Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, “IntegPRO”
Abstract
1. Introduction
2. Materials and Methods
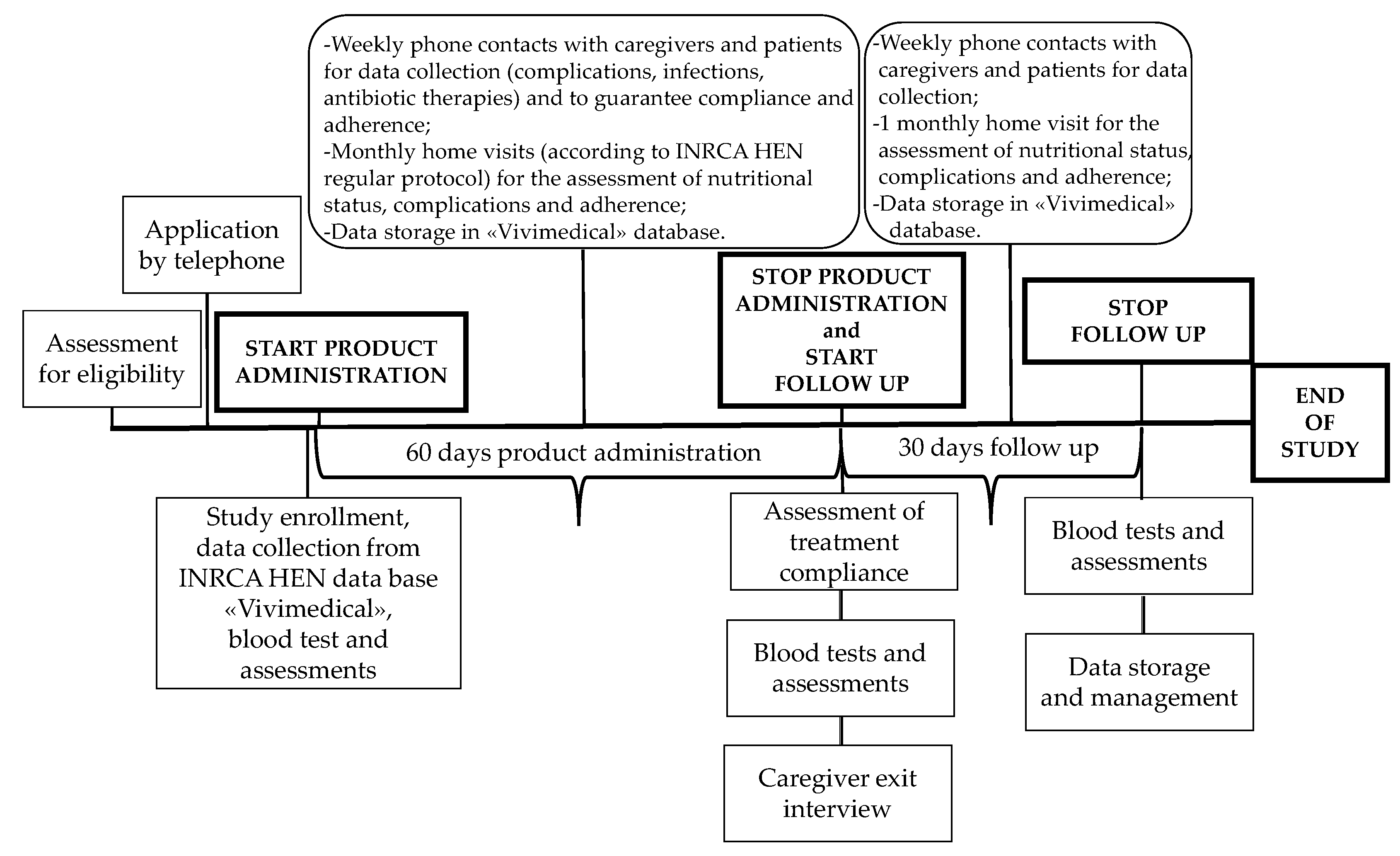
2.1. Study Design
2.2. Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
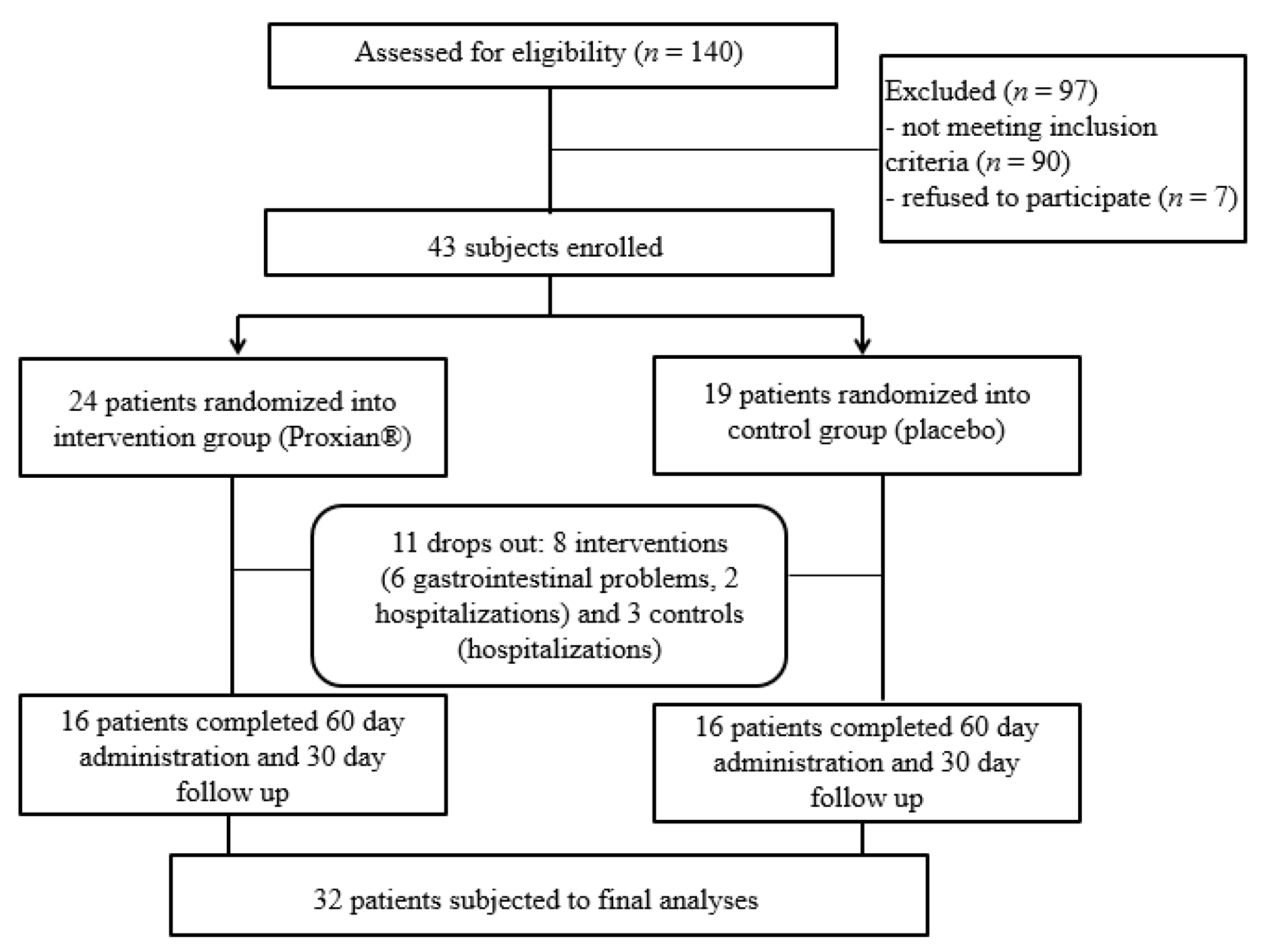
2.3. Sample Recruitment
2.4. Interventions
2.5. Assessments and Outcomes
Primary and Secondary Outcomes
2.6. Sample Size
2.7. Randomization, Allocation Concealment and Blinding
2.8. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Registration
Acknowledgments
Conflicts of Interest
References
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.O.; Lee, Y.K.; Kim, J.M.; Yoon, G. From cell senescence to age-related diseases: Differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2015, 48, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Giacosa, A.; Faliva, M.A. Review on microbiota and effectiveness of probiotic use in older. World J. Clin. Cases 2015, 3, 156–162. [Google Scholar] [CrossRef]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Probiotics and prebiotics in the elderly. Postgrad. Med. J. 2004, 80, 447–451. [Google Scholar] [CrossRef]
- Foligne, B.; Zoumpopoulou, G.; Dewulf, J.; Younes, A.B.; Chareyre, F.; Sirard, J.C.; Pot, B.; Grangette, C. A key role of dendritic cells in probiotic functionality. PLoS ONE 2007, 2, e313. [Google Scholar] [CrossRef]
