Csernus, Katalin Török, Szilvia Bokor, Mrs. Angster, Enikö Nagy, Orsolya Kovács, Judit Répasi.
- University of Crete School of Medicine (Greece)Anthony Kafatos, Caroline Codrington, María Plada, Angeliki Papadaki, Katerina Sarri, Anna Viskadourou, Christos Hatzis, Michael Kiriakakis, George Tsibinos, Constantine Vardavas, Manolis Sbokos, Eva Protoyeraki, Maria Fasoulaki.
- Institut für Ernährungs- und Lebensmittelwissenschaften – Ernährungphysiologie. Rheinische Friedrich Wilhelms Universität (Germany)Peter Stehle, Klaus Pietrzik, Marcela González-Gross, Christina Breidenassel, Andre Spinneker, Jasmin Al-Tahan, Miriam Segoviano, Anke Berchtold, Christine Bierschbach, Erika Blatzheim, Adelheid Schuch, Petra Pickert.
- University of Granada (Spain)Manuel J. Castillo, Ángel Gutiérrez, Francisco B Ortega, Jonatan R Ruiz, Enrique G Artero, Vanesa España, David Jiménez-Pavón, Palma Chillón, Cristóbal Sánchez-Muñoz, Magdalena Cuenca.
- Istituto Nazionalen di Ricerca per gli Alimenti e la Nutrizione (Italy)Davide Arcella, Elena Azzini, Emma Barrison, Noemi Bevilacqua, Pasquale Buonocore, Giovina Catasta, Laura Censi, Donatella Ciarapica, Paola D’Acapito, Marika Ferrari, Myriam Galfo, Cinzia Le Donne, Catherine Leclercq, Giuseppe Maiani, Beatrice Mauro, Lorenza Mistura, Antonella Pasquali, Raffaela Piccinelli, Angela Polito, Romana Roccaldo, Raffaella Spada, Stefania Sette, Maria Zaccaria.
- University of Napoli "Federico II" Dept of Food Science (Italy)Luca Scalfi, Paola Vitaglione, Concetta Montagnese.
- Ghent University (Belgium)Ilse De Bourdeaudhuij, Stefaan De Henauw, Tineke De Vriendt, Lea Maes, Christophe Matthys, Carine Vereecken, Mieke de Maeyer, Charlene Ottevaere, Inge Huybrechts.
- Medical University of Vienna (Austria)Kurt Widhalm, Katharina Phillipp, Sabine Dietrich, Birgit KubelkaMarion Boriss-Riedl.
- Harokopio University (Greece)Yannis Manios, Eva Grammatikaki, Zoi Bouloubasi, Tina Louisa Cook, Sofia Eleutheriou, Orsalia Consta, George Moschonis, Ioanna Katsaroli, George Kraniou, Stalo Papoutsou, Despoina Keke, Ioanna Petraki, Elena Bellou, Sofia Tanagra, Kostalenia Kallianoti, Dionysia Argyropoulou, Stamatoula Tsikrika, Christos Karaiskos.
- Institut Pasteur de Lille (France)Jean Dallongeville, Aline Meirhaeghe.
- Karolinska Institutet (Sweden) Michael Sjöstrom, Jonatan R Ruiz, Francisco B. Ortega, María Hagströmer, Anita Hurtig Wennlöf, Lena Hallström, Emma Patterson, Lydia Kwak, Julia Wärnberg, Nico Rizzo.
- Asociación de Investigación de la Industria Agroalimentaria (Spain) Jackie Sánchez-Molero, Sara Castelló, Elena Picó, Maite Navarro, Blanca Viadel, José Enrique Carreres, Gema Merino, Rosa Sanjuán, María Lorente, María José Sánchez.
- Campden BRI (United Kingdom)Chantal Gilbert, Sarah Thomas, Elaine Allchurch, Peter Burgess.
- SIK - Institutet foer Livsmedel och Bioteknik (Sweden)Gunnar Hall, Annika Astrom, Anna Sverkén, Agneta Broberg.
- Meurice Recherche & Development asbl (Belgium)Annick Masson, Claire Lehoux, Pascal Brabant, Philippe Pate, Laurence Fontaine.
- Campden & Chorleywood Food Development Institute (Hungary)Andras Sebok, Tunde Kuti, Adrienn Hegyi.
- Productos Aditivos SA (Spain)Cristina Maldonado, Ana Llorente.
