Gestation Food Restriction and Refeeding Compensate Maternal Energy Status and Alleviate Metabolic Consequences in Juvenile Offspring in a Rabbit Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
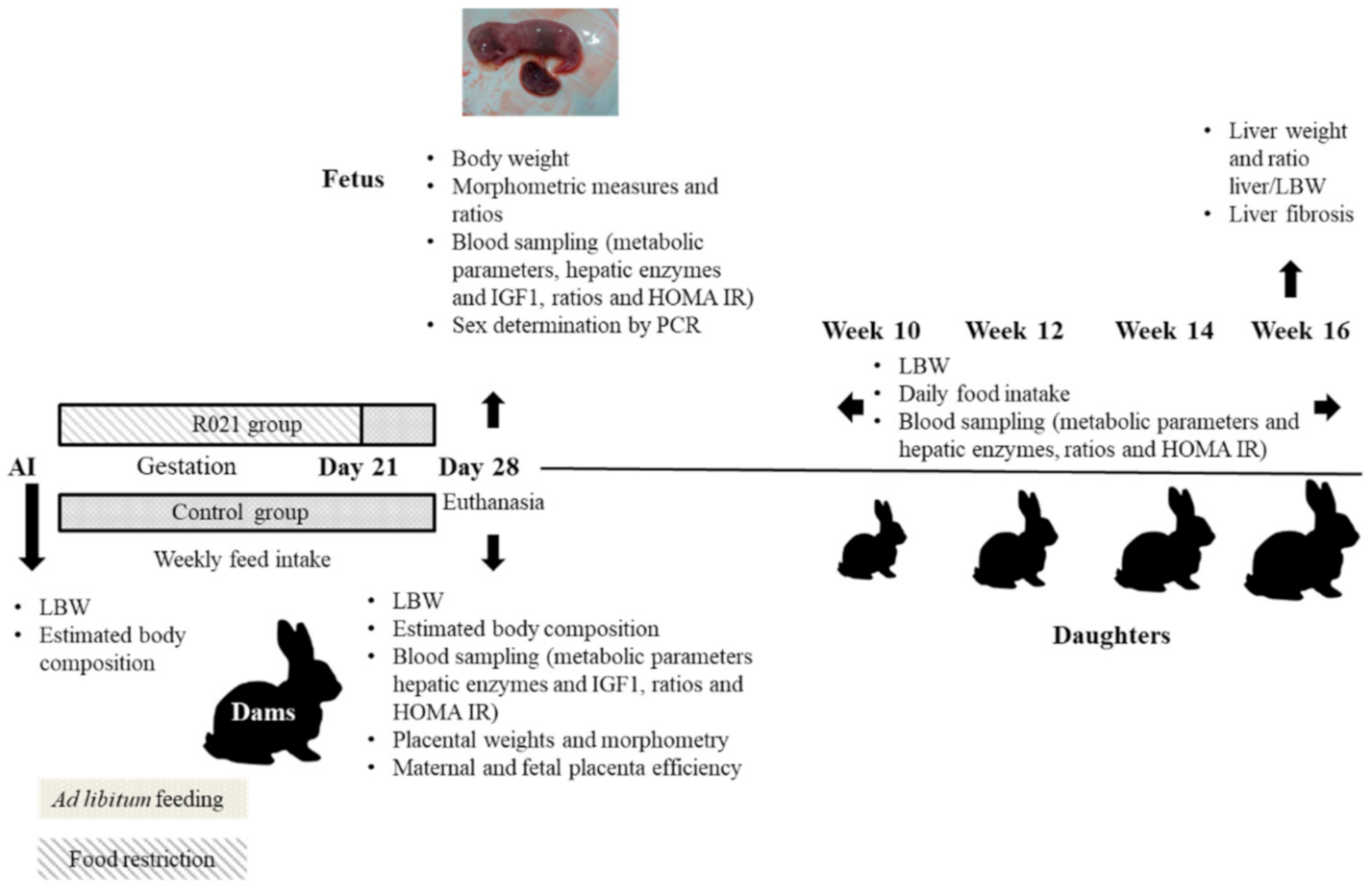
2.2. Experimental Design
2.2.1. Dams
2.2.2. Fetuses
2.2.3. Offspring
2.3. Blood Analysis
2.4. Sex Determination by PCR
2.5. Liver Fibrosis by Sirius Red Analysis
2.6. Statistical Analysis
3. Results
3.1. Maternal Response to Food Restriction during Gestation
3.1.1. Feed Intake, LBW, and Estimated Body Composition
3.1.2. Biochemical and Hormonal Serum Analysis of Does
3.1.3. Morphometric Study of Placental Structures
3.2. Fetal Response to Maternal Food Restriction during Gestation
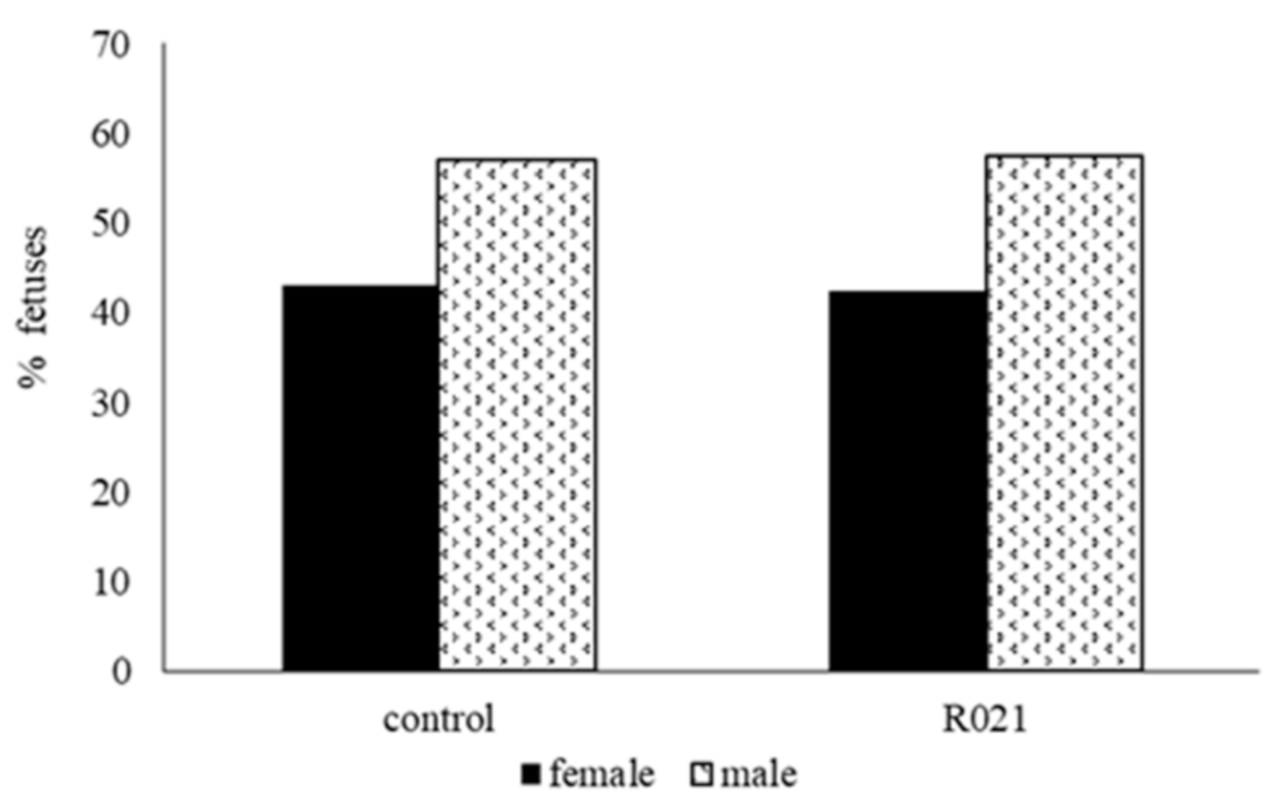
3.2.1. Morphometric Study of Fetuses and Sex Ratio
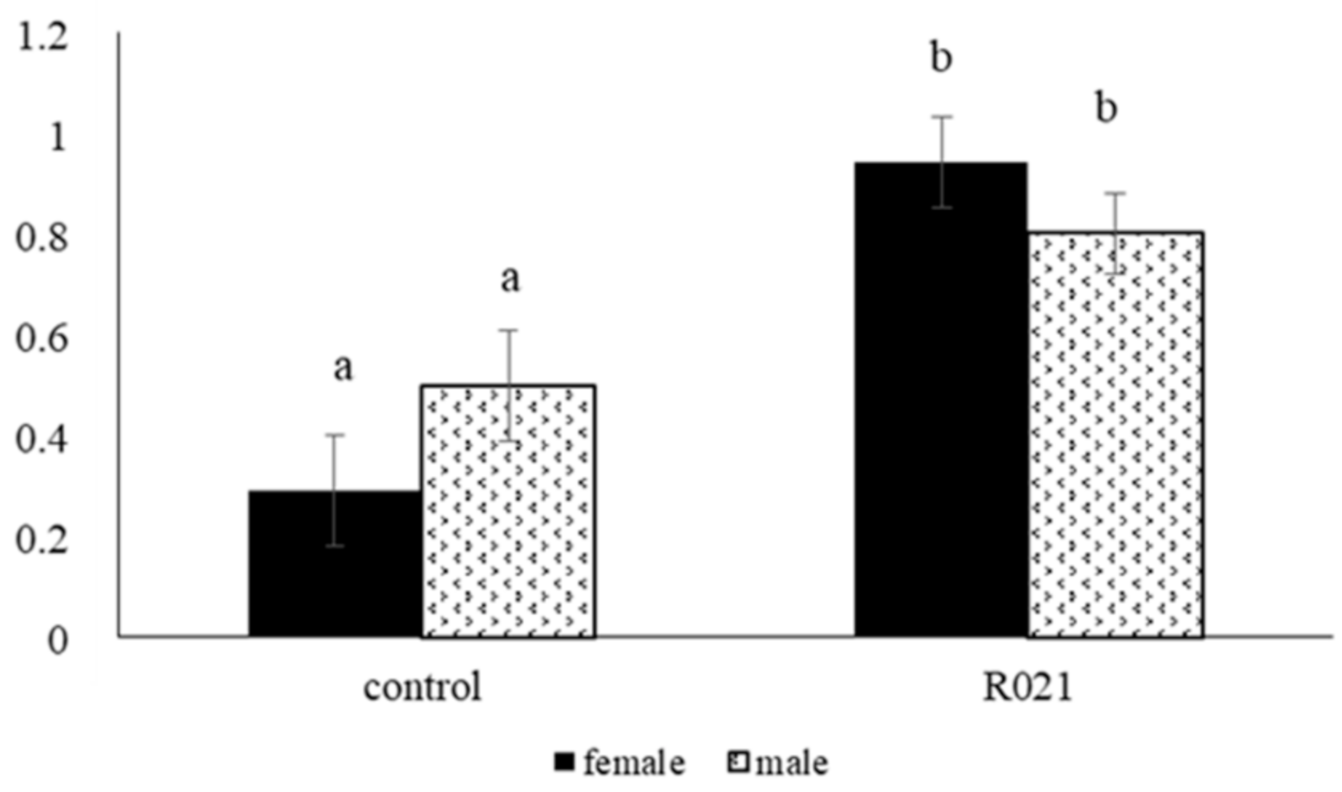
3.2.2. Metabolic and Hormonal Parameters
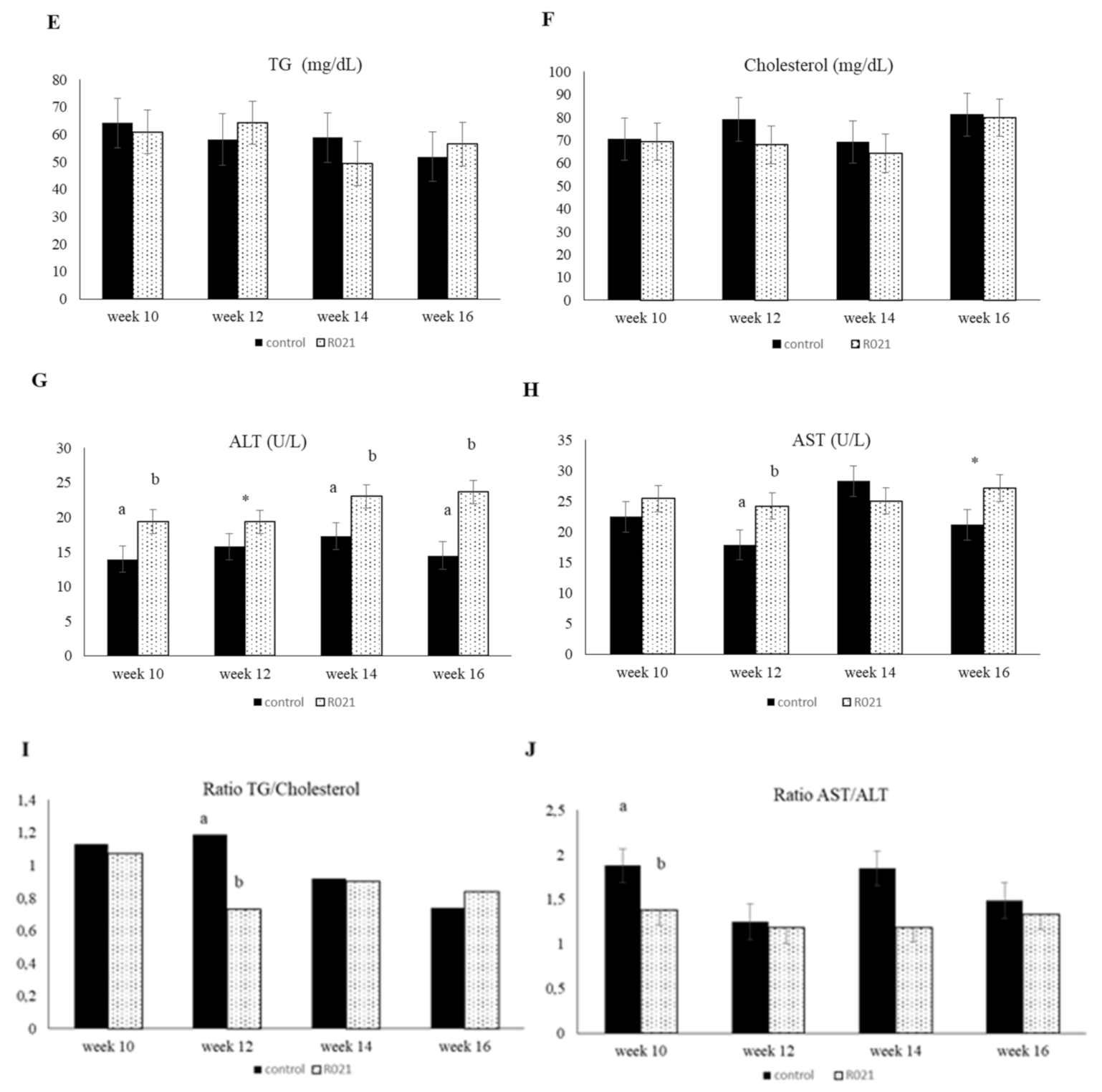