- Stadlbauer, V. Immunosuppression and probiotics: Are they effective and safe? Benef. Microbes 2015, 6, 823–828. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 11, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Toward, R.E.; Walton, G.E.; Gibson, G.R. Immunosenescence and gut microbiota. Nutr. Aging 2012, 1, 167–180. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 1–16. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Fairweather-Tait, S.; Franceschi, C.; Brigidi, P. Aging of the human metaorganism: The microbial counterpart. Age 2012, 34, 247–267. [Google Scholar] [CrossRef]
- Kecskés, G.; Belágyi, T.; Oláh, A. Early jejunal nutrition with combined pre- and probiotics in acute pancreatitis–prospective, randomized, double-blind investigations. Magy. Seb. 2003, 56, 3–8. [Google Scholar]
- McNaught, C.E.; Woodcock, N.P.; Anderson, A.D.; MacFie, J. A prospective randomised trial of probiotics in critically ill patients. Clin. Nutr. 2005, 24, 211–219. [Google Scholar] [CrossRef]
- Klarin, B.; Wullt, M.; Palmquist, I.; Molin, G.; Larsson, A.; Jeppsson, B. Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics. Acta Anaesthesiol. Scand. 2008, 52, 1096–1102. [Google Scholar] [CrossRef]
- Orlandoni, P.; Jukic Peladic, N.; Spazzafumo, L.; Venturini, C.; Cola, C.; Sparvoli, D.; Giorgini, N.; Basile, R.; Fagnani, D. Utility of video consultation to improve the outcomes of home enteral nutrition in a population of frail older patients. Geriatr. Gerontol. Int. 2016, 16, 762–767. [Google Scholar] [CrossRef]
