Abstract
Gut microbiota composition and function are major areas of research for functional gastrointestinal disorders. There is a connection between gastrointestinal tract and central nervous system and this is mediated by neurotransmitters, inflammatory cytokines, the vagus nerve and the hypothalamic-pituitary-adrenal axis. Functional gastrointestinal disorders are prevalent diseases affecting more than one third of the population. The etiology of these disorders is not clarified. Visceral hyperalgesia is the main hypothesis for explaining clinical symptoms, however gut-brain axis disorder is a new terminology for functional disorders. In this review, microbiota-gut-brain axis connection pathways and related disorders are discussed. Antibiotics are widely used in developed countries and recent evidence indicates antibiotic-induced dysbiosis as an important factor for functional disorders. Antibiotics exert negative effects on gut microbiota composition and functions. Antibiotic-induced dysbiosis is a major factor for occurrence of post-infectious irritable bowel syndrome. Cognitive and mood disorders are also frequent in functional gastrointestinal disorders. Animal and human trials show strong evidence for the causal relationship between gut microbiota and brain functions. Therapeutic implications of these newly defined pathogenic pathways are also discussed.
1. Introduction
The human microbiota is a collection of microorganisms that live in and on us. Over the past two decades, we have had an enormous amount of publications about gut microbiota in health and disease. Improvement in sequencing technologies coupled with bioinformatics science are making this research more feasible and cheaper. For instance, the estimated ratio of human:microbiota cells was recently revised from 10 times to 1.3 times more than human cells [1,2]. Even this 1.3 ratio (microbiota cells/human cells) is mind-blowing since these cells harbor their own genetic material. When we make a comparison between the number of genes between human and microbes in our body, over 99% of the genes in our body are microbial (numbering over 10 million). In the evolution process, we co-evolved with microbiota and they might have influenced our immune system and epigenetics [3,4,5,6,7]. Although we can modulate the expression of human genome through diet and lifestyle, human microbiome can also be directly modulated by these factors. Therefore, from a therapeutic perspective, gut microbiota can be manipulated by diet, drugs, lifestyle, etc. This opportunity gives us a new horizon for therapeutic aspect of chronic diseases. In the last decade, we had great progress in the field of molecular genetic tests and today there are evolving methods of analyzing gut microbiome [8].
Functional gastrointestinal disorders (FGID) are prevalent diseases affecting more than one third of the population [9]. Emerging evidence suggests a new definition for these disorders, involving gut-brain axis [10]. These syndromes are also pathogenetically related to disorders of gut-brain interaction [11]. Rome Criteria defines 26 distinct adult FGIDs, most well-known are irritable bowel syndrome (IBS) and functional dyspepsia (FD). The pathogenesis of these disorders is not well-defined [12] and these disorders are associated with psychiatric co-morbidities, including panic attack, anxiety disorder, depression, hypochondriac behavior and somatization [13,14,15,16,17,18]. Recent studies showed that these behavioral changes are related to disturbances in gut-brain axis, including gut microbiota [19].
Methodology
A search for peer-reviewed articles published before September 2020 was performed in PubMed, Google, Web of Science, and MEDLINE to identify studies observing the effects of antibiotics in gut-brain-microbiota axis. Search terms used were (“gut” or “gut-brain” or “antibiotic”) or and (“microbiota”) and (“gastrointestinal disorder”). All titles/abstracts of the occasioning articles were appraised manually. For relevant abstracts, full articles were achieved and reviewed.
2. Gut-Brain-Microbiota Axis
The brain-gut-microbiota axis is a bidirectional system enabling gut microorganisms to communicate with the central nervous system (CNS), and the CNS with the gut [19]. The mechanisms of signal transmission are complex and not fully understood, but include neural, endocrine, immune and metabolic pathways [19]. There are many factors affecting microbiome-gut-brain-axis. These are diet, genetics, drugs, environment, exercise, cognitive behavior, stress, social interactions, and fear (Figure 1) [20,21,22,23,24,25,26,27,28].

Figure 1.
Brain-gut-microbiome axis is a dynamic, interactive network. Factors affecting the communication of these elements are mainly genetics, diet, and lifestyle (Modified from reference [8]).
