Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight
Abstract
1. Introduction
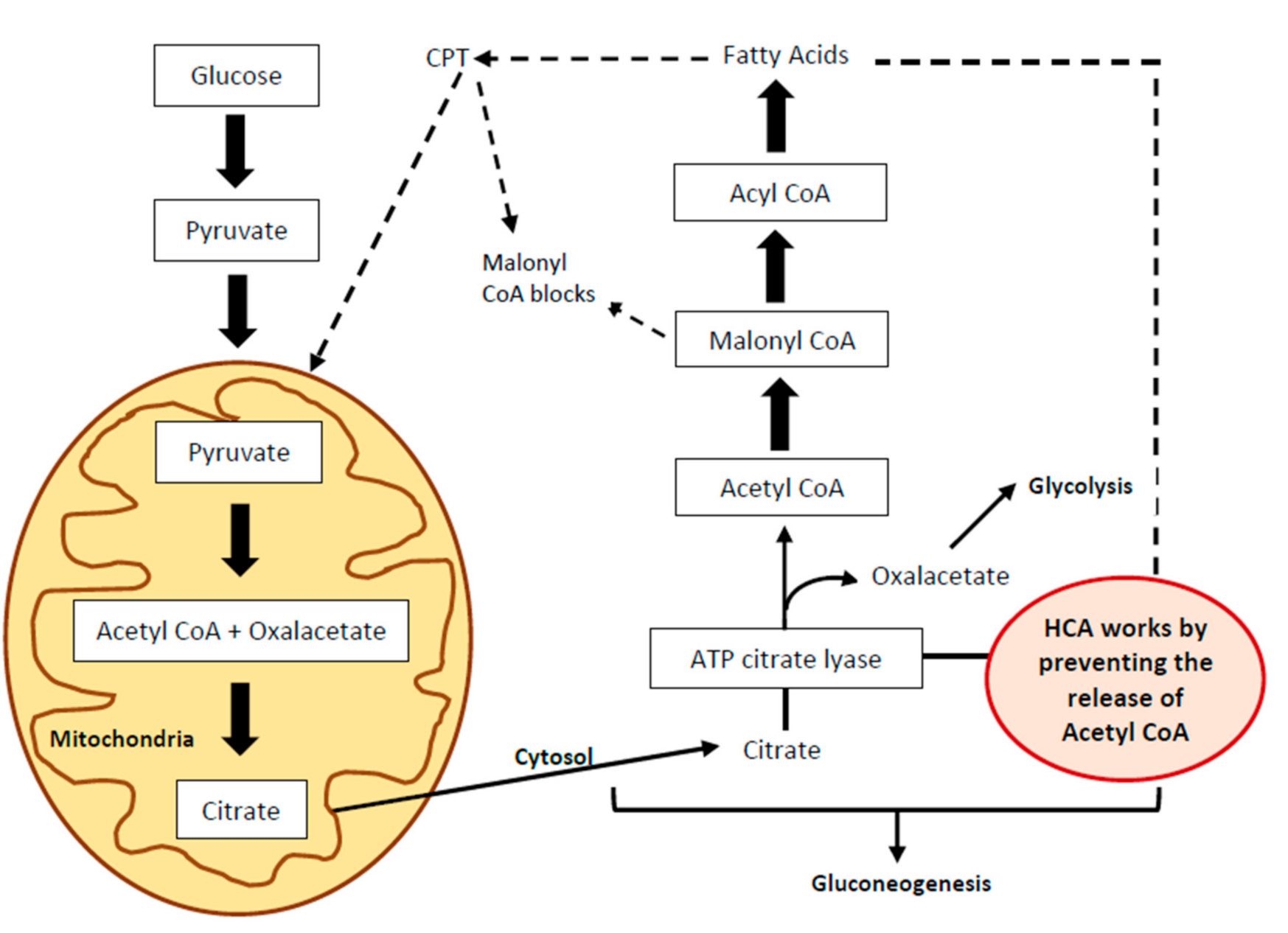
2. Active Principle and Mechanisms of Action
3. Effectiveness of Garcinia to Lose and Maintain Body Weight
4. Negative Effects on Health
4.1. Animal Toxicity Studies
4.2. Clinical Toxicity
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The heavy burden of obesity and the economics of prevention. In The Heavy Burden of Obesity; OECD Publishing: Paris, France, 2019.
- World Health Organization. Obesity. Preventing and Managing the Global Epidemic. In Report of a WHO Consultation on Obesity; WHO/NUT/NCD/981; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Haber, S.; Awwad, O.; Phillips, A.; Park, A.; Pham, T. Garcinia cambogia for weight loss. Am. J. Health-Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Egan, B.; de Klein, S.; Dima, L.; Maggi, F.M.; Isoniemi, M.; Ribas-Barba, L.; Raats, M.M.; Meissner, E.M.; Badea, M.; et al. Usage of plant food supplements across six European countries: Findings from the PlantLIBRA consumer survey. PLoS ONE 2014, 9, 537. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.; Niranjana, S.R.; Girish, K.S. An overview on genus garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on antiobesity effect of Garcinia Origin (-)-HCA. Evid. Based Complement. Altern. Med. 2013, 2013, 751658. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Oyaizu, S.; Onuki, K.; Lim, K.; Fushiki, T. Chronic (-)-Hydroxycitrate Administration Spares Carbohydrate Utilization and Promotes Lipid Oxidation during Exercise in Mice. J. Nutr. 2000, 130, 2990–2995. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.R.; Cleary, M.P.; Gruen, R.; Blasé, D.; Stern, J.S.; Triscari, J.; Sullivan, A.C. Effect of (-)-hydroxycitrate on development of obesity in the Zucker obese rat. Am. J. Physiol. Endocrinol. Metab. 1981, 240, 72–78. [Google Scholar] [CrossRef]
- Leonhardt, M.; Langhans, W. Hydroxycitrate has long-term effects on feeding behavior, body weight regain and metabolism after body weight loss in male rats. J. Nutr. 2002, 132, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ueno, M.; Ogino, S.; Kubo, K.; Nagata, J.; Takeuchi, M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food Chem. Toxicol. 2005, 43, 411–419. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Allison, D.B.; Vasselli, J.R.; Pietrobelli, A.; Greenfield, D.; Nunez, C. Garcinia cambogia (Hydroxycitric Acid) as a potential antiobesity agent: A randomized controlled trial. JAMA 1998, 280, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Bormann, L. Effects of (-)-hydroxycitric acid on appetitive variables. Physiol. Behav. 2000, 71, 87–94. [Google Scholar] [CrossRef]
- Thom, E. A randomized, double-blind, placebo-controlled trial of a new weight-reducing agent of natural origin. J. Int. Med. Res. 2000, 28, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.R.; Westerterp-Plantenga, M.S.; Saris, W.H.M. The effects of 2-week ingestion of (--)-hydroxycitrate and (--)-hydroxycitrate combined with medium-chain triglycerides on satiety, fat oxidation, energy expenditure and body weight. Int. J. Obes. 2001, 25, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.R.; Westerterp-Plantenga, M.S.; de Vries, M.; Brouns, F.; Saris, W.H.M. Effects of 2-week ingestion of (−)-hydroxycitrate and (−)-hydroxycitrate combined with medium-chain triglycerides on satiety and food intake. Physiol. Behav. 2001, 74, 543–549. [Google Scholar] [CrossRef]
- Hayamizu, K.; Ishii, Y.; Kaneko, I.; Shen, M.; Okuhara, Y.; Shigematsu, N.; Tomi, H.; Furuse, M.; Yoshino, G.; Shimasaki, H. Effects of Garcinia cambogia (Hydroxycitric Acid) on visceral fat accumulation: A double-blind, randomized, placebo-controlled trial. Curr. Ther. Res. 2003, 64, 551–567. [Google Scholar] [CrossRef]
- Preuss, H.G.; Bagchi, D.; Bagchi, M.; Rao, C.V.S.; Satyanarayana, S.; Dey, D.K. Efficacy of a novel, natural extract of (–)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX, niacin-bound chromium and Gymnema sylvestre extract in weight management in human volunteers: A pilot study. Nutr. Res. 2004, 24, 45–58. [Google Scholar] [CrossRef]
- Preuss, H.G.; Bagchi, D.; Bagchi, M.; Rao, C.V.S.; Dey, D.K.; Satyanarayana, S. Effects of a natural extract of (-)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX plus niacin-bound chromium and Gymnema sylvestre extract on weight loss. Diabetes Obes. Metab. 2004, 6, 171–180. [Google Scholar] [CrossRef]
- Roongpisuthipong, C.; Kantawan, R.; Roongpisuthipong, W. Reduction of adipose tissue and body weight: Effect of water soluble calcium hydroxycitrate in Garcinia atroviridis on the short term treatment of obese women in Thailand. Asia Pac. J. Clin. Nutr. 2007, 16, 25. [Google Scholar]