3.3. Influence of the Maternal Food Restriction during Gestation in the Juvenile Phase (from 10 to 16 Weeks of Age)
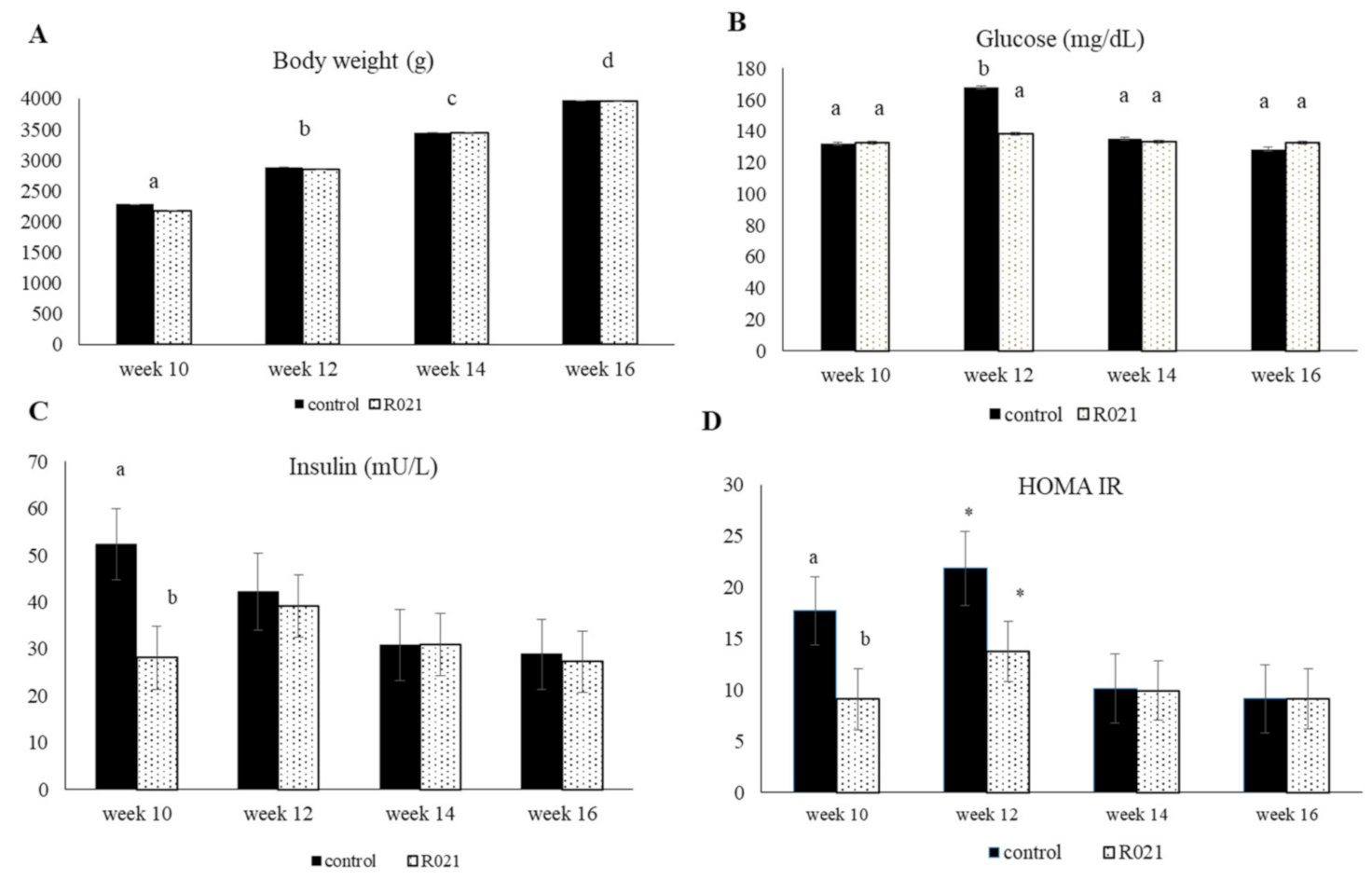
3.3.1. LBW, Feed Intake, Serum Metabolic and Hormonal Parameters, and Hepatic Enzymes
3.3.2. Liver Weight and Fibrosis at 16 Weeks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Vickers, M.H. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef]
- Herrera, E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur. J. Clin. Nutr. 2000, 54, S47–S51. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Arias-Álvarez, M.; Martinez, M.-A.J.; Garcia-Garcia, R.M.; Rodriguez, M.; Gonzalez, P.L.L.; Bermejo-Poza, R.; Gonzalez-Bulnes, A.; Rebollar, P.G. Competition for materno-fetal resource partitioning in a rabbit model of undernourished pregnancy. PLoS ONE 2017, 12, e0169194. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Yan, Q.; Ren, A.; Kong, Z.; Tang, S.; Han, X.; Tan, Z.L.; Salem, A.Z. Evidence for liver energy metabolism programming in offspring subjected to intrauterine undernutrition during midgestation. Nutr. Metab. 2019, 16. [Google Scholar] [CrossRef]
