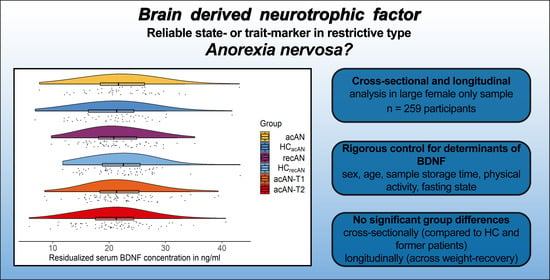
Is Serum BDNF Altered in Acute, Short- and Long-Term Recovered Restrictive Type Anorexia Nervosa?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. BDNF Measurements
2.4. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Comparisons of Serum BDNF Concentrations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treasure, J.; Zipfel, S.; Micali, N.; Wade, T.; Stice, E.; Claudino, A.; Schmidt, U.; Frank, G.K.; Bulik, C.M.; Wentz, E. Anorexia Nervosa. Nat. Rev. Dis. Primer 2015, 1, 15074. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia Nervosa: Aetiology, Assessment, and Treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Frank, G.K.W. The Perfect Storm—A Bio-Psycho-Social Risk Model for Developing and Maintaining Eating Disorders. Front. Behav. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Berner, L.A.; Brown, T.A.; Lavender, J.M.; Lopez, E.; Wierenga, C.E.; Kaye, W.H. Neuroendocrinology of Reward in Anorexia Nervosa and Bulimia Nervosa: Beyond Leptin and Ghrelin. Mol. Cell. Endocrinol. 2019, 497, 110320. [Google Scholar] [CrossRef] [PubMed]
- Eddy, K.T.; Lawson, E.A.; Meade, C.; Meenaghan, E.; Horton, S.E.; Misra, M.; Klibanski, A.; Miller, K.K. Appetite Regulatory Hormones in Women with Anorexia Nervosa: Binge-Eating/Purging versus Restricting Type. J. Clin. Psychiatry 2015, 76, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Merle, J.V.; Haas, V.; Burghardt, R.; Döhler, N.; Schneider, N.; Lehmkuhl, U.; Ehrlich, S. Agouti-Related Protein in Patients with Acute and Weight-Restored Anorexia Nervosa. Psychol. Med. 2011, 41, 2183–2192. [Google Scholar] [CrossRef]
- Monteleone, P.; Maj, M. Dysfunctions of Leptin, Ghrelin, BDNF and Endocannabinoids in Eating Disorders: Beyond the Homeostatic Control of Food Intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Tam, F.I.; Seidel, M.; Boehm, I.; Ritschel, F.; Bahnsen, K.; Biemann, R.; Weidner, K.; Roessner, V.; Ehrlich, S. Peptide YY3–36 Concentration in Acute- and Long-Term Recovered Anorexia Nervosa. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Barde, Y.-A. Trophic Factors and Neuronal Survival. Neuron 1989, 2, 1525–1534. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-Derived Neurotrophic Factor. Growth Factors Chur Switz. 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Waterhouse, E.G.; Xu, B. New Insights into the Role of Brain-Derived Neurotrophic Factor in Synaptic Plasticity. Mol. Cell. Neurosci. 2009, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Green, M.J.; Matheson, S.L.; Shepherd, A.; Weickert, C.S.; Carr, V.J. Brain-Derived Neurotrophic Factor Levels in Schizophrenia: A Systematic Review with Meta-Analysis. Mol. Psychiatry 2011, 16, 960–972. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.-M. Decreased Serum Brain-Derived Neurotrophic Factor Levels in Major Depressed Patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Hemmings, S.M.J.; Seedat, S. Brain-Derived Neurotrophic Factor (BDNF) Protein Levels in Anxiety Disorders: Systematic Review and Meta-Regression Analysis. Front. Integr. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Monteleone, P.; Fabrazzo, M.; Martiadis, V.; Serritella, C.; Pannuto, M.; Maj, M. Circulating Brain-Derived Neurotrophic Factor Is Decreased in Women with Anorexia and Bulimia Nervosa but Not in Women with Binge-Eating Disorder: Relationships to Co-Morbid Depression, Psychopathology and Hormonal Variables. Psychol. Med. 2005, 35, 897–905. [Google Scholar] [CrossRef]
- Nakazato, M.; Hashimoto, K.; Shimizu, E.; Kumakiri, C.; Koizumi, H.; Okamura, N.; Mitsumori, M.; Komatsu, N.; Iyo, M. Decreased Levels of Serum Brain-Derived Neurotrophic Factor in Female Patients with Eating Disorders. Biol. Psychiatry 2003, 54, 485–490. [Google Scholar] [CrossRef]
- Rios, M. BDNF and the Central Control of Feeding: Accidental Bystander or Essential Player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef]