- Toromanyan, E.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Panossian, A. Efficacy of Slim339® in reducing body weight of overweight and obese human subjects. Phytother. Res. 2007, 21, 1177–1181. [Google Scholar] [CrossRef]
- Vasques, C.A.R.; Rossetto, S.; Halmenschlager, G.; Linden, R.; Heckler, E.; Fernandez, M.S.P.; Alonso, J.L.L. Evaluation of the pharmacotherapeutic efficacy of Garcinia cambogia plus Amorphophallus konjac for the treatment of obesity. Phytother. Res. 2008, 22, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeon, S.; Park, K.H.; Lee, W.S.; Jeong, T.; McGregor, R.A.; Choi, M.-S. Does Glycine max leaves or Garcinia Cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: A randomized control trial. Nutr. J. 2011, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Yang, T.H.; Wu, C.C.; Doong, J.Y.; Lin, P.Y.; Chiang, C.M.; Lin, C.L.; Hsieh, S.L. Clinical evaluation of Garcinia cambogia and Phaseolus vulgaris extract for obese adults in taiwan. Nutr. Sci. J. 2012, 37, 75–84. [Google Scholar] [CrossRef]
- Stern, J.S.; Peerson, J.; Mishra, A.T.; Sadasiva Rao, M.V.; Rajeswari, K.P. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity 2013, 21, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.S.; Peerson, J.; Mishra, A.T.; Mathukumalli, V.S.R.; Konda, P.R. Efficacy and tolerability of an herbal formulation for weight management. J. Med. Food 2013, 16, 529–537. [Google Scholar] [CrossRef]
- Chong, P.; Beah, Z.; Grube, B.; Riede, L. IQP-GC-101 reduces body weight and body fat mass: A randomized, double-blind, placebo-controlled study. Phytother. Res. 2014, 28, 1520–1526. [Google Scholar] [CrossRef]
- Vasques, C.A.R.; Schneider, R.; Klein-Júnior, L.C.; Falavigna, A.; Piazza, I.; Rossetto, S. Hypolipemic Effect of Garcinia cambogia in Obese Women. Phytother. Res. 2014, 28, 887–891. [Google Scholar] [CrossRef]
- Kudiganti, V.; Kodur, R.R.; Kodur, S.R.; Halemane, M.; Deep, D.K. Efficacy and tolerability of Meratrim for weight management: A randomized, double-blind, placebo-controlled study in healthy overweight human subjects. Lipids Health Dis. 2016, 15, 136. [Google Scholar] [CrossRef][Green Version]
- Maia-Landim, A.; Ramírez, J.M.; Lancho, C.; Poblador, M.S.; Lancho, J.L. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC Complement. Altern. Med. 2018, 18, 26. [Google Scholar] [CrossRef]
- Watanabe, M.; Gangitano, E.; Francomano, D.; Addessi, E.; Toscano, R.; Costantini, D.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; et al. Mangosteen Extract shows a potent Insulin sensitizing effect in Obese female patients: A prospective randomized controlled pilot study. Nutrients 2018, 10, 586. [Google Scholar] [CrossRef]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. J. Obes. 2011, 2011, 509038. [Google Scholar] [CrossRef]
- Chuah, L.O.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Alitheen, N.B. In vitro and in vivo toxicity of Garcinia or hydroxycitric acid: A review. Evid. Based Complement. Altern. Med. 2012, 2012, 197920. [Google Scholar] [CrossRef]
- Soni, M.G.; Burdock, G.A.; Preuss, H.G.; Stohs, S.J.; Ohia, S.E.; Bagchi, D. Safety assessment of (−)-hydroxycitric acid and Super CitriMax®, a novel calcium/potassium salt. Food Chem. Toxicol. 2004, 42, 1513–1529. [Google Scholar] [CrossRef]
- Márquez, F.; Babio, N.; Bulló, M.; Salas-Salvadó, J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit. Rev. Food Sci. Nutr. 2012, 52, 585–594. [Google Scholar] [CrossRef]
- Ohia, S.; Opere, C.; LeDay, A.; Bagchi, M.; Bagchi, D.; Stohs, S. Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX). Mol. Cell. Biochem. 2002, 238, 89–103. [Google Scholar] [CrossRef]
- Clouatre, D.L.; Preuss, H.G. Hydroxycitric acid does not promote inflammation or liver toxicity. World J. Gastroenterol. 2013, 19, 8160–8162. [Google Scholar] [CrossRef]
- Shara, M.; Ohia, S.E.; Yasmin, T.; Zardetto-Smith, A.; Kincaid, A.; Bagchi, M.; Chatterjee, A.; Bagchi, D.; Stohs, S.J. Dose- and time-dependent effects of a novel (−)-hydroxycitric acid extract on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation and histopathological data over a period of 90 days. Mol. Cell. Biochem. 2003, 254, 339–346. [Google Scholar] [CrossRef]
- Shara, M.; Ohia, S.E.; Schmidt, R.E.; Yasmin, T.; Zardetto-Smith, A.; Kincaid, A.; Bagchi, M.; Chatterjee, A.; Bagchi, D.; Stohs, S.J. Physico-chemical properties of a novel (–)-hydroxy- citric acid extract and its effect on body weight, selected organ weights, hepatic lipid peroxidation and DNA fragmentation, hematology and clinical chemistry, and histopathological changes over a period of 90 days. Mol. Cell. Biochem. 2004, 260, 171–186. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, M.; Park, Y.B.; Kim, S.R.; Lee, M.; Jung, U.J. Garcinia cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013, 19, 4689–4701. [Google Scholar] [CrossRef]