- Menchetti, L.; Brecchia, G.; Cardinali, R.; Polisca, A.; Boiti, C. Food restriction during pregnancy: Effects on body condition and productive performance of primiparous rabbit does. World Rabbit. Sci. 2015, 23. [Google Scholar] [CrossRef]
- Limones, M.; Sevillano, J.; Sánchez-Alonso, M.G.; Herrera, E.; Ramos, M.P. Metabolic alterations associated with maternal undernutrition during the first half of gestation lead to a diabetogenic state in the rat. Eur. J. Nutr. 2018, 58, 2521–2533. [Google Scholar] [CrossRef]
- De Boo, H.A.; Harding, J.E. The developmental origins of adult disease (Barker) hypothesis. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 4–14. [Google Scholar] [CrossRef]
- Desai, M.; Hales, C.N. Role of fetal and infant growth in programming metabolism in later life. Biol. Rev. 2007, 72, 329–348. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Walker, D.A.; Stephenson, T.; Symonds, M.E. Ontogeny and nutritional manipulation of the hepatic prolactin–growth hormone–insulin-like growth factor axis in the ovine fetus and in neonate and juvenile sheep. Proc. Nutr. Soc. 2004, 63, 127–135. [Google Scholar] [CrossRef]
- Khanal, P.; Nielsen, M.O. Impacts of prenatal nutrition on animal production and performance: A focus on growth and metabolic and endocrine function in sheep. J. Anim. Sci. Biotechnol. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Giussani, D.A.; Forhead, A.J. Intrauterine programming of physiological systems: Causes and consequences. Physiology 2006, 21, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lumey, L.H. Reproductive outcomes in women prenatally exposed to undernutrition: A review of findings from the Dutch famine birth cohort. Proc. Nutr. Soc. 1998, 57, 129–135. [Google Scholar] [CrossRef] [PubMed]
- ACC/SCN. “Fourth Report on the World Nutrition Situation.” Geneva ACC/SCN Collab. with IFPRI, p. 140. 2000. Available online: https://www.unscn.org/layout/modules/resources/files/rwns4.pdf (accessed on 23 November 2020).
- Itoh, H.; Muramatsu-Kato, K.; Ferdous, U.J.; Kohmura-Kobayashi, Y.; Kanayama, N. Undernourishmentin uteroand hepatic steatosis in later life: A potential issue in Japanese people. Congenit. Anom. 2017, 57, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Robinson, S.; Godfrey, K.M.; Cooper, C.; Inskip, H. Women’s dietary patterns change little from before to during pregnancy. J. Nutr. 2009, 139, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- UN. “Progress in Nutriti N,” United Nations Adm. Comm. Coord. Comm. Nutr., no 6, pp. 1–131. 2010. Available online: www.unscn.org (accessed on 23 November 2020).
- IEG. Global Nutrition Report. Available online: https://globalnutritionreport.org/reports/global-nutrition-report-2018/executive-summary/ (accessed on 30 December 2020).