- Rosas-Vargas, H.; Martínez-Ezquerro, J.D.; Bienvenu, T. Brain-Derived Neurotrophic Factor, Food Intake Regulation, and Obesity. Arch. Med. Res. 2011, 42, 482–494. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A High-Fat, Refined Sugar Diet Reduces Hippocampal Brain-Derived Neurotrophic Factor, Neuronal Plasticity, and Learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Chan, K.L.; Tong, K.Y.; Yip, S.P. Relationship of Serum Brain-Derived Neurotrophic Factor (BDNF) and Health-Related Lifestyle in Healthy Human Subjects. Neurosci. Lett. 2008, 447, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Galbete, C.; Martinez-González, M.Á.; Martinez, J.A.; Razquin, C.; Salas-Salvadó, J.; Estruch, R.; Buil-Cosiales, P.; Martí, A. The Effect of the Mediterranean Diet on Plasma Brain-Derived Neurotrophic Factor (BDNF) Levels: The PREDIMED-NAVARRA Randomized Trial. Nutr. Neurosci. 2011, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- An, J.J.; Liao, G.-Y.; Kinney, C.E.; Sahibzada, N.; Xu, B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015, 22, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Numan, S.; Seroogy, K.B. Expression of TrkB and TrkC MRNAs by Adult Midbrain Dopamine Neurons: A Double-Label in Situ Hybridization Study. J. Comp. Neurol. 1999, 403, 295–308. [Google Scholar] [CrossRef]
- Okazawa, H.; Murata, M.; Watanabe, M.; Kamei, M.; Kanazawa, I. Dopaminergic Stimulation Up-Regulates the in vivo Expression of Brain-Derived Neurotrophic Factor (BDNF) in the Striatum. FEBS Lett. 1992, 313, 138–142. [Google Scholar] [CrossRef]
- Tyler, W.J.; Alonso, M.; Bramham, C.R.; Pozzo-Miller, L.D. From Acquisition to Consolidation: On the Role of Brain-Derived Neurotrophic Factor Signaling in Hippocampal-Dependent Learning. Learn. Mem. 2002, 9, 224–237. [Google Scholar] [CrossRef]
- Harrison, A.; O’Brien, N.; Lopez, C.; Treasure, J. Sensitivity to Reward and Punishment in Eating Disorders. Psychiatry Res. 2010, 177, 1–11. [Google Scholar] [CrossRef]
- Kaye, W.H.; Fudge, J.L.; Paulus, M. New Insights into Symptoms and Neurocircuit Function of Anorexia Nervosa. Nat. Rev. Neurosci. 2009, 10, 573–584. [Google Scholar] [CrossRef]
- O’Hara, C.B.; Campbell, I.C.; Schmidt, U. A Reward-Centred Model of Anorexia Nervosa: A Focussed Narrative Review of the Neurological and Psychophysiological Literature. Neurosci. Biobehav. Rev. 2015, 52, 131–152. [Google Scholar] [CrossRef]
- Steinglass, J.; Walsh, B.T. Habit Learning and Anorexia Nervosa: A Cognitive Neuroscience Hypothesis. Int. J. Eat. Disord. 2006, 39, 267–275. [Google Scholar] [CrossRef]
- Brandys, M.K.; Kas, M.J.H.; van Elburg, A.A.; Campbell, I.C.; Adan, R.A.H. A Meta-Analysis of Circulating BDNF Concentrations in Anorexia Nervosa. World J. Biol. Psychiatry 2011, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Izquierdo, A.; Slattery, M.; Becker, K.R.; Plessow, F.; Thomas, J.J.; Eddy, K.T.; Lawson, E.A.; Misra, M. Changes in Appetite-Regulating Hormones Following Food Intake Are Associated with Changes in Reported Appetite and a Measure of Hedonic Eating in Girls and Young Women with Anorexia Nervosa. Psychoneuroendocrinology 2020, 113, 104556. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Tortorella, A.; Martiadis, V.; Serritella, C.; Fuschino, A.; Maj, M. Opposite Changes in the Serum Brain-Derived Neurotrophic Factor in Anorexia Nervosa and Obesity. Psychosom. Med. 2004, 66, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Tyszkiewicz-Nwafor, M.; Rybakowski, F.; Dmitrzak-Weglarz, M.; Skibinska, M.; Paszynska, E.; Dutkiewicz, A.; Słopien, A. Brain-Derived Neurotrophic Factor and Oxytocin Signaling in Association with Clinical Symptoms in Adolescent Inpatients with Anorexia Nervosa—A Longitudinal Study. Front. Psychiatry 2020, 10, 1032. [Google Scholar] [CrossRef]
- Dalton, B.; Campbell, I.; Chung, R.; Breen, G.; Schmidt, U.; Himmerich, H. Inflammatory Markers in Anorexia Nervosa: An Exploratory Study. Nutrients 2018, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Watanabe, K.; Hashimoto, E.; Saito, T. Low Serum BDNF and Food Intake Regulation: A Possible New Explanation of the Pathophysiology of Eating Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 312–316. [Google Scholar] [CrossRef]