- Cárnicas Serrano SL (Spain)Emilio García.
- Cederroth International AB (Sweden)Holger von Fircks, Marianne Lilja Hallberg, Maria Messerer.
- Lantmännen Food R&D (Sweden)Mats Larsson, Helena Fredriksson, Viola Adamsson, Ingmar Börjesson.
- European Food Information Council (Belgium)Laura Fernández, Laura Smillie, Josephine Wills.
- Universidad Politécnica de Madrid (Spain)Marcela González-Gross, Raquel Pedrero-Chamizo, Agustín Meléndez, Jara Valtueña, David Jiménez-Pavón, Ulrike Albers, Pedro J. Benito, Juan José Gómez Lorente, David Cañada, Alejandro Urzanqui, Rosa María Torres, Paloma Navarro.
References
- World Health Organisation. Ginebra. Noncommunicable Diseases: Childhood Overweight and Obesity. 2020. Available online: https://www.who.int/news-room/q-a-detail/noncommunicable-diseases-childhood-overweight-and-obesity (accessed on 9 November 2020).
- Spinelli, A.; Buoncristiano, M.; Kovacs, V.; Yngve, A.; Spiroski, I.; Obreja, G.; Starc, G.; Pérez, N.; Rito, A.; Kunešová, M.; et al. Prevalence of Severe Obesity among Primary School Children in 21 European Countries. Obes. Facts 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.; Hu, F.; Després, J.; Matsuzawa, Y.; Loos, R.; Moreno, L.; Bray, G.; Martinez, J. Obesity. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Tognon, G.; Hebestreit, A.; Lanfer, A.; Moreno, L.; Pala, V.; Siani, A.; Tornaritis, M.; De Henauw, S.; Veidebaum, T.; Molnár, D.; et al. Mediterranean diet, overweight and body composition in children from eight European countries: Cross-sectional and prospective results from the IDEFICS study. Nutr. Metab. Cardiovasc. Dis. 2014, 24. [Google Scholar] [CrossRef] [PubMed]
- Notario-Barandiaran, L.; Valera-Gran, D.; Gonzalez-Palacios, S.; Garcia-de-la-Hera, M.; Fernández-Barrés, S.; Pereda-Pereda, E.; Fernández-Somoano, A.; Guxens, M.; Iñiguez, C.; Romaguera, D.; et al. High adherence to a mediterranean diet at age 4 reduces overweight, obesity and abdominal obesity incidence in children at the age of 8. Int. J. Obes. 2020, 44. [Google Scholar] [CrossRef]
- Vicente-Rodríguez, G.; Rey-López, J.; Martín-Matillas, M.; Moreno, L.; Wärnberg, J.; Redondo, C.; Tercedor, P.; Delgado, M.; Marcos, A.; Castillo, M.; et al. Television watching, videogames, and excess of body fat in Spanish adolescents: The AVENA study. Nutrition 2008, 24. [Google Scholar] [CrossRef]
- Steele, R.; van Sluijs, E.; Cassidy, A.; Griffin, S.; Ekelund, U. Targeting sedentary time or moderate- and vigorous-intensity activity: Independent relations with adiposity in a population-based sample of 10-y-old British children. Am. J. Clin. Nutr. 2009, 90. [Google Scholar] [CrossRef]
- Tremblay, M.; Carson, V.; Chaput, J.; Connor Gorber, S.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.; Gruber, R.; Janson, K.; et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016, 41. [Google Scholar] [CrossRef]
- Santaliestra-Pasías, A.; Mouratidou, T.; Verbestel, V.; Bammann, K.; Molnar, D.; Sieri, S.; Siani, A.; Veidebaum, T.; Mårild, S.; Lissner, L.; et al. Physical activity and sedentary behaviour in European children: The IDEFICS study. Public Health Nutr. 2014, 17. [Google Scholar] [CrossRef]
- LeBlanc, A.; Katzmarzyk, P.; Barreira, T.; Broyles, S.; Chaput, J.; Church, T.; Fogelholm, M.; Harrington, D.; Hu, G.; Kuriyan, R.; et al. Correlates of Total Sedentary Time and Screen Time in 9–11 Year-Old Children around the World: The International Study of Childhood Obesity, Lifestyle and the Environment. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Medrano, M.; Cadenas-Sanchez, C.; Oses, M.; Arenaza, L.; Amasene, M.; Labayen, I. Changes in lifestyle behaviours during the COVID-19 confinement in Spanish children: A longitudinal analysis from the MUGI project. Pediatr. Obes. 2020. [Google Scholar] [CrossRef]