- Godfrey, K.M.; Barker, D.J.; Aranceta, J.; Serra-Majem, L.; Ribas, L.; Pérez-Rodrigo, C. Fetal programming and adult health. Public Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef]
- Lumey, L. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944–1945. Paediatr. Périnat. Epidemiol. 1992, 6, 240–253. [Google Scholar] [CrossRef]
- Barker, D. The intrauterine origins of cardiovascular disease. Acta Paediatr. 1993, 82, 93–99. [Google Scholar] [CrossRef]
- Fleming, T.; Watkins, A.J.; Velazquez, M.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health Around the Time of Conception: Causes and Consequences. Obstet. Gynecol. Surv. 2018, 73, 555–557. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal undernutrition and disease in later life. Rev. Reprod. 1997, 2, 105–112. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Fleming, T.P.; Watkins, A.J. Periconceptional environment and the developmental origins of disease. J. Endocrinol. 2019, 242, T33–T49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Y.; Deng, M.-Q.; Zhang, Q.; Xiao, X.-H. Early-life nutrition and metabolic disorders in later life: A new perspective on energy metabolism. Chin. Med. J. 2020, 133, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.S.; Crouse, M.S.; Reynolds, L.P.; Neville, T.L.; Dahlen, C.R.; Ward, A.K.; Swanson, K.C. Maternal nutrition and programming of offspring energy requirements. Transl. Anim. Sci. 2019, 3, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Jaquiery, A.L.; Oliver, M.H.; Honeyfield-Ross, M.; Harding, J.E.; Bloomfield, F.H. Periconceptional undernutrition in sheep affects adult phenotype only in males. J. Nutr. Metab. 2012, 2012, 123610. [Google Scholar] [CrossRef]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Santos, A.N.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef]
- López-Tello, J.; Barbero, A.; González-Bulnes, A.; Astiz, S.; Rodríguez, M.; Formoso-Rafferty, N.; Arias-Álvarez, M.; Rebollar, P.G. Characterization of early changes in fetoplacental hemodynamics in a diet-induced rabbit model of IUGR. J. Dev. Orig. Health Dis. 2015, 6, 454–461. [Google Scholar] [CrossRef]
- Polisca, A.; Scotti, L.; Orlandi, R.; Brecchia, G.; Boiti, C. Doppler evaluation of maternal and fetal vessels during normal gestation in rabbits. Theriogenology 2010, 73, 358–366. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Couturier-Tarrade, A.; Thieme, R.; Brat, R.; Rolland, A.; Boileau, P.; Aubrière, M.-C.; Daniel, N.; Dahirel, M.; Derisoud, E.; et al. A short periconceptional exposure to maternal type-1 diabetes is sufficient to disrupt the feto-placental phenotype in a rabbit model. Mol. Cell. Endocrinol. 2019, 480, 42–53. [Google Scholar] [CrossRef]
- Chavatte-Palmer, P.; Richard, C.; Peugnet, P.; Robles, M.; Rousseau-Ralliard, D.; Tarrade, A. The developmental origins of health and disease: Importance for animal production. Anim. Reprod. 2015, 12, 505–520. [Google Scholar]
- Roseboom, T.J.; De Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and micronutrient intake during pregnancy: An overview of recent evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Sakr, O.; Garcia-Garcia, R.M.; Arias-Álvarez, M.; Millan, P.; Lorenzo, P.L.; Rebollar, P.G. Body reserves and ovarian performance in primiparous lactating rabbit does submitted to early weaning as a strategy to decrease energy deficit. Anim. Reprod. Sci. 2010, 121, 294–300. [Google Scholar] [CrossRef]
- Vašíček, D.; Vašíčková, K.; Parkányi, V.; Ondruška, Ľ.; Vašíček, D. Noninvasive PCR sexing of Neonatal Rabbits Selected for Islet Cell Culture. Slovak J. Anim. Sci. 2011, 44, 43–47. [Google Scholar]
- Parkányi, V.; Vašíček, D.; Ondruška, L.; Rafay, J. The Sex-Detection in Newborn Rabbits by X-Chromatin and Pcr-Sry. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008. [Google Scholar]
- León, A.L.-D.; Rojkind, M. Micromethod for Collagen and Total Protein Determination in Formalin-fixed. Histochemistry 1985, 33, 737–743. [Google Scholar]
- Mori, M.; Fujii, H.; Ogawa, T.; Kobayashi, S.; Iwai, S.; Morikawa, H.; Enomoto, M.; Tamori, A.; Sawada, A.; Takeda, S.; et al. Close correlation of liver stiffness with collagen deposition and presence of myofibroblasts in non-alcoholic fatty liver disease. Hepatol. Res. 2011, 41, 897–903. [Google Scholar] [CrossRef]