Gut microbes are capable of producing most neurotransmitters found in the human brain. While these neurotransmitters primarily act locally in the gut, modulating the enteric nervous system, there is also undeniable evidence indicating that gut microbes can influence CNS through multiple mechanisms. The treatment with probiotic Bifidobacteria for instance, can increase the amount of tryptophan, the precursor of serotonin [29]. Some Lactobacilli species alter gamma-aminobutyric acid (GABA) metabolism and change brain GABA receptor expression and behavior [30]. Preclinical studies show that the vagus nerve is the main route for exerting the effects of gut microbiota on CNS. Lactobacillus rhamnosus has a central effect in animals and this was ameliorated by vagotomy [31]. In fact, patients with a history of vagotomy have diminished risk for certain neurological diseases [32]. Synthesis and release of neurotransmitters from bacteria have been reported: Lactobacillus and Bifidobacterium species can produce GABA; Escherichia, Bacillus and Saccharomyces spp. can produce noradrenaline; Candida, Streptococcus, Escherichia and Enterococcus spp. can produce serotonin; Bacillus can produce dopamine; Lactobacillus can produce acetylcholine [33,34,35]. Although these neurotransmitters can cross inflamed intestinal mucosal barrier, they cannot cross blood–brain-barrier (BBB) in healthy conditions. Another way of gut-brain interaction is the stimulation of hypothalamic-pituitary-adrenal (HPA) axis, which induces cortisol secretion. This system is the main stressor system in the body and it is mainly regulated by gut-HPA axis [36,37,38,39,40,41,42,43,44,45,46,47]. Psychological or physical stress can affect HPA axis and subsequently gut microbiota/barrier function (e.g., IBS) [33].
Post-infectious IBS is a prototype for gut-brain axis disorders. Water-born gastroenteritis outbreak occurred in United States and people affected from this E.coli infection later developed IBS-like symptoms including co-morbid depression, anxiety disorder [48].
3. Pathways of Communication
There are diverse ways of communication between gut microbiota and brain such as autonomic nervous system, vagus nerve, enteric nervous system, neurotransmitters, and immune system (Figure 2).
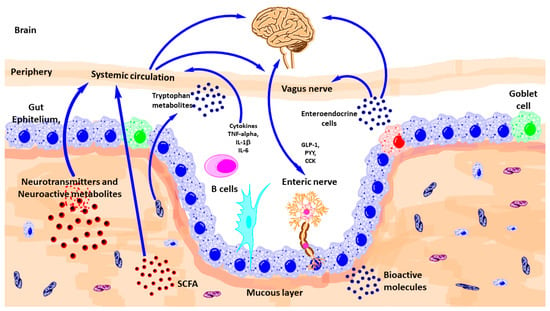
Figure 2.
Schematic outlining the various known pathways of communication between the gut-microbiota and the brain. CCK, Cholecystokinin; GLp-1, glucagon-like peptide-1; IL, interleukin; PYY, peptide YY; TNF, tumor necrosis factor; SCFA, short-chain fatty acid. (Modified from reference [8]).
3.1. Autonomic Nervous System (ANS)
The autonomic nervous system (ANS) comprises the sympathetic and parasympathetic branches. Combined with activity from the enteric nervous system (ENS) and influenced by the CNS, the ANS is responsible for physiological homeostasis, as well as responding to endocrine, motor, autonomic, and behavioral areas. Gut microbiota communicate with ANS bidirectional both antagonistic and synergistically [49,50]. Afferent nerves carry information from visceral organs to CNS and from CNS, important survival messages sent towards peripheral organs. ANS acts as the most immediate responder in health and disease states [51,52,53,54,55,56,57,58]. Local GI autonomic activation can be stimulated by afferent feedback loops from the microbiota and CNS efferent modulation [59]. Microbiota-related metabolites such as tryptophan (and end products, e.g., serotonin-5HT), GABA, catecholamine’s mediate ANS related effects. Sympathetic innervation has post-ganglionic vasoconstrictor effects and also suppressive effects on gut secretions and motility. Intestinal mucus layer is regulated by sympathetic innervation, by modulating mucosal immune system, microbial composition, and function [52,54,60].
3.2. Vagus Nerve
It is the tenth cranial nerve and the longest in the body with extensive connections and networks with peripheral organs. The vagus exerts anti-inflammatory actions via medullary dorsal motor nucleus. The vagal modulation of macrophage action an important factor for the inflammation in inflammatory bowel disease (IBD) [53].