- Dmitrzak-Weglarz, M.; Skibinska, M.; Slopien, A.; Tyszkiewicz, M.; Pawlak, J.; Maciukiewicz, M.; Zaremba, D.; Rajewski, A.; Hauser, J. Serum Neurotrophin Concentrations in Polish Adolescent Girls with Anorexia Nervosa. Neuropsychobiology 2013, 67, 25–32. [Google Scholar] [CrossRef]
- Zwipp, J.; Hass, J.; Schober, I.; Geisler, D.; Ritschel, F.; Seidel, M.; Weiss, J.; Roessner, V.; Hellweg, R.; Ehrlich, S. Serum Brain-Derived Neurotrophic Factor and Cognitive Functioning in Underweight, Weight-Recovered and Partially Weight-Recovered Females with Anorexia Nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 163–169. [Google Scholar] [CrossRef]
- Nakazato, M.; Hashimoto, K.; Yoshimura, K.; Hashimoto, T.; Shimizu, E.; Iyo, M. No Change between the Serum Brain-Derived Neurotrophic Factor in Female Patients with Anorexia Nervosa before and after Partial Weight Recovery. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1117–1121. [Google Scholar] [CrossRef]
- Phillips, K.E.; Jimerson, D.C.; Pillai, A.; Wolfe, B.E. Plasma BDNF Levels Following Weight Recovery in Anorexia Nervosa. Physiol. Behav. 2016, 165, 300–303. [Google Scholar] [CrossRef]
- Ehrlich, S.; Salbach-Andrae, H.; Eckart, S.; Merle, J.V.; Burghardt, R.; Pfeiffer, E.; Franke, L.; Uebelhack, R.; Lehmkuhl, U.; Hellweg, R. Serum Brain-Derived Neurotrophic Factor and Peripheral Indicators of the Serotonin System in Underweight and Weight-Recovered Adolescent Girls and Women with Anorexia Nervosa. J. Psychiatry Neurosci. JPN 2009, 34, 323–329. [Google Scholar] [PubMed]
- Bus, B.A.A.; Molendijk, M.L.; Penninx, B.J.W.H.; Buitelaar, J.K.; Kenis, G.; Prickaerts, J.; Elzinga, B.M.; Voshaar, R.C.O. Determinants of Serum Brain-Derived Neurotrophic Factor. Psychoneuroendocrinology 2011, 36, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between Serum and Plasma Brain-Derived Neurotrophic Factor and Influence of Storage Time and Centrifugation Strategy. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Bocchio-Chiavetto, L.; Bagnardi, V.; Zanardini, R.; Molteni, R.; Gabriela Nielsen, M.; Placentino, A.; Giovannini, C.; Rillosi, L.; Ventriglia, M.; Riva, M.A.; et al. Serum and Plasma BDNF Levels in Major Depression: A Replication Study and Meta-Analyses. World J. Biol. Psychiatry 2010, 11, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Elfving, B.; Plougmann, P.H.; Wegener, G. Detection of Brain-Derived Neurotrophic Factor (BDNF) in Rat Blood and Brain Preparations Using ELISA: Pitfalls and Solutions. J. Neurosci. Methods 2010, 187, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimine, S.; Sugawara, N.; Ishioka, M.; Yasui-Furukori, N. Preanalysis Storage Conditions Influence the Measurement of Brain-Derived Neurotrophic Factor Levels in Peripheral Blood. Neuropsychobiology 2014, 69, 83–88. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor: A Systematic Review of Experimental Studies in Human Subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A Meta-Analytic Review of the Effects of Exercise on Brain-Derived Neurotrophic Factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Naegelin, Y.; Dingsdale, H.; Säuberli, K.; Schädelin, S.; Kappos, L.; Barde, Y.-A. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Trajkovska, V.; Marcussen, A.B.; Vinberg, M.; Hartvig, P.; Aznar, S.; Knudsen, G.M. Measurements of Brain-Derived Neurotrophic Factor: Methodological Aspects and Demographical Data. Brain Res. Bull. 2007, 73, 143–149. [Google Scholar] [CrossRef]
- Frank, G.K.W. Altered Brain Reward Circuits in Eating Disorders: Chicken or Egg? Curr. Psychiatry Rep. 2013, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Jimerson, D.C.; Wolfe, B.E. Neuropeptides in Eating Disorders. CNS Spectr. 2004, 9, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.; Quadflieg, N. Strukturiertes Inventar für Anorektische und Bulimische Essstörungen (SIAB); Fragebogen (SIABS) Und Interview (SIAB-EX) Nach DSM-IV Und ICD-10; Handanweisung; Hogrefe: Göttingen, Germany, 1999. [Google Scholar]