- Kothadia, J.P.; Kaminski, M.; Samant, H.; Olivera-Martinez, M. Hepatotoxicity associated with use of the weight loss supplement Garcinia cambogia: A case report and review of the literature. Case Rep. Hepatol. 2018, 2018, 6483605. [Google Scholar] [CrossRef]
- Farombi, E.O.; Adedara, I.A.; Oyenihi, A.B.; Ekakitie, E.; Kehinde, S. Hepatic, testicular and spermatozoa antioxidant status in rats chronically treated with Garcinia kola seed. J. Ethnopharmacol. 2013, 146, 536–542. [Google Scholar] [CrossRef]
- Saiyed, Z.M.; Sengupta, K.; Krishnaraju, A.V.; Trimurtulu, G.; Lau, F.C.; Lugo, J.P. Safety and toxicological evaluation of Meratrim®: An herbal formulation for weight management. Food Chem. Toxicol. 2015, 78, 122–129. [Google Scholar] [CrossRef]
- Stevens, T.; Qadri, A.; Zein, N.N. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann. Intern. Med. 2005, 142, 477–478. [Google Scholar] [CrossRef]
- Dara, L.; Hewett, J.; Lim, J.K. Hydroxycut hepatotoxicity: A case series and review of liver toxicity from herbal weight loss supplements. World J. Gastroenterol. 2008, 14, 6999–7004. [Google Scholar] [CrossRef]
- Shuster, J. Priapism and risperidone; Psoriasis after certolizumab therapy; bruxism with two different SSRIs; Nasal septum deviation associated with bevacizumab treatment; Hepatic failure with another herbal weight loss supplement; Bupropion-related dystonia and parkinsonism; bendamustine-associated hemolytic anemia. Hosp. Pharm. 2010, 45, 100–109. [Google Scholar] [CrossRef]
- Sharma, T.; Wong, L.; Tsai, N.; Wong, R.D. Hydroxycut(®) (herbal weight loss supplement) induced hepatotoxicity: A case report and review of literature. Hawaii Med. J. 2010, 69, 188–190. [Google Scholar]
- Mancano, M.A. Garcinia cambogia–Induced Acute Hepatitis; Varenicline-Induced Parkinsonism; Resistant Hypocalcemia after Zoledronic Acid Administration; Zonisamide-Induced Acute Kidney Injury; Psychosis Associated with Guanfacine. Hosp. Pharm. 2015, 50, 564–568. [Google Scholar] [CrossRef]
- Melendez-Rosado, J.; Snipelisky, D.; Matcha, G.; Stancampiano, F. Acute hepatitis induced by pure Garcinia cambogia. J. Clin. Gastroenterol. 2015, 49, 449–450. [Google Scholar] [CrossRef]
- Araujo, J.L.; Worman, H.J. Acute liver injury associated with a newer formulation of the herbal weight loss supplement Hydroxycut. BMJ Case Rep. 2015, 2015, bcr2015210303. [Google Scholar] [CrossRef]
- Smith, R.J.; Bertilone, C.; Robertson, A.G. Fulminant liver failure and transplantation after use of dietary supplements. Med. J. Aust. 2016, 204, 30–32. [Google Scholar] [CrossRef]
- Corey, R.; Werner, K.T.; Singer, A.; Moss, A.; Smith, M.; Noelting, J.; Rakela, J. Acute liver failure associated with Garcinia cambogia use. Ann. Hepatol. 2016, 15, 123. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Bodzin, A.S.; Reino, D.C.; Wang, H.L.; Busuttil, R.W. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J. Gastroenterol. 2016, 22, 10071–10076. [Google Scholar] [CrossRef]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Marconi, E.; Risaliti, F.; Bertoni, M.; Ippolito, F.M.; Maggini, V.; Gallo, E.; Firenzuoli, F.; et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: Case series and literature review. Intern. Emerg. Med. 2018, 13, 857–872. [Google Scholar] [CrossRef]
- Sharma, A.; Akagi, E.; Njie, A.; Goyal, S.; Arsene, C.; Krishnamoorthy, G.; Ehrinpreis, M. Acute hepatitis due to Garcinia cambogia extract, an herbal weight loss supplement. Case Rep. Gastrointest. Med. 2018, 2018, 9606171. [Google Scholar] [CrossRef]
- Philips, C.A.; Augustine, P. Chemical analysis of weight loss herbal supplement safe lean™ associated with acute liver injury—A concern for spurious drug, misbranding and adulteration. J. Clin. Exp. Hepatol. 2018, 8, 471–473. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Chaudhary, F.S.; Hodanazari, S.M.; Sittambalam, C.D. Hepatotoxicity associated with Garcinia cambogia: A case report. World J. Hepatol. 2019, 11, 735–742. [Google Scholar] [CrossRef]
- Khetpal, N.; Mandzhieva, B.; Shahid, S.; Khetpal, A.; Jain, A.G. Not All Herbals are Benign: A Case of Hydroxycut-induced Acute Liver Injury. Cureus 2020, 12, e6870. [Google Scholar] [CrossRef]
- Ferreira, V.; Mathieu, A.; Soucy, G.; Giard, J.M.; Erard-Poinsot, D. Acute severe liver injury related to long-term Garcinia cambogia intake. ACG Case Rep. J. 2020, 7, e00429. [Google Scholar] [CrossRef]
- García-Cortés, M.; Robles-Díaz, M.; Ortega-Alonso, A.; Medina-Caliz, I.; Andrade, R.J. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int. J. Mol. Sci. 2016, 17, 537. [Google Scholar] [CrossRef]