- Besselink, M.G.; Van Santvoort, H.C.; Buskens, E. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Del Piano, M.; Carmagnola, S.; Ballarè, M.; Sartori, M.; Orsello, M.; Balzarini, M.; Pagliarulo, M.; Tari, R.; Anderloni, A.; Strozzi, G.P.; et al. Is microencapsulation the future of probiotic preparations? The increased efficacy of gastro-protected probiotics. Gut Microbes 2011, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Dianawati, D.; Mishra, V.; Shah, N.P. Survival of Microencapsulated Probiotic Bacteria after Processing and during Storage: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1685–1716. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Rowsey, P.J.; Bishop, B.L.; Ward, W.O.; MacPhail, R.C. Serum biomarkers of aging in the Brown Norway rat. Exp. Gerontol. 2011, 46, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Ferns, G. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analyses of Randomized Controled Trials. Nutrients 2017, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Myers, C.E. Safety of probiotics in patients receiving nutritional support: A systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am. J. Clin. Nutr. 2010, 91, 687–703. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Müller, A.R.; Hansen, S.; Bengmark, S.; Neuhaus, P. Einfluss von Probiotika und Ballaststoffen auf die Inzidenz bakterieller Infektionen nach viszeralchirurgischen Eingriffen—Ergebnisse einer prospektiven Studie [Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery—Results of a prospective trial]. Z. Gastroenterol. 2002, 40, 869–876. (In German) [Google Scholar] [CrossRef]