- Bernardoni, F.; King, J.A.; Geisler, D.; Stein, E.; Jaite, C.; Nätsch, D.; Tam, F.I.; Boehm, I.; Seidel, M.; Roessner, V.; et al. Weight Restoration Therapy Rapidly Reverses Cortical Thinning in Anorexia Nervosa: A Longitudinal Study. NeuroImage 2016, 130, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Doose, A.; King, J.A.; Bernardoni, F.; Geisler, D.; Hellerhoff, I.; Weinert, T.; Roessner, V.; Smolka, M.N.; Ehrlich, S. Strengthened Default Mode Network Activation during Delay Discounting in Adolescents with Anorexia Nervosa after Partial Weight Restoration: A Longitudinal FMRI Study. J. Clin. Med. 2020, 9, 900. [Google Scholar] [CrossRef]
- Thiel, A.; Jacobi, C.; Horstmann, S.; Paul, T.; Nutzinger, D.O.; Schüssler, G. A German version of the Eating Disorder Inventory EDI-2. Psychother. Psychosom. Med. Psychol. 1997, 47, 365–376. [Google Scholar]
- Hautzinger, M.; Kühner, C.; Keller, F. Beck-Depressions-Inventar (BDI-II); Hogrefe Publishing Corp: Göttingen, Germany, 2006. [Google Scholar]
- von Aster, M.; Neubauer, A.; Horn, R. Wechsler Intelligenztest für Erwachsene WIE. Deutschsprachige Bearbeitung und Adaptation des WAIS-III von David Wechsler (2. Korrigierte Auflage); Hogrefe Publishing Corp: Göttingen, Germany, 2006. [Google Scholar]
- Petermann, F.; Petermann, U. Hamburg Wechsler Intelligenztest Für Kinder IV (HAWIK-IV), 4th ed.; Huber: Göttingen, Germany, 2007. [Google Scholar]
- Ehrlich, S.; Burghardt, R.; Schneider, N.; Broecker-Preuss, M.; Weiss, D.; Merle, J.V.; Craciun, E.M.; Pfeiffer, E.; Mann, K.; Lehmkuhl, U.; et al. The Role of Leptin and Cortisol in Hyperactivity in Patients with Acute and Weight-Recovered Anorexia Nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 658–662. [Google Scholar] [CrossRef]
- Holtkamp, K.; Herpertz-Dahlmann, B.; Mika, C.; Heer, M.; Heussen, N.; Fichter, M.; Herpertz, S.; Senf, W.; Blum, W.F.; Schweiger, U.; et al. Elevated Physical Activity and Low Leptin Levels Co-Occur in Patients with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2003, 88, 5169–5174. [Google Scholar] [CrossRef]
- Hemmelmann, C.; Brose, S.; Vens, M.; Hebebrand, J.; Ziegler, A. Perzentilen des Body-Mass-Index auch für 18- bis 80-Jährige? Daten der Nationalen Verzehrsstudie II. DMW Dtsch. Med. Wochenschr. 2010, 135, 848–852. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Seidel, M.; King, J.A.; Ritschel, F.; Döpmann, J.; Bühren, K.; Seitz, J.; Roessner, V.; Westphal, S.; Egberts, K.; Burghardt, R.; et al. Serum Visfatin Concentration in Acutely Ill and Weight-Recovered Patients with Anorexia Nervosa. Psychoneuroendocrinology 2015, 53, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hellerhoff, I.; King, J.A.; Tam, F.I.; Pauligk, S.; Seidel, M.; Geisler, D.; Bahnsen, K.; Kretschmann, N.; Akgün, K.; Roessner, V.; et al. Differential Longitudinal Changes of Neuronal and Glial Damage Markers in Anorexia Nervosa after Partial Weight Restoration. Transl. Psychiatry 2021. [Google Scholar] [CrossRef]
- Munkres, J. Algorithms for the Assignment and Transportation Problems. J. Soc. Ind. Appl. Math. 1957, 5, 32–38. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.-J. Using Bayes Factor Hypothesis Testing in Neuroscience to Establish Evidence of Absence. Nat. Neurosci. 2020, 23, 788–799. [Google Scholar] [CrossRef]
- Quintana, D.S.; Williams, D.R. Bayesian Alternatives for Common Null-Hypothesis Significance Tests in Psychiatry: A Non-Technical Guide Using JASP. BMC Psychiatry 2018, 18, 178. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; ISBN 3-900051-07-0. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Hlavac, M. Stargazer: Well-Formatted Regression and Summary Statistics Tables; Central European Labour Studies Institute (CELSI): Bratislava, Slovakia, 2018. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Lüdecke, D. Automated Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. J. Open Source Softw 2020, 5, 2815. [Google Scholar] [CrossRef]
- Allen, M.; Poggiali, D.; Whitaker, K.; Marshall, T.R.; Kievit, R.A. Raincloud Plots: A Multi-Platform Tool for Robust Data Visualization. Wellcome Open Res. 2019, 4, 63. [Google Scholar] [CrossRef]
- JASP Team. JASP (Version 0.14) [Computer Software]. Available online: https://jasp-stats.org/faq/how-do-i-cite-jasp/ (accessed on 30 October 2020).