- Santaliestra-Pasías, A.; Mouratidou, T.; Huybrechts, I.; Beghin, L.; Cuenca-García, M.; Castillo, M.; Galfo, M.; Hallstrom, L.; Kafatos, A.; Manios, Y.; et al. Increased sedentary behaviour is associated with unhealthy dietary patterns in European adolescents participating in the HELENA study. Eur. J. Clin. Nutr. 2014, 68. [Google Scholar] [CrossRef] [PubMed]
- Archero, F.; Ricotti, R.; Solito, A.; Carrera, D.; Civello, F.; Di Bella, R.; Bellone, S.; Prodam, F. Adherence to the Mediterranean Diet among School Children and Adolescents Living in Northern Italy and Unhealthy Food Behaviors Associated to Overweight. Nutrients 2018, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni Mdel, M.; Martínez, E.; Llull, R.; Pons, A.; Tur, J. Western and Mediterranean dietary patterns among Balearic Islands’ adolescents: Socio-economic and lifestyle determinants. Public Health Nutr. 2012, 15. [Google Scholar] [CrossRef] [PubMed]
- Labayen Goñi, I.; Arenaza, L.; Medrano, M.; García, N.; Cadenas-Sanchez, C.; Ortega, F.B. Associations between the adherence to the Mediterranean diet and cardiorespiratory fitness with total and central obesity in preschool children: The PREFIT project. Eur. J. Nutr. 2018, 57. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Hernandez, V.; Arenaza, L.; Gracia-Marco, L.; Medrano, M.; Merchan Ramirez, E.; Martinez Avila, W.D.; Oses, M.; Ruiz, J.R.; Ortega, F.B.; Labayen, I. Influence of Physical Activity on Bone Mineral Content and Density in Overweight and Obese Children with Low Adherence to the Mediterranean Dietary Pattern. Nutrients 2018, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino Idelson, P.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in children and adolescents: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2017, 27. [Google Scholar] [CrossRef]
- Papadaki, S.; Mavrikaki, E. Greek adolescents and the Mediterranean diet: Factors affecting quality and adherence. Nutrition 2015, 31. [Google Scholar] [CrossRef]
- Moreno, L.A.; De Henauw, S.; Gonzalez-Gross, M.; Kersting, M.; Molnar, D.; Gottrand, F.; Barrios, L.; Sjostrom, M.; Manios, Y.; Gilbert, C.C.; et al. Design and implementation of the Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-Sectional Study. Int. J. Obes. 2008, 32 (Suppl. S5), S4–S11. [Google Scholar] [CrossRef]
- Moreno, L.A.; Gottrand, F.; Huybrechts, I.; Ruiz, J.R.; Gonzalez-Gross, M.; DeHenauw, S. Nutrition and lifestyle in european adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Adv. Nutr. 2014, 5, 615s–623s. [Google Scholar] [CrossRef]
- Moreno, L.; González-Gross, M.; Kersting, M.; Molnár, D.; de Henauw, S.; Beghin, L.; Sjöström, M.; Hagströmer, M.; Manios, Y.; Gilbert, C.; et al. Assessing, understanding and modifying nutritional status, eating habits and physical activity in European adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr. 2008, 11. [Google Scholar] [CrossRef]
- Beghin, L.; Castera, M.; Manios, Y.; Gilbert, C.C.; Kersting, M.; De Henauw, S.; Kafatos, A.; Gottrand, F.; Molnar, D.; Sjöström, M.; et al. Quality assurance of ethical issues and regulatory aspects relating to good clinical practices in the HELENA Cross-Sectional Study. Int. J. Obes. 2008, 32 (Suppl. S5), S12–S18. [Google Scholar] [CrossRef]