- Iqbal, U.; Anwar, H.; Siddiqui, H.U.; Mehmood, A. Acute Pancreatitis Secondary to Use of Appetite Suppressant: Garcinia cambogia. Cureus 2019, 11, e4676. [Google Scholar] [CrossRef]
- Lopez, A.; Kornegay, J.; Hendrickson, R. Serotonin toxicity associated with Garcinia cambogia over-the-counter supplement. J. Med. Toxicol. 2014, 10, 399–401. [Google Scholar] [CrossRef]
- Hendrickson, B.P.; Shaikh, N.; Occhiogrosso, M.; Penzner, J.B. Mania induced by Garcinia cambogia: A case series. Prim. Care Companion CNS Disord. 2016, 18. [Google Scholar] [CrossRef]
- Cotovio, G.; Oliveira-Maia, A.J. Hypomania induced by a Garcinia cambogia supplement. Aust. N. Z. J. Psychiatry 2017, 51, 641–642. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Timmer, T.K.; Davison, B.C.; McGrane, I.R. Possible Garcinia cambogia-induced mania with psychosis: A case report. J. Pharm. Pract. 2019, 32, 99–102. [Google Scholar] [CrossRef]
- Bystrak, T.; Cervera-Hernandez, M.E.; Reddy, N.; King, Z.; Bratberg, J. Garcinia cambogia, Diabetic Ketoacidosis, and Pancreatitis. RI Med. J. 2017, 100, 48–50. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Ohia, S.E.; Kaats, G.R.; Keen, C.L.; Williams, L.D.; Burdock, G.A. No evidence demonstrating hepatotoxicity associated with hydroxycitric acid. World J. Gastroenterol. 2009, 15, 4087–4089. [Google Scholar] [CrossRef]
- Teschke, R.; Wolff, A.; Eickhoff, A.; Danan, G. Is obesity rather than the dietary supplement used for weight reduction the cause of liver injury? JGH Open 2018, 2, 152–157. [Google Scholar] [CrossRef]
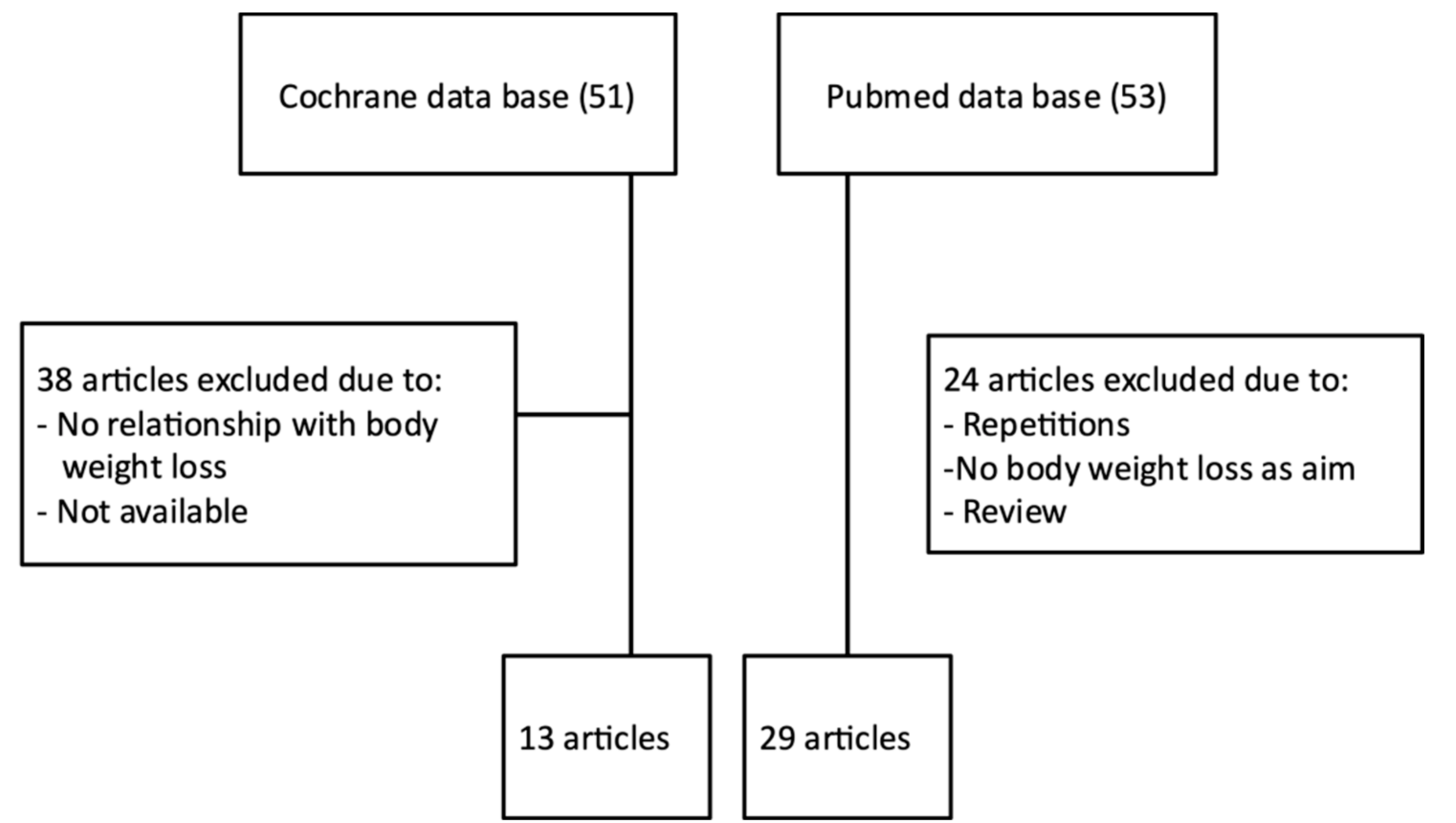

| Reference | Type of Study | Participants | Diet and Physical Activity | Treatment | Treatment Duration | Results | |
|---|---|---|---|---|---|---|---|
| Weight loss and Related Parameters | Other Results Observed after the Intervention | ||||||
| Heymsfield et al., 1998 [13] | Randomized, double-blind, placebo-controlled study. | 135 subjects BMI 27–38 aged 18 to 65 years. Control: 69 Intervention: 66. | 1200 Kcal/d diethigh in fibre: -Proteins: 30% -Lipids: 20% -Carbohydrates: 50% Regular physical activity | Pills: 500 mg Garcinia cambogia (50% HCA), 2 pills, 3 times/day | 3 months | No significant differences. | |
| Mattes et al., 2000 [14] | Double-blind, placebo-controlled parallel group study. | 89 women mean BMI of 28.6 aged 18 to 65 years. Control: 42 Intervention: 47 | 1200 Kcal/diet (30% lipids) Exercise was encouraged, but no formal regimen was prescribed. | Capsules: 400 mg of Garcinia cambogia (50% HCA), 3 times/day | 3 months | Significant weight loss. Reduction in waist circumference. | |
| Thom et al., 2000 [15] | Randomized double-blind study | 40 subjects BMI 27.5–39.0 aged ≥18 years. Control: 20 Intervention: 20. | Participants were given diet lists with advice on low-fat foods, supplying an energy intake of approximately 1200 Kcal/d, and were recommended to use this diet during the study. | Suco-Blo™ (tablets): 200 mg Phaseolus vulgaris extract, 200 mg inulin and 50 mg Garcinia cambogia extract 3 times/day. | 3 months | Significant reductionin body weight andbody mass. | |
| Kovacs et al., 2001 [16] | Double-blind, placebo-controlled, randomized, and cross-over study. | 11 obese men mean BMI of 27.4 mean age of 47 years. | Diet was divided in 3 meals without restrictions on the type and quantity of food and a maximum of one glass of alcohol drink per day. | Isoenergetic snack (cereal bar): 4 times/d Intervention 1:500 mg HCA Intervention 2:500 mg HCA + 3 g MCT | 3 Intervention periods of 2 weeks separated by washout periods of 4 weeks. | No significant differences. | |
| Kovacs et al., 2001 [17] | Double-blind, placebo-controlled, randomized, and crossover study. | 21 obese subjects mean BMI of 27 mean of age 43 years. | Diet was divided in 3 meals without restrictions on the type and quantity of food and a maximum of one glass of alcoholic drink per day. | Isoenergetic snack (cereal bar): 4 times/d Control: no supplementation Intervention 1:500 mg HCA Intervention 2:500 mg HCA + 3 g MCT | 3 intervention periods of 2 weeks separated by washout periods of 2 or 6 weeks | No significant differences. | |
| Hayamizu et al., 2003 [18] | Double blind, randomized, placebo-controlled, parallel-group study. | 44 subjects aged 20 to 65 years visceral fat area >90 cm2 Control: 21 Intervention: 23 | Maximun 2250 Kcal/d for men and 1800 Kcal/d for women. | Tablets: 185.25 mg of Garcinia cambogia extract (60% HCA) 3 tablets before each meal (9 tablets/day) | 3 months + 1 month of placebo in both groups at the end. | Reduction in visceral fat area, subcutaneous fat area and total fat area. | |