3.3. Enteric Nervous System (ENS)
At the interface of microbiota and host, there is a network of gut neurons called ENS. Anatomically divided as submucosal and myenteric plexus, ENS regulates gut motility and secretions [61]. Factors affecting neurodevelopment and health status of CNS may also affect ENS integrity. Gut microbiota influences development and function of ENS via pattern recognition receptors (PRR) and including Toll-like receptors (TLRs), especially TLR-2 and TLR-4. These TLRs are involved in the recognition of microbial molecules [62]. Bacteroides fragilis and the capsular exopolysaccharide, are good examples, which can influence ENS function [63]. L. rhamnosus strain (JB-1) performs this action via a G protein-coupled receptor-mediated pathway [31]. Recent data showed that stress-induced alterations in ENS activity, via stimulated acetylcholine release, were influenced by both maternal separation and the microbiota [64]. This proves that the microbiota may affect ENS-related gut dysfunction associated with early-life stress. ENS abnormalities are associated with life-threatening GI disorders including Hirsch sprung disease and chronic intestinal pseudo-obstruction [65]. Moreover, the ENS is also involved in disorders of the CNS, including ASD, Alzheimer’s disease (AD), and Parkinson’s disease (PD) [66].
3.4. Immune System
Gastrointestinal tract has the highest number of immune cells in the body and there is a delicate, complex communication with the gut microbiota [67]. Mucus produced by epithelial Goblet cells provide a barrier against contact with host cells and microbial elements. The gut microbiota influences regulation of subsets of immune cells including T helper (Th), T regulatory (Treg), natural killer (NK), mononuclear phagocytes, and innate lymphoid cells [68]. The mechanisms by which the gut microbiota influences innate and adaptive responses during health and disease is still being investigated.
Environmental factors affect immune function. The release of cytokines promotes the activation and recruitment of eosinophils, B cells, and mast cells. In addition to the traditional T-helper 2 pathway, secretion of IL-23 from antigen-presenting cells, such as dendritic cells, B cells, and macrophages, promotes T helper-cell 17 differentiation. Degranulation of these cells disrupt intestinal barrier and enteric nerves. This results in visceral hypersensitivity and dysmotility [19]. α4β7 gut homing T cells are a marker of intestinal inflammation in both functional dyspepsia and irritable bowel syndrome. The site and extend of intestinal immune activation can define the phenotype such as functional heartburn, functional dyspepsia, irritable bowel syndrome, functional constipation, or functional diarrhea [19] (Figure 3).
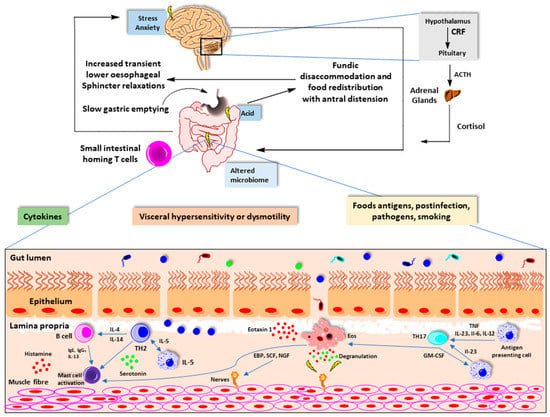
Figure 3.
Mucosal immune system activation and functional gastrointestinal disorders. (Adapted from reference 19). CRF, corticotrophin-releasing factor; ACTH, adrenocorticotropic hormone; Ig, immunoglobulin; IL, interleukin; TH, T-helper cell.
4. Impact of Antibiotic Use on Gut Microbiome-Brain Axis
The bi-directional communication between gut microbiota and brain ensures the maintenance of intestinal homeostasis and likely affects CNS functions in different time points of life, from early life neurodevelopment to aging and neurodegeneration [66]. Studies on the effect of antibiotics against gut microbiota-brain axis should be interpreted by considering the well-defined age-related differences in gut microbiota composition and diversity as well as its resilience and stability [69]. Moreover, the effect of antibiotics may be specific to an antibiotic group, course, and duration. Their absorption from GI tract is also critical in order to evaluate the direct effect of gut microbial perturbations without the neurologic effect of antibiotic itself [70]. For instance, metronidazole is a widely prescribed absorbable antibiotic for GI disorders, which can cross the blood–brain barrier [71]. Non-absorbable antibiotics such as vancomycin does not cross blood–brain barrier and become concentrated in the gastrointestinal tract, excluding the direct effect of antibiotics on CNS [72]. Route of antibiotic administration is also another important factor in experimental gut-brain axis studies, since oral gavage may induce stress in animals and may lead to misinterpretation of behavioral parameters [73].