- Daoud, N.M.; Mahrous, K.F.; Ezzo, O. Feed restriction as a biostimulant of the production of oocyte, their quality and GDF-9 gene expression in rabbit oocytes. Anim. Reprod. Sci. 2012, 136, 121–127. [Google Scholar] [CrossRef]
- Robinson, J. Changes in Body Composition during Pregnancy. Proc. Nutr. Soc. 1986, 45, 71–80. [Google Scholar] [CrossRef]
- Jaque-Fortunato, S.V.; Khodiguian, N.; Artal, R.; Wiswell, R.A. Body composition in pregnancy. Semin. Perinatol. 1996, 20, 340–342. [Google Scholar] [CrossRef]
- Fortun-Lamothe, L. Energy balance and reproductive performance in rabbit does. Anim. Reprod. Sci. 2006, 93, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Goldberg, G.R. Energy adaptations in human pregnancy: Limits and long-term consequences. Am. J. Clin. Nutr. 2000, 71, 1226S–1232S. [Google Scholar] [CrossRef]
- Sonagra, A.D. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M. Pregnancy and lactation in relation to range of acceptable carbohydrate and fat intake. Eur. J. Clin. Nutr. 1999, 53, s124–s135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffman, F.; Boretto, E.; Vitale, S.; Gonzalez, V.; Vidal, G.; Pardo, M.; Flores, M.; Garcia, F.; Bagnis, G.; Queiroz, O.; et al. Maternal nutritional restriction during late gestation impairs development of the reproductive organs in both male and female lambs. Theriogenology 2018, 108, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Bourke, D.; Da Silva, P.; Aitken, R. Nutrient partitioning during adolescent pregnancy. Reproduction 2001, 122, 347–357. [Google Scholar] [CrossRef]
- Fortun, L.; Prunier, A.; Etienne, M.; LeBas, F. Influence of the nutritional deficit of foetal survival and growth and plasma metabolites in rabbit does. Reprod. Nutr. Dev. 1994, 34, 201–211. [Google Scholar] [CrossRef]
- Brameld, J.M.; Mostyn, A.; Dandrea, J.; Stephenson, T.; Dawson, J.; Buttery, P.; Symonds, M.; Symonds, M.E. Maternal nutrition alters the expression of insulin-like growth factors in fetal sheep liver and skeletal muscle. J. Endocrinol. 2000, 167, 429–437. [Google Scholar] [CrossRef]
- Zócalo, Y.; Ungerfeld, R.; Pérez-Clariget, R.; Bia, D. Maternal nutritional restriction during gestation impacts differently on offspring muscular and elastic arteries and is associated with increased carotid resistance and ventricular afterload in maturity. J. Dev. Orig. Health Dis. 2019, 11, 7–17. [Google Scholar] [CrossRef]
- Anderson, J.; Henck, J. Fetal development. In The Biology of the Laboratory Rabbit; Manning, P.J., Ringler, D.H., Newcomer, C.E., Eds.; Academic Press: Cambridge, MA, USA, 1994; p. 457. [Google Scholar]
- Sferruzzi-Perri, A.N.; Camm, E.J. The programming power of the placenta. Front. Physiol. 2016, 7, 33. [Google Scholar] [CrossRef]
- Ford, S.P.; Hess, B.W.; Schwope, M.M.; Nijland, M.J.; Gilbert, J.S.; Vonnahme, K.A.; Means, W.J.; Han, H.; Nathanielsz, P.W. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 2007, 85, 1285–1294. [Google Scholar] [CrossRef]
- Richter, H.G.; Hansell, J.A.; Raut, S.; Giussani, D.A. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 2009, 46, 357–364. [Google Scholar] [CrossRef]
- Freetly, H.C.; Ferrell, C.L.; Jenkins, T.G. Nutritionally altering weight gain patterns of pregnant heifers and young cows changes the time that feed resources are offered without any differences in production. J. Anim. Sci. 2005, 83, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Niederecker, K.N.; Larson, J.M.; Kallenbach, R.L.; Meyer, A.M. Effects of feeding stockpiled tall fescue versus summer-baled tall fescue-based hay to late gestation beef cows: I. Cow performance, maternal metabolic status, and fetal growth. J. Anim. Sci. 2018, 96, 4618–4632. [Google Scholar] [CrossRef] [PubMed]
- Borwick, S.; Rae, M.; Brooks, J.; McNeilly, A.; Racey, P.; Rhind, S.M. Undernutrition of ewe lambs in utero and in early post-natal life does not affect hypothalamic-pituitary function in adulthood. Anim. Reprod. Sci. 2003, 77, 61–70. [Google Scholar] [CrossRef]