| Preuss et al., 2004 [19] | Randomized, double-blind, placebo-controlled study. | 30 subjects BMI > 26 aged 21 to 50 years Control:10 Intervention 1:10 Intervention 2:10 | 2000 Kcal/d divided in 3 meals: - Proteins: 17% - Lipids: 25% - Carbohydrates: 58% 30 min supervised walking exercise program (5 days a week). | HCA-SX (4667 mg) divided in 3 doses: Intervention 1:2800 mg/d of HCA Intervention 2:2800 mg/d of HCA, 4 mg niacin-bound chromium and 400 mg Gymnema sylvestre extract. | 2 months | Significant weight loss in both intervention groups. Reduction in food intake. | Reduction in total cholesterol, LDL-c, TG, and leptin levels. Increase in fat oxidation, HDL levels and serotonin levels. |
| Preuss et al., 2004 [20] | Randomized, double-blind, placebo-controlled study. | 60 subjects BMI > 26 aged 21 to 50 years Control: 20 Intervention 1:20 Intervention 2:20 | 2000 Kcal/d - Proteins: 17% - Lipids: 25% - Carbohydrates: 58% 30 min supervised walking exercise program (5 days a week). | HCA-SX (4667 mg) divided in 3 doses: Intervention 1:2800 mg/d of HCA Intervention 2:2800 mg/d of HCA, 4 mg niacin-bound chromium and 400 mg Gymnema sylvestre extract. | 2 months | Significant weight lossand reduction in food intake in both intervention groups. | Reduction in total cholesterol, LDL-c, TG and serum leptin levels in both intervention groups. Increase in HDL-c and excretion of urinary fat metabolites in both intervention groups. |
| Roongpisuthipong et al., 2007 [21] | Randomized, double-blind, placebo-controlled study | 50 women BMI 25–30 aged 18 to 75 years Control: 25 Intervention: 25 | 1000 Kcal/d - Proteins: 50 g - Fats: 33 g - Carbohydrates: 125 g | Sachets: 1.15 g of Garcinia artroviridis (HCA) 3 times/day | 2 months | Significant weight loss during the first 4 weeks. No significant differences over the following 4 weeks. Decrease in fat mass, bicipital, subscapular and suprailiac folds and upper arm circumference. | Increase in lean mass and body water. Decrease in TG. |
| Toromanyan et al., 2007 [22] | Double blind, randomized, parallel group, placebo-controlled study. | 60 subjects BMI 25–44 aged 25 to 65 years Control: 30 Intervention: 30. | Diet and exercise performed regularly. | Slim339™ (tablets): 132 mg of Garcinia cambogia (HCA) + Matricaria chamomilla, Rosa damascena, Lavandula officinalis and Cananga odorata 3 times/d. | 2 months | Significant weight reduction. | |
| Vasques et al., 2008 [23] | Randomized double-blind study | 58 subjects BMI 30–39.9 aged 25 to 60 years Control: 26 Intervention: 32 | Capsules: 800 mg of Garcinia cambogia (HCA) + 500 mg de Amorphophallus konjac → 3 times/day | 3 months | No significant reduction in body weight. | Reduction in total cholesterol and LDL-c. | |
| Kim et al., 2011 [24] | Randomized, double-blind, placebo-controlled study | 86 subjects BMI 23–29 aged 20 to 60 years. Control: 29 Glycine max leaves (GML): 28 Garcinia cambogia (GC): 29 | Diet and habitual physical activity | Pills: 2 g/d of the substances corresponding to each group. In the case of the placebo and GML 4 pills/d and for Garcinia cambogia 8 pills/day. | 2.5 months | No significant reduction in body weight. | GML reduced total cholesterol and increased HDL-c (significant differences compared to the placebo group and the GC and placebo group, respectively). |
| Lu et al., 2012 [25] | Randomized double-blind study | 114 overweight subjects | Nutritional education | Super CitriMax™ (HCA) 2800 mg/day | 2 months | No significant reduction in body weight. | |
| Stern et al., 2013 [26] | Randomized, double-blind, placebo-controlled clinical study | 60 subjects BMI 30–40 aged 21 to 50 years Control: 30 Intervention: 30 | Participants were given free prepared meals. 2000 Kcal/d - Proteins: 14% - Lipids: 25% - Carbohydrates: 61% Physical activity (walking) 30 min, 5 times/d. | Capsules: 400 mg of Sphaeranthus indicus + Garcinia mangostana, ratio 3:1 2 times/day | 2 months | Significant reduction in body weight, BMI and waist circumference. | Decrease in total cholesterol and TG and increase in adiponectin. |
| Stern et al., 2013 [27] | Randomized, double-blind, placebo-controlled clinical study | 95 subjects BMI 30–40 aged 36 to 40 years Control: 46 Intervention: 49 | Same diet and physical activityas in reference 26. In this case the diet is divided into 3 intakes. | Same treatment as in reference 26. | 2 months | Reduction in body weight, BMI, waist and hip circumferences. | Decrease in total cholesterol, TG and fasting glucose. Increase in adiponectin. Improvement in physical function and self-esteem (IWQOL questionnaire). |