- Falcão de Arruda, I.S.; de Aguilar-Nascimento, J.E. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin. Sci. 2004, 106, 287–292. [Google Scholar] [CrossRef]
- Spindler-Vesel, A.; Bengmark, S.; Vovk, I.; Cerovic, O.; Kompan, L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: A randomized study in trauma patients. JPEN J. Parenter. Enter. Nutr. 2007, 31, 119–126. [Google Scholar] [CrossRef]
- Mogna, L.; Nicola, S.; Pane, M.; Lorenzini, P.; Strozzi, G.; Mogna, G. Selenium and zinc internalized by Lactobacillus buchneri Lb26 (DSM 16341) and Bifidobacterium lactis Bb1 (DSM 17850): Improved bioavailability using a new biological approach. J. Clin. Gastroenterol. 2012, 46 (Suppl. S41–S45). [Google Scholar] [CrossRef]
| Total (n = 32) | Intervention (n = 16) | Placebo (n = 16) | p | |
|---|---|---|---|---|
| Age, Mean ± SD ^ (range) * | 79.7 ± 10.3 (min 66; max 97) | 80.0 ± 10.2 (min 67; max 93) | 79.3 ± 10.0 (min 66; max 97) | 0.853 |
| Gender, n (%) ** | F 24 (75%), M 8 (25%) | F 13 (81%), M 3 (19%) | F 11 (69%), M 5 (31%) | 0.343 |
| Living, n (%) ** | Home 13 (41)%, NH 19 (59)% | Home 6 (38%), NH 10 (62%) | Home 7 (44%), NH 9 (56%) | 0.500 |
| Dementia, n (%) ** | 19 (59%) | 11 (69%) | 8 (50%) | 0.236 |
| Comorbidity, n (%) ** | 21 (66%) | 12 (75%) | 9 (56%) | 0.229 |
| BMI ^^, Mean ± SD ^ * | 19.6 ± 5.1 | 19.5 ± 5.4 | 19.7 ± 5.2 | 0.898 |