Initial colonization of the gut begins in early life and mainly shaped by gestational age [74], mode of delivery (vaginal birth vs. Caesarean section) [75], method of feeding (breastfeeding vs. formula feeding) [76], and antibiotic exposure [77]. The early postnatal period is critical for microbial community and immune cell development. During this critical window, morphological and functional development in CNS also takes place, which is likely to be directly or indirectly influenced by gut microbiota [78,79]. A growing body of evidence from both epidemiological [80,81,82] and experimental studies [71,83,84] indicates that the gut microbiota has a crucial role in modulating brain function and behavior via gut-brain axis.
The majority of the studies providing evidence for the role of the gut microbiota in neurodevelopment come from germ-free animal experiments including altered hippocampal neurogenesis and decreased expression of brain-derived neurotrophic factors in germ-free mice [66,85,86]. Unlike germ-free conditions, antibiotics can selectively deplete microbial populations which is advantageous to design flexible experimental models and to determine targeted approaches for specific bacterial taxa [73]. Disruptive effect of antibiotic use especially in early life has known to cause long lasting consequences on microbial community and immunity, which may lead to increased predisposition and susceptibility to further disease progression such as IBD [77,87]. Majority of the studies using broad spectrum antibiotic cocktails showed comparable results with those observed in germ-free mice involving impaired social behaviors, neurogenesis and cognitive function together with the perturbations in gut microbiota [88,89].
In addition to evidence from experimental studies, a recent longitudinal population-based study reported that early-life antibiotic exposure is associated with an increased risk for psychiatric disorders [81]. Another recent clinical study also showed an altered auditory processing and recognition memory responses in infants who received intravenous antibiotics after delivery, supporting the importance of microbiota-gut-brain axis in humans during early life [80]. As a neurodevelopmental condition, autism spectrum disorder has also been linked to gut microbiota-brain axis dysfunction [90]. According to a recent meta-analysis, prenatal antibiotic exposure significantly increased the ASD risk in children. Interestingly, non-absorbable antibiotic vancomycin treatment, which mainly target Gram positive bacteria such as Clostridium spp., resulted in improved eye contact behavior and less constipation in autistic children [82].
Although not specifically focused in this review, neurodegenerative disorders such as Alzheimer’s disease (AD) are another consequence of gut microbiota-brain axis impairment. Minter et al. [78] reported that broad-spectrum postnatal antibiotic use reduced the amyloid plaque deposition in aged APPSWE/PS1ΔE9 transgenic mice suggesting that selective, targeted microbiome-based treatments can be used in early stage AD. A possible potential mechanism might be blooming in Lachnospiraceae family, one of the main butyrate-producers of the gut microbiota, after antibiotic treatment [91]. The increased butyrate production may induce T-reg differentiation, which has beneficial effect in AD pathogenesis by modulating microglial response amyloid-β deposition [92].
In addition to early life being of critical importance in terms of disruptive antibiotic effect on gut microbiota, antibiotic studies on adult animals also showed impaired behavior and brain function [71,83,93,94,95] and neurodegenerative disorders [96] both in prolonged and short courses, supporting the crucial role of gut microbiota-brain axis during lifespan. Some of the studies showed that these disruptive effects can be rescued by probiotic administrations [89,97].
Experimental reports summarizing the effect of antibiotics by considering the type of antibiotic, duration, route and age of exposure on gut-brain axis are depicted in Table 1. These experimental studies can be evaluated by considering broad-spectrum antibiotic cocktail strategies, which can broadly target gut microbiota including anaerobic bacteria causing germ-free like conditions [73], and targeted therapies using specific antibiotic groups such as Vancomycin. Both approaches lead to brain and behavioral impairments suggesting the role of disruption in both structure and function of the microbiota together, rather than a specific taxonomic group. A study by Erny et al. [98] also highlights the importance of gut microbial metabolites in microglia maturation and function. They showed that gut microbiota depletion by antibiotics resulted in defective microglia, which can be restored by microbiota-derived metabolites such as SCFA. Accordingly, antibiotic-driven disruption in gut microbiota may cause perturbation in functional gut microbiota, leading to alterations in microbial metabolites such as SCFA, as possible regulators of CNS inflammation [99].