| Chong et al., 2014 [28] | Randomized, placebo-controlled, double-blind parallel group study | 91 caucasian subjects BMI 25–32 aged 18 to 60 years Control: 45 Intervention: 46 | Dietary advice + balanced diet with a deficit of 500 Kcal. −30% lipids. | Tablets: 850 mg 3 tablets 2 times/day. Composition: 650 mg of Garcinia cambogia (HCA) + 100 mg of Camellia sinensis + 75 mg of Coffea arabica + 25 mg of Lagerstroemia speciosa | 3.5 months | Significant weight loss and reduction in BMI, body fat, waist and hip circumferences | |
| Vasques et al., 2014 [29] | Randomized double-blind study | 43 women BMI > 25) aged 25 to 60 years Control: 13 Intervention: 30 | Individualized diet, with an average caloric restriction of 1523 ± 185 Kcal/day Regular physical activity. | Capsules: 800 mg of Garcinia cambogia (HCA) 3 times/day | 2 months | No statistically significant differences. | Reduction in TG level. |
| Kudiganti et al., 2016 [30] | Randomized, double-blind, placebo-controlled clinical study | 60 subjects mean BMI of 28.3 aged 21 to 50 years Control: 30 Intervention: 30. | 2000 Kcal/d - Proteins: 17% - Lipids: 25% - Carbohydrates: 58% | Capsules: 400 mg of Meratrim™: extracts from the flower heads of Sphaeranthus indicus and the fruit rinds of Garcinia mangostana 2 times/day | 4 months | Significant weight loss and reduction in BMI, waist and hip circumferences. | Reduction in TG and LDL-c cholesterol. Increase in HDL-c. |
| Maia-Landim et al., 2018 [31] | Non-randomized prospective controlled intervention study | 214 subjects BMI > 25 older than 18 years | Balanced diet and regular physical activity, smoking not permitted and controlof alcohol intake. | Capsules: 500 mg of Garcinia cambogia (HCA) + 500 mg of Amorphophallus konjac 2 times/day | 6 months | Reduction in total fat mass and visceral fat mass after 3 and 6 months of intervention. | Increase in basal metabolic rate Reduction in glucose, total cholesterol and TG. |
| Watanabe et al., 2018 [32] | Prospective, randomized, controlled, parallel study | 22 obese women with insulin resistance aged 18 to 65 years Control: 11 Intervention: 11 | Hypocaloric diet (300 Kcal restriction) + physical activity of moderate intensity - Proteins: 20–25% - Lipids: 30% - Carbohydrates: 45–50% | Capsules: 400 mg of Garcinia mangostana 1 time/day | 6.5 months | No significant reduction in body weight. | Reduction in insulin concentration and HOMA-IR. |
| Reference | Ageyears | Sex | Type of Supplement | Duration | Symptoms | Test Performed | Diagnosis/Type of Liver Injury |
|---|---|---|---|---|---|---|---|
| Stevens et al., 2005 [45] | 27 | M | Hydroxycut™ 3 capsules, 3 times/d. | 5 weeks | Fatigue and jaundice. | Laboratory analysis: elevated AST, ALT, AF and PT. Serological study: negative. | Hepatotoxicity Cholestatic liver injury pattern. |
| Stevens et al., 2005 [45] | 30 | M | Hydroxycut™ 9 capsules/d. | 5 days | Fever, vomiting, fatigue, and jaundice. | Laboratory analysis: AST, ALT, AF and PT elevated and low albumin. Serological study: negative. CT and cholangiography: normal. | Hepatotoxicity Hepatocyte necrosis was the likely pattern of injury. |
| Dara et al., 2008 [46] | 40 | W | Hydroxycut™ 6 capsules/d. | 1 week | Mid-epigastric abdominal pain, non-bloody diarrhea, fevers, chills, nausea, vomiting, anorexia and profound fatigue. | Laboratory analysis: acute hepatitis (elevated AST, ALT and AF) Serological study: negative. | Acute hepatitis. |
| Dara et al., 2008 [46] | 33 | W | Hydroxycut™ | 2 weeks | Nausea, crampy abdominal pain, jaundice, acholic stools, dark-colored urine, pruritus and profound fatigue. | Physical examination: jaundice and scleral icterus. Laboratory analysis: elevated AST, ALT, TB and DB. Serological study: negative. | Acute hepatitis. |
| Shuster et al., 2010 [47] | 25 | M | Exilis: Garcinia cambogia, Garcinia sylvestre, L-carnintine and chrome | 3 weeks | Two weeks after starting treatment: fatigue and dark urine. In the third week: fever, nausea, vomiting and pain. | Laboratory analysis: elevated ALT, AST, TB and INRA comprehensive study was conducted to determine the etiology of liver damage, but all tests were negative. | Hepatic encephalopathy Liver transplantation required. |
| Sharma et al., 2010 [48] | 19 | M | Hydroxycut™ | 1 week | Fever, severe fatigue, myalgia, arthralgia, and erythematous rash over in lower extremities. | Physical examination: toxic appearance, marked jaundice and fever (39.4 °C). Laboratory analysis: elevated ALT, AF, bilirubin and PT. low blood cell count and hemoglobin. Blood culture, urinalysis, X-rays, abdominal ultrasound, CT and MRCP: normal. Serological study: negative. Hepatic biopsy: acute cholangitis. | Acute cholangitis |