- Nagy, E.; Vicente-Rodriguez, G.; Manios, Y.; Beghin, L.; Iliescu, C.; Censi, L.; Dietrich, S.; Ortega, F.B.; De Vriendt, T.; Plada, M.; et al. Harmonization process and reliability assessment of anthropometric measurements in a multicenter study in adolescents. Int. J. Obes. 2008, 32 (Suppl. S5), S58–S65. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.; Lohman, T.; Boileau, R.; Horswill, C.; Stillman, R.; Van Loan, M.; Bemben, D. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988, 60. [Google Scholar]
- VanItallie, T.; Yang, M.; Heymsfield, S.; Funk, R.; Boileau, R. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Whitehouse, R. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51. [Google Scholar] [CrossRef]
- Diethelm, K.; Huybrechts, I.; Moreno, L.; De Henauw, S.; Manios, Y.; Beghin, L.; Gonzalez-Gross, M.; Le Donne, C.; Cuenca-Garcia, M.; Castillo, M.J.; et al. Nutrient intake of European adolescents: Results of the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr. 2014, 17, 486–497. [Google Scholar] [CrossRef]
- Vereecken, C.A.; Covents, M.; Matthys, C.; Maes, L. Young adolescents’ nutrition assessment on computer (YANA-C). Eur. J. Clin. Nutr. 2005, 59, 658–667. [Google Scholar] [CrossRef]
- Vereecken, C.A.; Covents, M.; Sichert-Hellert, W.; Alvira, J.M.F.; Le Donne, C.; De Henauw, S.; De Vriendt, T.; Phillipp, M.K.; Béghin, L.; Manios, Y.; et al. Development and evaluation of a self-administered computerized 24-h dietary recall method for adolescents in Europe. Int. J. Obes. 2008, 32 (Suppl. S5). [Google Scholar] [CrossRef]
- Andersen, L.F.; Lioret, S.; Brants, H.; Kaic-Rak, A.; de Boer, E.J.; Amiano, P.; Trolle, E. Recommendations for a trans-European dietary assessment method in children between 4 and 14 years. Eur. J. Clin. Nutr. 2011, 65 (Suppl. S1), S58–S64. [Google Scholar] [CrossRef]
- Haubrock, J.; Nöthlings, U.; Volatier, J.; Dekkers, A.; Ocké, M.; Harttig, U.; Illner, A.; Knüppel, S.; Andersen, L.; Boeing, H. Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J. Nutr. 2011, 141. [Google Scholar] [CrossRef] [PubMed]
- Arenaza, L.; Huybrechts, I.; Ortega, F.B.; Ruiz, J.R.; De Henauw, S.; Manios, Y.; Marcos, A.; Julián, C.; Widhalm, K.; Bueno, G.; et al. Adherence to the Mediterranean diet in metabolically healthy and unhealthy overweight and obese European adolescents: The HELENA study. Eur. J. Nutr. 2019, 58. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.A.; Bel-Serrat, S.; Santaliestra-Pasias, A.; Bueno, G. Dairy products, yogurt consumption, and cardiometabolic risk in children and adolescents. Nutr. Rev. 2015, 73 (Suppl. S1), 8–14. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A. Traditional Mediterranean diet and longevity in the elderly: A review. Public Health Nutr. 2004, 7, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Rey-López, J.; Ruiz, J.; Ortega, F.B.; Verloigne, M.; Vicente-Rodriguez, G.; Gracia-Marco, L.; Gottrand, F.; Molnar, D.; Widhalm, K.; Zaccaria, M.; et al. Reliability and validity of a screen time-based sedentary behaviour questionnaire for adolescents: The HELENA study. Eur. J. Public Health 2012, 22. [Google Scholar] [CrossRef] [PubMed]
- Rey-López, J.; Bel-Serrat, S.; Santaliestra-Pasías, A.; de Moraes, A.; Vicente-Rodríguez, G.; Ruiz, J.; Artero, E.; Martínez-Gómez, D.; Gottrand, F.; De Henauw, S.; et al. Sedentary behaviour and clustered metabolic risk in adolescents: The HELENA study. Nutr. Metab. Cardiovasc. Dis. 2013, 23. [Google Scholar] [CrossRef]