- Polyakova, M.; Schlögl, H.; Sacher, J.; Schmidt-Kassow, M.; Kaiser, J.; Stumvoll, M.; Kratzsch, J.; Schroeter, M.L. Stability of BDNF in Human Samples Stored Up to 6 Months and Correlations of Serum and EDTA-Plasma Concentrations. Int. J. Mol. Sci. 2017, 18, 1189. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The Effects of Physical Activity and Exercise on Brain-Derived Neurotrophic Factor in Healthy Humans: A Review: Physical Activity and BDNF. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Ribasés, M.; Gratacòs, M.; Armengol, L.; de Cid, R.; Badía, A.; Jiménez, L.; Solano, R.; Vallejo, J.; Fernández, F.; Estivill, X. Met66 in the Brain-Derived Neurotrophic Factor (BDNF) Precursor Is Associated with Anorexia Nervosa Restrictive Type. Mol. Psychiatry 2003, 8, 745–751. [Google Scholar] [CrossRef]
- Nakazato, M.; Tchanturia, K.; Schmidt, U.; Campbell, I.C.; Treasure, J.; Collier, D.A.; Hashimoto, K.; Iyo, M. Brain-Derived Neurotrophic Factor (BDNF) and Set-Shifting in Currently Ill and Recovered Anorexia Nervosa (AN) Patients. Psychol. Med. 2009, 39, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Keane, K.; Wolfe, B.E. Peripheral Brain Derived Neurotrophic Factor (BDNF) in Bulimia Nervosa: A Systematic Review. Arch. Psychiatr. Nurs. 2014, 28, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yoshimura, C.; Nakajima, T.; Nagata, T. Recovery of Low Plasma BDNF over the Course of Treatment among Patients with Bulimia Nervosa. Psychiatry Res. 2012, 198, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Homan, P.; Grob, S.; Milos, G.; Schnyder, U.; Eckert, A.; Lang, U.; Hasler, G. The Role of BDNF, Leptin, and Catecholamines in Reward Learning in Bulimia Nervosa. Int. J. Neuropsychopharmacol. 2015, 18, pyu092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Front. Psychiatry 2018, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Halmi, K.A.; Sunday, S.R.; Klump, K.L.; Strober, M.; Leckman, J.F.; Fichter, M.; Kaplan, A.; Woodside, B.; Treasure, J.; Berrettini, W.H.; et al. Obsessions and Compulsions in Anorexia Nervosa Subtypes. Int. J. Eat. Disord. 2003, 33, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.K. Comorbid Depression and Anxiety in Childhood and Adolescent Anorexia Nervosa: Prevalence and Implications for Outcome: Depression and Anxiety in Anorexia Nervosa. Clin. Psychol. 2012, 16, 15–24. [Google Scholar] [CrossRef]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-Derived Neurotrophic Factor-Deficient Mice Develop Aggressiveness and Hyperphagia in Conjunction with Brain Serotonergic Abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional Deletion Of Brain-Derived Neurotrophic Factor in the Postnatal Brain Leads to Obesity and Hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Kernie, S.G. BDNF Regulates Eating Behavior and Locomotor Activity in Mice. EMBO J. 2000, 19, 1290–1300. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Hefti, F. BDNF and NGF treatment in lesioned rats: Effects on cholinergic function and weight gain. NeuroReport 1992, 3, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Yeo, G.S.H.; Cox, J.J.; Morton, J.; Adlam, A.-L.R.; Keogh, J.M.; Yanovski, J.A.; El Gharbawy, A.; Han, J.C.; Tung, Y.C.L.; et al. Hyperphagia, Severe Obesity, Impaired Cognitive Function, and Hyperactivity Associated with Functional Loss of One Copy of the Brain-Derived Neurotrophic Factor (BDNF) Gene. Diabetes 2006, 55, 3366–3371. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.S.H.; Connie Hung, C.-C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de Novo Mutation Affecting Human TrkB Associated with Severe Obesity and Developmental Delay. Nat. Neurosci. 2004, 7, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The Aging Hippocampus: Interactions between Exercise, Depression, and BDNF. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2012, 18, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-Derived Neurotrophic Factor in Patients with Huntington’s Disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of Brain-Derived Neurotrophic Factor across the Blood–Brain Barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Karege, F.; Schwald, M.; Cisse, M. Postnatal Developmental Profile of Brain-Derived Neurotrophic Factor in Rat Brain and Platelets. Neurosci. Lett. 2002, 328, 261–264. [Google Scholar] [CrossRef]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal Long-Term Potentiation Is Impaired in Mice Lacking Brain-Derived Neurotrophic Factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999, 22, 295–318. [Google Scholar] [CrossRef]
- King, J.A.; Geisler, D.; Ritschel, F.; Boehm, I.; Seidel, M.; Roschinski, B.; Soltwedel, L.; Zwipp, J.; Pfuhl, G.; Marxen, M.; et al. Global Cortical Thinning in Acute Anorexia Nervosa Normalizes Following Long-Term Weight Restoration. Biol. Psychiatry 2015, 77, 624–632. [Google Scholar] [CrossRef]
- Seitz, J.; Bühren, K.; von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological Changes in the Brain of Acutely Ill and Weight-Recovered Patients with Anorexia Nervosa. A Meta-Analysis and Qualitative Review. Z. Kinder. Jugendpsychiatr. Psychother. 2014, 42, 7–17, quiz 17–18. [Google Scholar] [CrossRef]
- McPhee, G.M.; Downey, L.A.; Stough, C. Neurotrophins as a Reliable Biomarker for Brain Function, Structure and Cognition: A Systematic Review and Meta-Analysis. Neurobiol. Learn. Mem. 2020, 175, 107298. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, L.-K.; Hänggi, J.; Jäncke, L.; Baur, V.; Piccirelli, M.; Kollias, S.; Schnyder, U.; Martin-Soelch, C.; Milos, G. Age Influences Structural Brain Restoration during Weight Gain Therapy in Anorexia Nervosa. Transl. Psychiatry 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Zanardini, R.; Gennarelli, M.; Bocchio-Chiavetto, L. Influence of Clotting Duration on Brain-Derived Neurotrophic Factor (BDNF) Dosage in Serum. BioTechniques 2014, 57. [Google Scholar] [CrossRef]