| Pressure ulcers, n (%) ** | 5 (16%) | 4 (25%) | 1 (6%) | 0.166 |
| Polypharmacy, n (%) ** | 21 (67%) | 9 (57%) | 12 (78%) | 0.694 |
| CRP ^^^ ≥ 0.8 mg/L, n (%) ** | 17 (53%) | 9 (56%) | 8 (50%) | 0.500 |
| Total (n = 32) | Intervention (n = 16) | Placebo (n = 16) | p | |
|---|---|---|---|---|
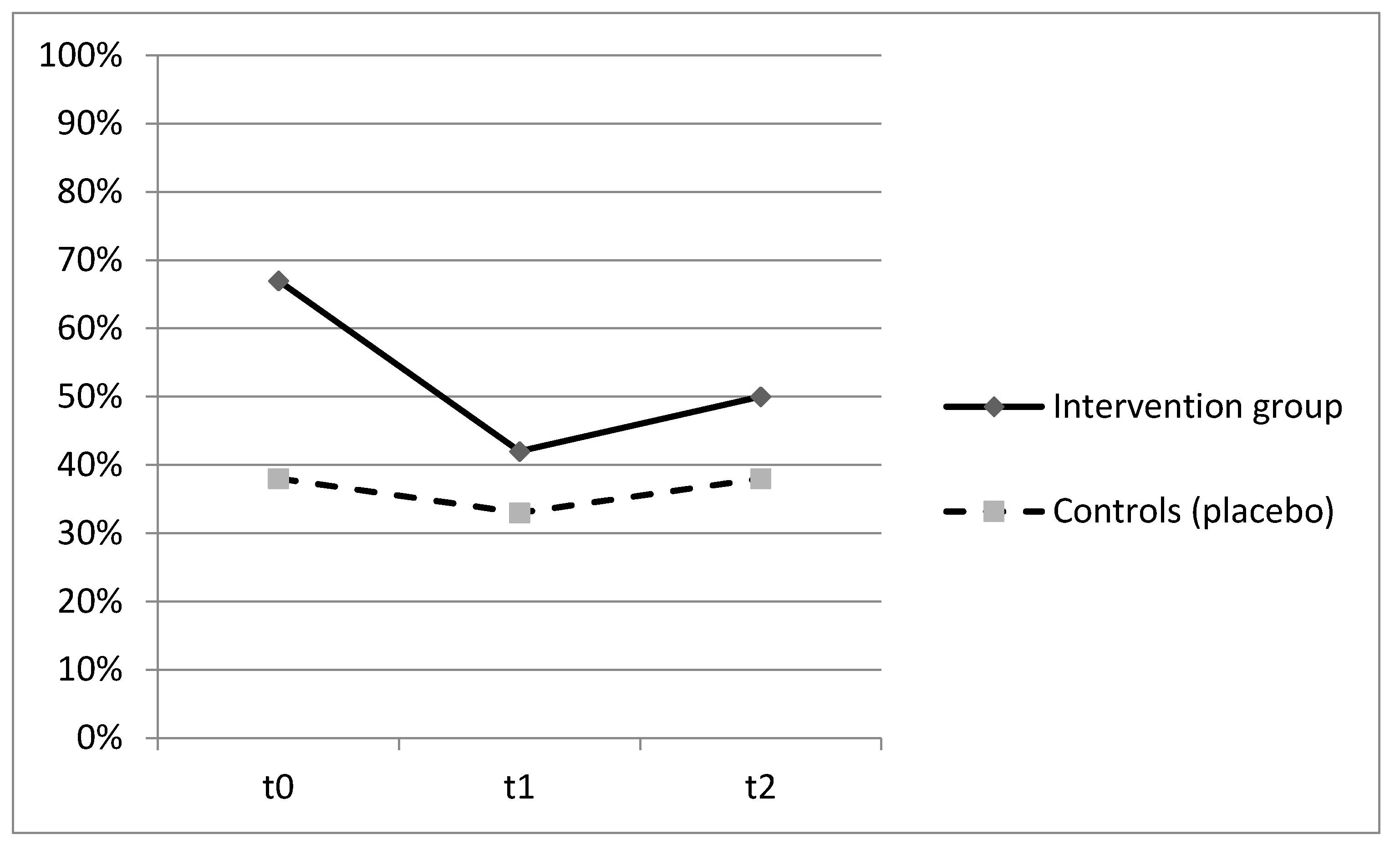
| Clinical manifestations of infections, n (%) ** | 11(34%) | 4(25%) | 7(44%) | 0.229 |
| Antibiotic therapy, n (%) ** | 8(25%) | 2(12%) | 6(37%) | 0.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandoni, P.; Jukic Peladic, N.; Amoruso, A.; Pane, M.; Di Rosa, M.; Vedruccio, J.; Santini, F. Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, “IntegPRO”. Nutrients 2021, 13, 391. https://doi.org/10.3390/nu13020391
Orlandoni P, Jukic Peladic N, Amoruso A, Pane M, Di Rosa M, Vedruccio J, Santini F. Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, “IntegPRO”. Nutrients. 2021; 13(2):391. https://doi.org/10.3390/nu13020391
Chicago/Turabian StyleOrlandoni, Paolo, Nikolina Jukic Peladic, Angela Amoruso, Marco Pane, Mirko Di Rosa, Jennifer Vedruccio, and Franco Santini. 2021. "Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, “IntegPRO”" Nutrients 13, no. 2: 391. https://doi.org/10.3390/nu13020391
APA StyleOrlandoni, P., Jukic Peladic, N., Amoruso, A., Pane, M., Di Rosa, M., Vedruccio, J., & Santini, F. (2021). Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, “IntegPRO”. Nutrients, 13(2), 391. https://doi.org/10.3390/nu13020391