- Currie, C.; Molcho, M.; Boyce, W.; Holstein, B.; Torsheim, T.; Richter, M. Researching health inequalities in adolescents: The development of the Health Behaviour in School-Aged Children (HBSC) family affluence scale. Soc. Sci. Med. 2008, 66. [Google Scholar] [CrossRef]
- Jiménez Pavón, D.; Ortega, F.B.; Ruiz, J.; España Romero, V.; García Artero, E.; Moliner Urdiales, D.; Gómez Martínez, S.; Vicente Rodríguez, G.; Manios, Y.; Béghin, L.; et al. Socioeconomic status influences physical fitness in European adolescents independently of body fat and physical activity: The HELENA study. Nutr. Hosp. 2010, 25. [Google Scholar]
- Strong, W.; Malina, R.; Blimkie, C.; Daniels, S.; Dishman, R.; Gutin, B.; Hergenroeder, A.; Must, A.; Nixon, P.; Pivarnik, J.; et al. Evidence based physical activity for school-age youth. J. Pediatr. 2005, 146. [Google Scholar] [CrossRef]
- Bibiloni, M.M.; Pich, J.; Córdova, A.; Pons, A.; Tur, J. Association between sedentary behaviour and socioeconomic factors, diet and lifestyle among the Balearic Islands adolescents. BMC Public Health 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Štefan, L.; Prosoli, R.; Emeljanovas, A.; Mieziene, B.; Milanović, I.; Radisavljević-Janić, S. Mediterranean Diet and Its Correlates among Adolescents in Non-Mediterranean European Countries: A Population-Based Study. Nutrients 2017, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Santaliestra-Pasías, A.; Mouratidou, T.; Reisch, L.; Pigeot, I.; Ahrens, W.; Mårild, S.; Molnár, D.; Siani, A.; Sieri, S.; Tornatiris, M.; et al. Clustering of lifestyle behaviours and relation to body composition in European children. The IDEFICS study. Eur. J. Clin. Nutr. 2015, 69. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; McNaughton, S.; Timperio, A. Clustering of diet, physical activity and sedentary behaviour among Australian children: Cross-sectional and longitudinal associations with overweight and obesity. Int. J. Obes. 2015, 39. [Google Scholar] [CrossRef]
- Koning, M.; Hoekstra, T.; de Jong, E.; Visscher, T.; Seidell, J.; Renders, C. Identifying developmental trajectories of body mass index in childhood using latent class growth (mixture) modelling: Associations with dietary, sedentary and physical activity behaviors: A longitudinal study. BMC Public Health 2016, 16. [Google Scholar] [CrossRef]
- Sánchez-Oliva, D.; Grao-Cruces, A.; Carbonell-Baeza, A.; Cabanas-Sánchez, V.; Veiga, O.; Castro-Piñero, J. Lifestyle Clusters in School-Aged Youth and Longitudinal Associations with Fatness: The UP & DOWN Study. J. Pediatr. 2018, 203. [Google Scholar] [CrossRef]
- Ottevaere, C.; Huybrechts, I.; Benser, J.; De Bourdeaudhuij, I.; Cuenca-Garcia, M.; Dallongeville, J.; Zaccaria, M.; Gottrand, F.; Kersting, M.; Rey-López, J.; et al. Clustering patterns of physical activity, sedentary and dietary behavior among European adolescents: The HELENA study. BMC Public Health 2011, 11. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Ojeda-Rodríguez, A.; Heinen, M.; Buoncristiano, M.; Abdrakhmanova, S.; Duleva, V.; Sant’Angelo, V.; Fijałkowska, A.; Hejgaard, T.; Huidumac, C.; et al. Clustering of Multiple Energy Balance-Related Behaviors in School Children and its Association with Overweight and Obesity-WHO European Childhood Obesity Surveillance Initiative (COSI 2015–2017). Nutrients 2019, 11, 511. [Google Scholar] [CrossRef]
- Dumuid, D.; Olds, T.; Lewis, L.; Martin-Fernández, J.; Barreira, T.; Broyles, S.; Chaput, J.; Fogelholm, M.; Hu, G.; Kuriyan, R.; et al. The adiposity of children is associated with their lifestyle behaviours: A cluster analysis of school-aged children from 12 nations. Pediatr. Obes. 2018, 13. [Google Scholar] [CrossRef]