Table 1.
Summary of studies demonstrating the effect of antibiotics (abx) on gut-brain axis in early life and adulthood.
While majority of the experimental studies in Table 1 highlight the complex role of gut microbiota in brain function both in early-life and adult age, the study by Minter et al. suggested a potential protective role of postnatal antibiotics in further AD progression, which provides a mechanism for Aβ deposition as a consequence of gut microbiota driven neuroinflammation [78].
5. Conclusions
Gut microbiota plays a vital role in the pathogenesis of functional gastrointestinal disorder. Gut microbiota and brain interactions are important factors for prevention and therapy. Further clinical studies are required for understanding the true effect of gut microbiota on disease progression in humans. While antibiotics are essential treatment strategies in most conditions, the long-lasting effects on host microbiome and immune functions, especially during early life should be interpreted with caution. We need prospective randomized trials for the therapeutic potential of gut microbiota modulation in functional gastrointestinal disorders.
Author Contributions
Conceptualization, T.K., C.O., E.K.A., S.B.; drafting the manuscript, T.K., C.O., E.K.A. and S.B.; drawing figures, E.S.-S. and E.K.A.; review and editing the paper, T.K. and E.K.A.; revising, T.K., E.K.A., E.S.-S. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Cimermancic, P.; Schulze, C.J.; Wieland Brown, L.C.; Martin, J.; Mitreva, M.; Clardy, J.; Linington, R.G.; Fischbach, M.A. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 2014, 158, 1402–1414. [Google Scholar] [CrossRef]
- Gill, R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Koloski, N.A.; Talley, N.J.; Boyce, P.M. Epidemiology and health care seeking in the functional GI disorders: A population-based study. Am. J. Gastroenterol. 2002, 97, 2290–2299. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J. Gastroenterol. 2019, 25, 552–566. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV—functional GI disorders: Disorders of gut-brain interaction. Gastroenterol 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Aziz, I.; Palsson, O.S.; Tornblom, H.; Sperber, A.D.; Whitehead, W.E.; Simren, M. The prevalence and impact of overlapping Rome IV¬ diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: A cross¬sectional general population study in three countries. Am. J. Gastroenterol. 2018, 113, 86–96. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Lee, C.; Doo, E.; Choi, J.M.; Jang, S.; Ryu, H.-S.; Lee, J.Y.; Oh, J.H.; Park, J.H.; Kim, Y.S. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: Systematic review and meta-analysis. J. Neurogastroenterol. Motil. 2017, 23, 349–362. [Google Scholar] [CrossRef]
- Zhang, Q.-E.; Wang, F.; Qin, G.; Zheng, W.; Ng, C.H.; Ungvari, G.S.; Yuan, Z.; Mei, S.; Wang, G.; Xiang, Y.-T. Depressive symptoms in patients with irritable bowel syndrome: A meta-analysis of comparative studies. Int. J. Biol. Sci. 2018, 14, 1504–1512. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef]
- Hausteiner-Wiehle, C.; Henningsen, P. Irritable bowel syndrome: Relations with functional, mental, and somatoform disorders. World J. Gastroenterol. 2014, 20, 6024–6030. [Google Scholar] [CrossRef]
- Farzaneh, N.; Ghobakhlou, M.; Moghimi-Dehkordi, B.; Naderi, N.; Fadai, F. Evaluation of psychological aspects among subtypes of irritable bowel syndrome. Indian J. Psychol. Med. 2012, 234, 144–148. [Google Scholar] [CrossRef]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 9, S0140–S6736. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- Xu, F.; Fu, Y.; Sun, T.Y.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.; Yang, J.; Wang, J.; et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 2020, 8, 145. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Gareau, M.G. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med. Biol. 2014, 817, 357–371. [Google Scholar] [CrossRef]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef]