- Amadio, P.; Sandrini, L.; Ieraci, A.; Tremoli, E.; Barbieri, S.S. Effect of Clotting Duration and Temperature on BDNF Measurement in Human Serum. Int. J. Mol. Sci. 2017, 18, 1987. [Google Scholar] [CrossRef]
- Sylvia, L.G.; Bernstein, E.E.; Hubbard, J.L.; Keating, L.; Anderson, E.J. Practical Guide to Measuring Physical Activity. J. Acad. Nutr. Diet. 2014, 114, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Ogiso, K.; Asakawa, A.; Amitani, H.; Inui, A. Ghrelin and Anorexia Nervosa: A Psychosomatic Perspective. Nutrition 2011, 27, 988–993. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. The Role of Ghrelin in Anorexia Nervosa. Int. J. Mol. Sci. 2018, 19, 2117. [Google Scholar] [CrossRef]
- Hebebrand, J.; Muller, T.D.; Holtkamp, K.; Herpertz-Dahlmann, B. The Role of Leptin in Anorexia Nervosa: Clinical Implications. Mol. Psychiatry 2007, 12, 23–35. [Google Scholar] [CrossRef]
- Misra, M.; Klibanski, A. Endocrine Consequences of Anorexia Nervosa. Lancet Diabetes Endocrinol. 2014, 2, 581–592. [Google Scholar] [CrossRef]
- Maguire, S.; O’Dell, A.; Touyz, L.; Russell, J. Oxytocin and Anorexia Nervosa: A Review of the Emerging Literature. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2013, 21, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Maguire, S.; Hunt, G.E.; Kesby, A.; Suraev, A.; Stuart, J.; Booth, J.; McGregor, I.S. Intranasal Oxytocin in the Treatment of Anorexia Nervosa: Randomized Controlled Trial during Re-Feeding. Psychoneuroendocrinology 2018, 87, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Grieco, K.A.; Klibanski, A. Testosterone Administration in Women with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2005, 90, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W. Pharmacotherapeutic Strategies for the Treatment of Anorexia Nervosa—Too Much for One Drug? Expert Opin. Pharmacother. 2020, 21, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, A.M.; Castellini, G.; Volpe, U.; Ricca, V.; Lelli, L.; Monteleone, P.; Maj, M. Neuroendocrinology and Brain Imaging of Reward in Eating Disorders: A Possible Key to the Treatment of Anorexia Nervosa and Bulimia Nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 132–142. [Google Scholar] [CrossRef] [PubMed]
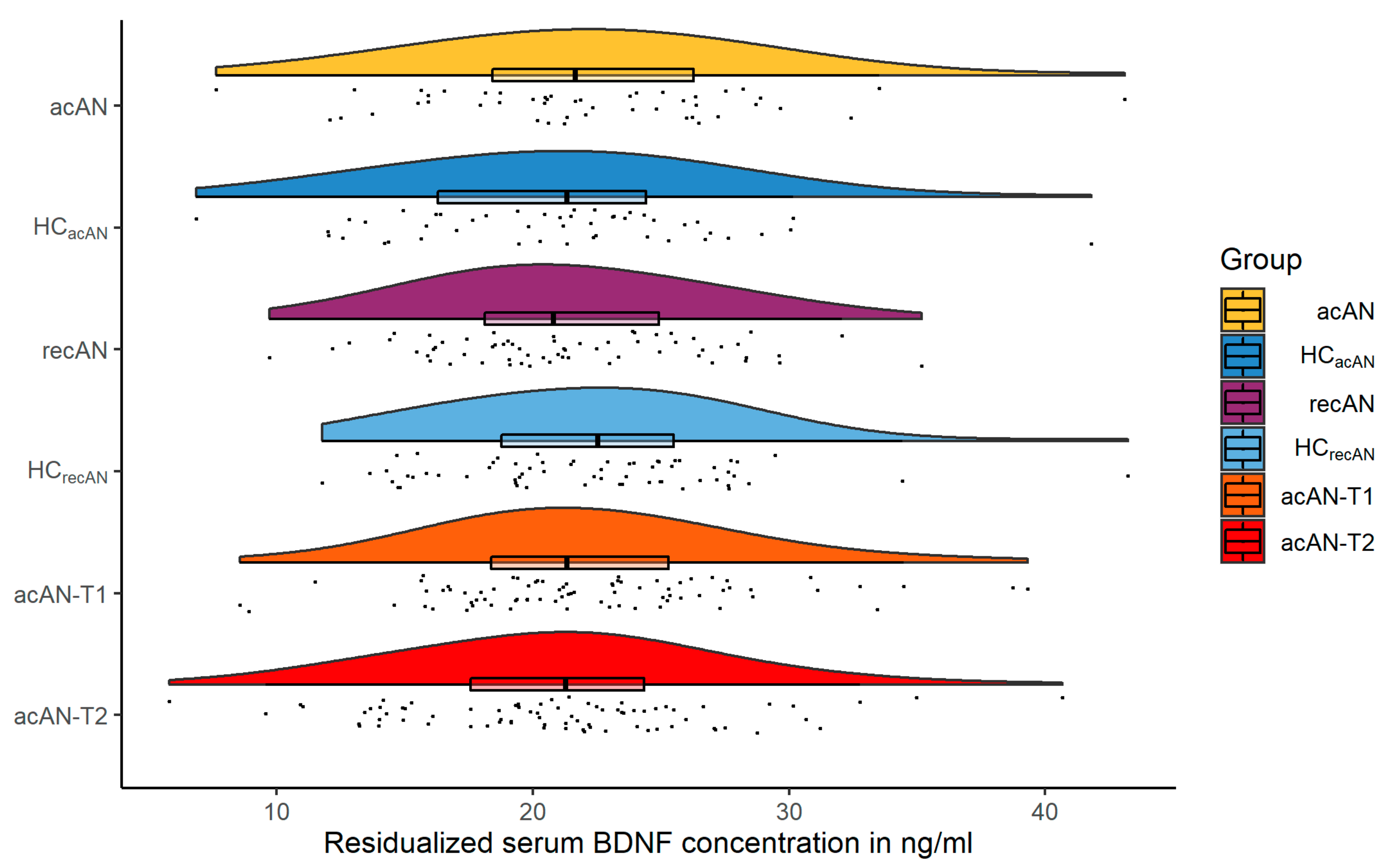
| N | acAN | HCacAN | t/W | p | recAN | HCrecAN | t/W | p | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 77/77/62/62 | 16.5 ± 3.3 | 16.5 ± 3.3 | <0.01 | 0.996 | 22.2 ± 3.5 | 22.2 ± 3.5 | −0.01 | 0.99 |
| IQ | 69/77/62/61 | 113.6 ± 12.3 | 112 ± 9.2 | −0.89 | 0.376 | 110.2 ± 9.4 | 110.1 ± 9.5 | −0.06 | 0.955 |
| BMI (kg/m²) | 77/77/62/62 | 14.9 ± 1.4 | 20.6 ± 2.2 | 19.19 | <0.001 | 20.8 ± 1.8 | 21.8 ± 2.2 | 2.68 | 0.008 |
| BMI-SDS | 77/77/62/62 | −3.1 ± 1.3 | −0.1 ± 0.7 | 18.05 | <0.001 | −0.5 ± 0.6 | −0.2 ± 0.7 | 2.74 | 0.007 |