- Leech, R.; McNaughton, S.; Timperio, A. The clustering of diet, physical activity and sedentary behavior in children and adolescents: A review. Int. J. Behav. Nutr. Phys. Act. 2014, 11. [Google Scholar] [CrossRef]
- Seghers, J.; Rutten, C. Clustering of multiple lifestyle behaviours and its relationship with weight status and cardiorespiratory fitness in a sample of Flemish 11- to 12-year-olds. Public Health Nutr. 2010, 13. [Google Scholar] [CrossRef] [PubMed]
- Myszkowska-Ryciak, J.; Harton, A.; Lange, E.; Laskowski, W.; Wawrzyniak, A.; Hamulka, J.; Gajewska, D. Reduced Screen Time is Associated with Healthy Dietary Behaviors but Not Body Weight Status among Polish Adolescents. Report from the Wise Nutrition-Healthy Generation Project. Nutrients 2020, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chan, N.; Lam, S.; Li, S.; Liu, Y.; Chan, J.; Kong, A.; Ma, R.; Chan, K.; Li, A.; et al. Emergence of Sex Differences in Insomnia Symptoms in Adolescents: A Large-Scale School-Based Study. Sleep 2016, 39. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Ortega, F.; Ruiz, J.; Rey-López, J.; Béghin, L.; Manios, Y.; Cuenca-García, M.; Plada, M.; Diethelm, K.; Kafatos, A.; et al. Short sleep duration is associated with increased obesity markers in European adolescents: Effect of physical activity and dietary habits. The HELENA study. Int. J. Obes. 2011, 35. [Google Scholar] [CrossRef] [PubMed]
- Bel, S.; Michels, N.; De Vriendt, T.; Patterson, E.; Cuenca-García, M.; Diethelm, K.; Gutin, B.; Grammatikak, I.E.; Manios, Y.; Leclercq, C.; et al. Association between self-reported sleep duration and dietary quality in European adolescents. Br. J. Nutr. 2013, 110. [Google Scholar] [CrossRef]
- Agostinis-Sobrinho, C.; Gómez-Martínez, S.; Nova, E.; Hernandez, A.; Labayen, I.; Kafatos, A.; Gottand, F.; Molnár, D.; Ferrari, M.; Moreno, L.; et al. Lifestyle patterns and endocrine, metabolic, and immunological biomarkers in European adolescents: The HELENA study. Pediatr. Diabetes 2019, 20. [Google Scholar] [CrossRef]
- Vereecken, C.; Dohogne, S.; Covents, M.; Maes, L. How accurate are adolescents in portion-size estimation using the computer tool Young Adolescents’ Nutrition Assessment on Computer (YANA-C)? Br. J. Nutr. 2010, 103. [Google Scholar] [CrossRef]
- van Grieken, A.; Ezendam, N.; Paulis, W.; van der Wouden, J.; Raat, H. Primary prevention of overweight in children and adolescents: A meta-analysis of the effectiveness of interventions aiming to decrease sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2012, 9. [Google Scholar] [CrossRef]
- Brown, T.; Moore, T.; Hooper, L.; Gao, Y.; Zayegh, A.; Ijaz, S.; Elwenspoek, M.; Foxen, S.; Magee, L.; O’Malley, C.; et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2019, 7. [Google Scholar] [CrossRef]

| Total | Male | Female | p | |
|---|---|---|---|---|
| n = 2047 | n = 925 | n = 1122 | ||
| Age (years) | 14.7 (13.7–15.7) | 14.8 (13.7–15.7) | 14.7 (13.7–15.7) | 0.590 |
| Height (cm) | 165.9 (159.3–172.0) | 170.1 (163.9–177.1) | 162.4 (157.7–167.0) | <0.001 |
| Weight (kg) | 58.4 (50.3–64.3) | 61.5 (52.3–68.9) | 55.8 (49.0–61.2) | <0.001 |
| BMI (kg/m2) | 21.1 (18.7–22.8) | 21.1 (18.6–22.8) | 21.1 (18.8–22.8) | 0.282 |
| WC (cm) | 71.8 (66.2–75.8) | 73.8 (67.8–78.3) | 70.2 (65.0–74.4) | <0.001 |
| FMI (kg/m2) | 5.15 (3.1–6.3) | 4.5 (2.4–5.3) | 5.7 (4.0–6.8) | <0.001 |
| Pubertal stage [n (%)] | <0.001 | |||
| I | 7 (0.3%) | 7 (0.8%) | 0 (0%) | |
| II | 134 (6.5%) | 84 (9.1%) | 50 (4.5%) | |