- Moeller, A.H.; Foerster, S.; Wilson, M.L.; Pusey, A.E.; Hahn, B.H.; Ochman, H. Social behavior shapes the chimpanzee pan-microbiome. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016, 63, 217–227. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteriainfantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Nat. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Svensson, E.; Horvath-Puho, E.; Thomsen, R.W.; Djurhuus, J.C.; Pedersen, L.; Borghammer, P.; Sørensen, H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015, 78, 522–529. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-alteringmicroorganisms: Theimpact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Galland, L. The gut microbiome and thebrain. J. Med. Food. 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology in the microbiome-gut-brain axis: How bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013, 9, e1003726. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 3–24. [Google Scholar] [CrossRef]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA Axis and Anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153. [Google Scholar] [CrossRef]
- vanBodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef]
- García-León, M.Á.; Pérez-Mármol, J.M.; Gonzalez-Pérez, R.; García-Ríos, M.D.C.; Peralta-Ramírez, M.I. Relationship between resilience and stress: Perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiol. Behav. 2019, 202, 87–93. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 5, 476–494. [Google Scholar] [CrossRef]
- Shapero, B.G.; Curley, E.E.; Black, C.L.; Alloy, L.B. The interactive association of proximal life stress and cumulative HPA axis functioning with depressive symptoms. Depress. Anxiety 2019, 36, 1089–1101. [Google Scholar] [CrossRef]
- Roos, L.G.; Janson, J.; Sturmbauer, S.C.; Bennett, J.M.; Rohleder, N. Higher trait reappraisal predicts stronger HPA axis habituation to repeated stress. Psychoneuroendocrinology 2019, 101, 12–18. [Google Scholar] [CrossRef]
- Young, E.S.; Doom, J.R.; Farrell, A.K.; Carlson, E.A.; Englund, M.M.; Miller, G.E.; Gunnar, M.R.; Roisman, G.I.; Simpson, J.A. Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Dev. Psychopathol. 2020, 1, 12. [Google Scholar] [CrossRef]
- Lovelock, D.F.; Deak, T. Acute stress imposed during adolescence has minimal effects on hypothalamic-pituitary-adrenal (HPA) axis sensitivity in adulthood in female Sprague Dawley rats. Physiol. Behav. 2020, 213, 112707. [Google Scholar] [CrossRef]
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar] [CrossRef]
- Starr, L.R.; Stroud, C.B.; Shaw, Z.A.; Vrshek-Schallhorn, S. Stress sensitization to depression following childhood adversity: Moderation by HPA axis and serotonergic multilocus profile scores. Dev. Psychopathol. 2020, 1–15. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. The human hypothalamus in mood disorders: The HPA axis in the center. IBRO Rep. 2018, 6, 45–53. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Annamalai, C.; Rao, S.S.C. Post-Infectious Irritable Bowel Syndrome. Curr. Gastroenterol. Rep. 2017, 19, 56. [Google Scholar] [CrossRef]
- Jänig, W. Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Mulak, A.; Bonaz, B. Irritable bowel syndrome: A model of the brain-gut interactions. Med. Sci. Monit. 2004, 10, RA55–RA62. [Google Scholar]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Bienenstock, J.; Kunze, W.; Forsythe, P. Microbiota and the gut-brainaxis. Nutr. Rev. 2015, 73, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K. The brain-gut axis in abdominal pain syndromes. Ann. Rev. Med. 2011, 62, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Chey, W.Y.; Jin, H.O.; Lee, M.H.; Sun, S.W.; Lee, K.Y. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am. J. Gastroenterol. 2001, 96, 1499–1506. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.I.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.K.; Kasper, D.L.; Wang, B.; Forsythe, P.; Bienenstock, J.; Kunze, W.A. Bacteroidesfragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun. 2013, 4, 1465. [Google Scholar] [CrossRef]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, C. Intestinal Motility Disorders and Development of the Enteric Nervous System. Pediatr. Res. 2001, 49, 605–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ganal-Vonarburg, S.C.; Duerr, C.U. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology 2020, 159, 39–51. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; O’Toole, P.W. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef]
- Frohlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jacan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Soto, M.; Herzog, C.; Pacheco, J.A.; Fujisaka, S.; Bullock, K.; Clish, C.B.; Kahn, C.R. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol. Psychiatry 2018, 23, 2287–2301. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Kerr, C.; Murphy, K.; O’Sullivan, O.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.P.; O’Toole, P.W.; Cotter, P.D.; Fitzgerald, G.F.; et al. The individual-specific and diverse nature of the preterm infant microbiota. Arch. Dis. Child. Fetal. Neonatal. Ed. 2013, 98, F334–F340. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Salvatori, G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Ruiz, V.E.; Battaglia, T.; Xu, J.; Roubaud-Baudron, C.; Cadwell, K.; Perez-Perez, G.I.; Blaser, M.J. A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis. Genome Med. 2020, 12, 65. [Google Scholar] [CrossRef]
- Minter, M.R.; Hinterleitner, R.; Meisel, M.; Zhang, C.; Leone, V.; Zhang, X.; Musch, M.W.; Shen, X.; Jabri, B.; Chang, E.B.; et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1DeltaE9 murine model of Alzheimer’s disease. Sci. Rep. 2017, 7, 10411. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Hickey, M.K.; Miller, N.C.; Haapala, J.; Demerath, E.W.; Pfister, K.M.; Georgieff, M.K.; Gale, C.A. Infants exposed to antibiotics after birth have altered recognition memory responses at one month of age. Pediatr. Res. 2020, 12. [Google Scholar] [CrossRef]
- Lavebratt, C.; Yang, L.L.; Giacobini, M.; Forsell, Y.; Schalling, M.; Partonen, T.; Gissler, M. Early exposure to antibiotic drugs and risk for psychiatric disorders: A population-based study. Transl. Psychiatry 2019, 9, 317. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, D.; Wang, Y.; Zeng, T.; Peng, Y. Urinary 3-(3-Hydroxyphenyl)-3-hydroxypropionic Acid, 3-Hydroxyphenylacetic Acid, and 3-Hydroxyhippuric Acid Are Elevated in Children with Autism Spectrum Disorders. Biomed. Res. Int. 2016, 2016, 9485412. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 15062. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry 2015, 78, e7–e9. [Google Scholar] [CrossRef]
- Roubaud-Baudron, C.; Ruiz, V.E.; Swan, A.M.; Vallance, B.A.; Ozkul, C.; Pei, Z.; Li, J.; Battaglia, T.W.; Perez-Perez, G.I.; Blaser, M.J. Long-Term Effects of Early-Life Antibiotic Exposure on Resistance to Subsequent Bacterial Infection. mBio 2019, 10, e02820-19. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.; Dinan, T.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Mohle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef]
- Dansokho, C.; Ait Ahmed, D.; Aid, S.; Toly-Ndour, C.; Chaigneau, T.; Calle, V.; Cagnard, N.; Holzenberger, M.; Piaggio, E.; Aucouturier, P.; et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 2016, 139 Pt 4, 1237–1251. [Google Scholar] [CrossRef]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 2017, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, B.; Mu, L.; Wang, H.; Luo, J.; Yang, Y.; Yang, H.; Li, M.; Zhou, L.; Tao, C. Long-Term Exposure to Ceftriaxone Sodium Induces Alteration of Gut Microbiota Accompanied by Abnormal Behaviors in Mice. Front. Cell Infect. Microbiol. 2020, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Kayyal, M.; Javkar, T.; FirozMian, M.; Binyamin, D.; Koren, O.; Neufeld, K.M.; Forsythe, P. Sex dependent effects of post-natal penicillin on brain, behavior and immune regulation are prevented by concurrent probiotic treatment. Sci. Rep. 2020, 10, 10318. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef]
- Degroote, S.; Hunting, D.J.; Baccarelli, A.A.; Takser, L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 76–82. [Google Scholar] [CrossRef]
- Tochitani, S.; Ikeno, T.; Ito, T.; Sakurai, A.; Yamauchi, T.; Matsuzaki, H. Administration of Non-Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS ONE 2016, 11, e0138293. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Felice, V.D.; Nally, K.; Savignac, H.M.; Claesson, M.J.; Scully, P.; Woznicki, J.; Hyland, N.P.; Shanahan, F.; Quigley, E.M.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; De Martin, S.; Montopoli, M.; Dall’Acqua, S.; et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br. J. Pharmacol. 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).