| Minimum lifetime BMI (kg/m²) | 77/69/62/60 | 14.4 ± 1.4 | 19.3 ± 1.7 | 18.79 | <0.001 | 14.2 ± 1.5 | 20.1 ± 1.9 | 18.54 | <0.001 |
| BDI-II | 76/76/62/61 | 23.1 ± 10.9 | 5.7 ± 5.5 | −12.45 | <0.001 | 9 ± 8.6 | 3.9 ± 5.6 | −3.88 | <0.001 |
| EDI-2 total | 72/75/60/61 | 210.5 ± 46.1 | 143.3 ± 26.1 | −10.82 | <0.001 | 169.5 ± 47.6 | 134.7 ± 27.4 | −4.92 | <0.001 |
| EDI-2 Drive for thinness | 74/76/61/61 | 28.1 ± 9.6 | 13.9 ± 5.5 | −11.15 | <0.001 | 20.7 ± 9.1 | 12.8 ± 4.8 | −6.01 | <0.001 |
| EDI-2 Body dissatisfaction | 75/76/61/61 | 36.9 ± 11 | 24.1 ± 8 | −8.14 | <0.001 | 31 ± 11.4 | 23.1 ± 8.2 | −4.42 | <0.001 |
| EDI-2 Bulimia | 74/76/61/61 | 10.6 ± 4.5 | 9.5 ± 2.6 | −1.77 | 0.079 | 10.5 ± 4 | 9.7 ± 3.1 | −1.24 | 0.219 |
| Physical activity | 75/76/61/62 | 3 (1) | 2 (1) | 4104 | <0.001 | 2 (1) | 2 (1) | 2054 | 0.382 |
| N | acAN-T1 | acAN-T2 | t/V | p | |
|---|---|---|---|---|---|
| Age (years) | 47/47 | 16.2 ± 2.2 | 16.4 ± 2.2 | −22.88 | <0.001 |
| IQ | 44 | 115 ± 12.8 | ---- | ---- | ---- |
| BMI (kg/m²) | 47/47 | 15 ± 1.2 | 19 ± 1.1 | −30.12 | <0.001 |
| BMI-SDS | 47/47 | −2.9 ± 1 | −0.7 ± 0.6 | −21.29 | <0.001 |
| Minimum lifetime BMI (kg/m²) | 47 | 14.8 ± 1.2 | ---- | ---- | ---- |
| BDI-II | 46/46 | 22.1 ± 10.2 | 12.7 ± 9.9 | 7.05 | <0.001 |
| EDI-2 total | 43/43 | 203 ± 44.3 | 182.6 ± 46.3 | 3.52 | 0.001 |
| EDI-2 Drive for thinness | 45/45 | 26.7 ± 9.8 | 22.8 ± 10.3 | 3.18 | 0.003 |
| EDI-2 Body dissatisfaction | 45/45 | 35.4 ± 11.6 | 35.1 ± 13 | 0.11 | 0.908 |
| EDI-2 Bulimia | 44/44 | 10.5 ± 4.4 | 8.8 ± 3 | 2.54 | 0.015 |
| Physical activity | 46/46 | 2 (1) | 1 (1) | 510 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhäuser, J.L.; King, J.A.; Tam, F.I.; Seidel, M.; Biemann, R.; Wronski, M.-L.; Geisler, D.; Roessner, V.; Ehrlich, S. Is Serum BDNF Altered in Acute, Short- and Long-Term Recovered Restrictive Type Anorexia Nervosa? Nutrients 2021, 13, 432. https://doi.org/10.3390/nu13020432
Steinhäuser JL, King JA, Tam FI, Seidel M, Biemann R, Wronski M-L, Geisler D, Roessner V, Ehrlich S. Is Serum BDNF Altered in Acute, Short- and Long-Term Recovered Restrictive Type Anorexia Nervosa? Nutrients. 2021; 13(2):432. https://doi.org/10.3390/nu13020432
Chicago/Turabian StyleSteinhäuser, Jonas L., Joseph A. King, Friederike I. Tam, Maria Seidel, Ronald Biemann, Marie-Louis Wronski, Daniel Geisler, Veit Roessner, and Stefan Ehrlich. 2021. "Is Serum BDNF Altered in Acute, Short- and Long-Term Recovered Restrictive Type Anorexia Nervosa?" Nutrients 13, no. 2: 432. https://doi.org/10.3390/nu13020432
APA StyleSteinhäuser, J. L., King, J. A., Tam, F. I., Seidel, M., Biemann, R., Wronski, M.-L., Geisler, D., Roessner, V., & Ehrlich, S. (2021). Is Serum BDNF Altered in Acute, Short- and Long-Term Recovered Restrictive Type Anorexia Nervosa? Nutrients, 13(2), 432. https://doi.org/10.3390/nu13020432