| III | 502 (24.5%) | 226 (24.4%) | 276 (24.6%) | |
| IV | 881 (43.0%) | 381 (41.2%) | 500 (44.6%) | |
| V | 523 (25.5%) | 227 (24.5%) | 296 (26.4%) | |
| FAS [n (%)] | 0.112 | |||
| Low | 199 (9.7%) | 76 (8.2%) | 123 (11.0%) | |
| Medium | 1133 (55.3%) | 519 (56.1%) | 614 (54.7%) | |
| High | 715 (35.0%) | 330 (35.7%) | 385 (34.3%) | |
| MDS * (points) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.071 |
| Energy intake (kcal/day) | 2180.1 (1634.9–2569.7) | 2517.9 (1921.0–2984.3) | 1901.6 (1492.4–2244.9) | <0.001 |
| Screen time (min/day) | 256.2 (139.3–330.0) | 288.1 (171.4–367.4) | 229.9 (126.4–300.0) | <0.001 |
| Screen Time (min/day) | |||||
|---|---|---|---|---|---|
| Model I a | Model II b | ||||
| β | p | β | p | R2 | |
| Male | 0.029 | ||||
| MDS (point) | −12.535 | <0.001 | −12.402 | <0.001 | |
| Energy Intake (kcal/day) | - | - | 0.026 | <0.001 | |
| Female | 0.016 | ||||
| MDS (point) | −12.402 | <0.001 | −12.402 | <0.001 | |
| Energy Intake (kcal/day) | - | - | - | - | |
| Males | (p-Values) | Females | (p-Values) | |||
|---|---|---|---|---|---|---|
| BMI (kg/m2) | WC (cm) | FMI (kg/m2) | BMI (kg/m2) | WC (cm) | FMI (kg/m2) | |
| Covariates | ||||||
| Pubertal Stage | ||||||
| II * | 0.502 | 0.139 | 0.048 | - | - | - |
| III | 0.547 | 0.209 | 0.010 | 0.026 | 0.012 | 0.126 |
| IV | 0.534 | 0.936 | 0.047 | <0.001 | <0.001 | <0.001 |
| V | 0.638 | 0.866 | 0.008 | <0.001 | <0.001 | <0.001 |
| FAS | ||||||
| Medium | 0.048 | 0.604 | 0.045 | <0.001 | 0.138 | <0.001 |
| High | 0.024 | 0.695 | 0.019 | <0.001 | 0.058 | <0.001 |
| Energy Intake (kcal/day) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Studied Variables | ||||||
| MDS (point) | 0.049 | 0.260 | 0.198 | 0.043 | 0.160 | 0.042 |
| Screen time: MDS | 0.062 | 0.617 | 0.122 | 0.025 | 0.002 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seral-Cortes, M.; Sabroso-Lasa, S.; Bailo-Aysa, A.; Gonzalez-Gross, M.; Molnár, D.; Censi, L.; Molina-Hidalgo, C.; Gottrand, F.; Henauw, S.D.; Manios, Y.; et al. Mediterranean Diet, Screen-Time-Based Sedentary Behavior and Their Interaction Effect on Adiposity in European Adolescents: The HELENA Study. Nutrients 2021, 13, 474. https://doi.org/10.3390/nu13020474
Seral-Cortes M, Sabroso-Lasa S, Bailo-Aysa A, Gonzalez-Gross M, Molnár D, Censi L, Molina-Hidalgo C, Gottrand F, Henauw SD, Manios Y, et al. Mediterranean Diet, Screen-Time-Based Sedentary Behavior and Their Interaction Effect on Adiposity in European Adolescents: The HELENA Study. Nutrients. 2021; 13(2):474. https://doi.org/10.3390/nu13020474
Chicago/Turabian StyleSeral-Cortes, Miguel, Sergio Sabroso-Lasa, Alexandro Bailo-Aysa, Marcela Gonzalez-Gross, Dénes Molnár, Laura Censi, Cristina Molina-Hidalgo, Frederic Gottrand, Stefaan De Henauw, Yannis Manios, and et al. 2021. "Mediterranean Diet, Screen-Time-Based Sedentary Behavior and Their Interaction Effect on Adiposity in European Adolescents: The HELENA Study" Nutrients 13, no. 2: 474. https://doi.org/10.3390/nu13020474
APA StyleSeral-Cortes, M., Sabroso-Lasa, S., Bailo-Aysa, A., Gonzalez-Gross, M., Molnár, D., Censi, L., Molina-Hidalgo, C., Gottrand, F., Henauw, S. D., Manios, Y., Mavrogianni, C., Widhalm, K., Kafatos, A., Dallongeville, J., Moreno, L. A., Esteban, L. M., Labayen, I., De Miguel-Etayo, P., & on behalf of the HELENA Study Group. (2021). Mediterranean Diet, Screen-Time-Based Sedentary Behavior and Their Interaction Effect on Adiposity in European Adolescents: The HELENA Study. Nutrients, 13(2), 474. https://doi.org/10.3390/nu13020474