| Mancano et al., 2015 [49] | 42 | W | Garcinia cambogia pure | 1 week | Right upper quadrant abdominal pain and nausea (without emesis). | Laboratory analysis: elevated ALT, AST, AF, ferritin and INR Serological study: negative. Abdominal ultrasound: normal. | Acute hepatitis |
| Melendez-Rosado et al., 2015 [50] | 42 | W | Garcinia cambogia pure | 1 week | Abdominal pain in the right upper quadrant, nausea without emesis and clamminess. | Laboratory analysis: elevated ALT, AST, AF and ferritin. Serological study: negative. Abdominal ultrasound: mildy coarse hepatic echotexture. CT: normal. | Acute hepatitis |
| Araujo et al., 2015 [51] | 41 | M | Hydroxycut SX-7 Clean Sensory™ 2 capsules/day 4 times/week | 2 months | Malaise, jaundice, fatigue, nausea, vomiting and asterixis | Physical examination: jaundice and liver edge percussed. Laboratory analysis: elevated AST, ALT, TB, DB, PT and creatinine. Serological study: negative. Abdominal ultrasound: increased liver echogenicity and liver length. CIOMS/RUCAM: 9 | Acute hepatocellular liver injury |
| Smith et al., 2016 [52] | 26 | M | Multi-ingredient protein supplement with Garcinia cambogia (70%). | 1 week | Jaundice, fatigue and asterixis. | Laboratory analysis: elevated ALT, AST, AFand bilirubin. Serological study: negative. Hepatic biopsy: liver necrosis. CIOMS: 6 | Hepatotoxicity Liver transplantation required. |
| Corey et al., 2016 [53] | 52 | W | Garcinia cambogia supplement: Garcinia cambogia extract (936 mg, 60% HCA), calcium, chromium, potassium 2 capsules/d (1000 mg/day) | 3.5 weeks | Decreased appetite, worsening fatigue, and intermittent confusion. | Physical examination: abdominal distention and jaundice. Laboratory analysis: elevated ALT, AST, AF, TB, DB and INR, and low platelet count. CT: nodular liver compatible with necrosis and ascites. Serological study: negative. Biopsy: severe acute hepatitis with necrosis and parenchymal collapse. MELD: it was evolving until it reached a score of 40. CIOMS: 7 | Acute liver failure. Liver transplantation required. |
| Lunsford et al., 2016 [54] | 34 | M | Garcinia cambogia pure 2 capsules of 80 mg, 3 times/day | 6.5 months. | Nausea, vomiting, abdominal pain, and dark urine. | Laboratory analysis: elevated transaminases and bilirubin. Asterixis, jaundice, and confusion. Elevated transaminases, bilirubin, elevated INR. Images: cirrhosis or hepatocellular carcinoma. MR: no tumor process.Serological study: positive antinuclear antibody.Hepatic biopsy: necrosis with collapse of the liver architecture. | Severe liver injury. Liver transplantation required. |
| Crescioli et al., 2018 [55] | 61 | W | SUPER ANANAS SLIM™: Garcinia cambogia (60%), Ananas comosus and Ilex paraguariensis. | 2 months | Abdominal pain, nausea, progressive weakness, jaundice, dark stools, and acholic stools. | Laboratory analysis: ALT, AST, TB, DB, albumin, AF, GGT out of normal range. Serological study: negative. Abdominal ultrasound, MRI. Doppler: normal. CT: small peritoneal effusion and perihepatic lymphadenopathy. Biopsy: cholestatic hepatitis. CIOMS: 7. | Herbal-induced liver damage. |
| Crescioli et al., 2018 [55] | 39 | W | Two sipplements: OBLESS™: Garcinia cambogia (72 mg of HCA) and other components: 1 capsule/day and | 1 month | Jaundice, asthenia, loss of appetite, and right hypochondrial pain. | Laboratory analysis: elevated ALT, AST, TB, DB, AF, GGT, CRP and lactate dehydrogenase. Serological study: nonspecific antinuclear antibodies and positive bile antibodies. Abdominal ultrasound: normal. CIOMS: 6 | Acute cholestatic |
| Magistral preparation of different herbs extracts: 1 capsule/day | 15 days | ||||||
| Crescioli et al., 2018 [55] | 47 | W | THERMO GIALLO™: Garcinia cambogia (200 mg HCA) and chromium: y 2 capsules/da. | 1 month | Severe abdominal pain. | Laboratory analysis: elevated AST, ALT and TB. Serological study: negative. CIOMS: 6. | Acute hepatitis. |
| Crescioli et al., 2018 [55] | 52 | W | 2 JILL COOPER BE SLIM™: Garcinia cambogia (240 mg) and Green Coffee extract 1 capsule / d of each product | 1 month | Laboratory analysis: elevated AST, ALT, BT, GGT and AF. Serological test: negative. CIOMS: 6. | Acute hepatitis. | |
| Sharma et al., 2018 [56] | 57 | W | Garcinia cambogia (100%) and vitamin A and D supplement 2 capsules/d (2800 mg/d) | 1 month | Abdominal pain (more intense in the right upper quadrant) and vomiting. | Laboratory analysis: elevated ALT, AST, TB, DB, INR, PT. Normal vitamin A and D levels. Serological study: negative Abdominal ultrasound: normal liver CIOMS/RUCAM: 11 | Hepatitis secondary to the consumption of Garcinia cambogia. After withdrawal of the supplement the levels of the altered enzymes normalized. After six months they elevated again, coinciding with the reintroduction of the supplement. |
| Philips et al., 2018 [57] | 33 | W | Safe Lean™: Garcinia cambogia (600 mg), Allium sativum (250 mg) and Trigonella foenum graecum (100 mg) 1 capsule, 2 times/day | 1 month | Nausea, loss of appetite | Laboratory analysis: elevated AST, ALT, AF, TB, gamma-glutamyl transferase, albumin and INR. Serological study: negative. CT: hepatomegaly. RUCAM: 8 | Drug induced liver injury, secondary to Safe Lean™. |
| Calcium, vitamin A and folic acid supplement 1 time/day | 3 months | ||||||
| Yousaf et al., 2019 [58] | 21 | W | Garcinia cambogia 1400 mg/day | 4 weeks | Abdominal pain for 1 wk associated with nausea, vomiting, anorexia and myalgias. | Abdominal ultrasound Laboratory analysis: elevated ALT, AST, alkaline phosphatase | Hepatomegaly Acute liver failure. |
| Khetpal et al., 2020 [59] | 22 | W | Hydroxycut™ 2 capsules/day | 3 months | Chest pain, fatigue, palpitations and shortness of breath | Physical examination: tachycardia, low oxygen saturation and asterixis. Laboratory analysis: elevated AST, ALT, INR, leukocytes and white blood cells. Serological study: negative. Abdominal ultrasound: hepatomegaly. RUCAM: 9. | Acute drug-induced liver injury likely due to Hydroxycut™. |
| Ferreira et al., 2020 [60] | 26 | W | 1800 mg of Garcinia cambogia (900 mg HCA), 1275 mg of green tea extract with 450 mg of Veldt raisin and 1200 mg of Coffea arabica daily. | 7 months | Fatigue, nausea and jaundice. | Laboratory analysis: acute hepatitis (elevated AST, ALT, TB and INR). Abdominal Ultrasound: normal. MRCP: normal. Transjugular liver biopsy: acute hepatitis. Serological study: negative. RUCAM: 6. | Subacute liver failure secondary to the consumption of Garcinia cambogia. Liver transplantation required. |
| Reference | Ageyears | Sex | Previous Psychiatric History | Type of Supplement and Treatment Duration | Psychotropic/Antidepressant Drugs | Symptoms | Diagnosis |
|---|---|---|---|---|---|---|---|
| Lopez et al., 2014 [63] | 35 | W | No | 1000 mg Garcinia cambogia (60% HCA), chromium, potassium and calcium 2 capsules, 3 times/day. 2–3 months. | Yes: escitalopram (SSRI). 1 year. | Stuttering speech pattern, spontaneous ankle clonus, bilateral ocular clonus, rhythmic jaw movements, profuse sweating, hypertension, tachycardia, and hyperreflexia. | Serotonin toxicity associated with Garcinia cambogia ingestion. |
| Hendrickson et al., 2016 [64] | 50 | M | Type I bipolar disorder | Garcinia cambogia: 2 capsules/day. 2 months. | No: he had been stable off medications for 6 years. | Irritability, pressured speech, grandiosity, excessive spending, increased social activity and decreased need for sleep. | Bipolar I disorder, manic, severe. |
| 25 | M | No | Garcinia cambogia: 1–2 capsules/day.2 months. | No | Inflated self-esteem, grandiosity, decreased need for sleep, increased activity, excessive spending, pressured speech, paranoia and religious delusions. | Bipolar I disorder, manic, severe with psychosis. | |
| 34 | W | Type II bipolar disorder and past SSRI-induced hypomania | Garcinia cambogia For 1–2 months. | Yes: aripiprazole, bupropion and topiramate. | Irritability, pressured speech, decreased need for sleep and agitation. | Recurrence of bipolar disorder type II, hypomania, moderate. | |
| Cotovio et al., 2017 [65] | 51 | W | Type I bipolar disorder | Garcinia cambogia, calcium, chromium and potassium. | Yes: paroxetine (SSRI) and valproic acid | Irritability, agitation, increased energy and decreased need for sleep. | Hypomanic episode associated with ingestion of Garcinia cambogia. |
| Nguyen et al., 2019 [66] | 22 | W | No | Garcinia cambogia PlusTM: 500 mg Garcinia cambogia per capsule (60% HCA) 1 capsule/day during de first 5 days and then 3 capsules/d during the next 5 days. | No | Expansive mood, psychomotor agitation, disorganized and pressured speech, flight of ideas, grandiosity, delusions and auditory hallucinations. | Mania and psychosis secondary to Garcinia cambogia ingestion. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andueza, N.; Giner, R.M.; Portillo, M.P. Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight. Nutrients 2021, 13, 450. https://doi.org/10.3390/nu13020450
Andueza N, Giner RM, Portillo MP. Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight. Nutrients. 2021; 13(2):450. https://doi.org/10.3390/nu13020450
Chicago/Turabian StyleAndueza, Naroa, Rosa M. Giner, and Maria P. Portillo. 2021. "Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight" Nutrients 13, no. 2: 450. https://doi.org/10.3390/nu13020450
APA StyleAndueza, N., Giner, R. M., & Portillo, M. P. (2021). Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight. Nutrients, 13(2), 450. https://doi.org/10.3390/nu13020450