- Crowe, C.; Bennet, L.; Hanson, M. Blood pressure and cardiovascular reflex development in fetal sheep. Relation to hypoxaemia, weight, and blood glucose. Reprod. Fertil. Dev. 1995, 7, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Q.; Tang, S.; Tan, Z.; Fitzsimmons, C.J.; Yi, K. Effects of maternal feed intake restriction during pregnancy on the expression of growth regulation, imprinting and epigenetic transcription-related genes in foetal goats. Anim. Reprod. Sci. 2018, 198, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Astiz, S.; Torres-Rovira, L.; Sánchez-Sánchez, R.; Gómez-Fidalgo, E.; Gonzalez, J.; Isabel, B.; Rey, A.; Óvilo, C.; et al. Ontogeny of sex-related differences in foetal developmental features, lipid availability and fatty acid composition. Int. J. Mol. Sci. 2017, 18, 1171. [Google Scholar] [CrossRef]
- Todd, S.E.; Oliver, M.H.; Jaquiery, A.L.; Bloomfield, F.H.; Harding, J.E. Periconceptional undernutrition of ewes impairs glucose tolerance in their adult offspring. Pediatr. Res. 2009, 65, 409–413. [Google Scholar] [CrossRef]
- George, L.A.; Zhang, L.; Tuersunjiang, N.; Ma, Y.; Long, N.M.; Uthlaut, A.B.; Smith, D.T.; Nathanielsz, P.W.; Ford, S.P. Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R795–R804. [Google Scholar] [CrossRef]
- Lie, S.; Morrison, J.L.; Williams-Wyss, O.; Suter, C.M.; Humphreys, D.T.; Ozanne, S.E.; Zhang, S.; MacLaughlin, S.M.; Kleemann, D.O.; Walker, S.K.; et al. Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep. Am. J. Physiol. Metab. 2014, 306, E1013–E1024. [Google Scholar] [CrossRef]
- De Koster, J.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]
- Lee, S.; You, Y.-A.; Kwon, E.J.; Jung, S.-C.; Jo, I.; Kim, Y.J. Maternal food restriction during pregnancy and lactation adversely affect hepatic growth and lipid metabolism in three-week-old rat offspring. Int. J. Mol. Sci. 2016, 17, 2115. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.K.; LeMaster, C.T.; Mangrum, K.S.; Ricks, R.E.; Long, N.M. Effects of maternal nutrient restriction during early or mid-gestation without realimentation on maternal physiology and foetal growth and development in beef cattle. Animal 2018, 12, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhu, M.J.; Uthlaut, A.B.; Nijland, M.J.; Nathanielsz, P.W.; Hess, B.W.; Ford, S.P. Upregulation of growth signaling and nutrient transporters in cotyledons of early to mid-gestational nutrient restricted ewes. Placenta 2011, 32, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Valentino, S.A.; Tarrade, A.; Aioun, J.; Mourier, E.; Richard, C.; Dahirel, M.; Rousseau-Ralliard, D.; Fournier, N.; Aubrière, M.-C.; Lallemand, M.-S.; et al. Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part. Fibre Toxicol. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- McAuley, K.A.; Williams, S.M.; Mann, J.I.; Walker, R.J.; Lewis-Barned, N.J.; Temple, L.A.; Duncan, A.W. Diagnosing insulin resistance in the general population. Diabetes Care 2001, 24, 460–464. [Google Scholar] [CrossRef]
- Wesolowski, S.R.; Mulligan, C.M.; Janssen, R.C.; Baker, P.R.; Bergman, B.C.; D’Alessandro, A.; Nemkov, T.; MacLean, K.N.; Jiang, H.; Dean, T.A.; et al. Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates. Mol. Metab. 2018, 18, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.C.; Floyd, R.R.; Sabir, S.; Jayasekera, D.W.; Leon-Mimila, P.V.; Jones, A.E.; Cortez, A.A.; Shravah, V.; Péterfy, M.; Stiles, L.; et al. Liver Pyruvate Kinase Promotes NAFLD/NASH in Both Mice and Humans in a Sex-Specific Manner. Cell. Mol. Gastroenterol. Hepatol. 2020. [CrossRef]
- Symeon, G.K.; Goliomytis, M.; Bizelis, I.; Papadomichelakis, G.; Pagonopoulou, O.; Abas, Z.; Deligeorgis, S.G.; Chadio, S.E. Effects of gestational maternal undernutrition on growth, carcass composition and meat quality of rabbit offspring. PLoS ONE 2015, 10, e0118259. [Google Scholar] [CrossRef][Green Version]
- Amacher, D.E. A toxicologist’s guide to biomarkers of hepatic response. Hum. Exp. Toxicol. 2002, 21, 253–262. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, B.; Liang, B.; Zhao, H.; Li, H.; Qian, J.; Liang, H.-M.; Feng, G.-S.; Zheng, C.-S. Hepatic Parenchymal Changes following Transcatheter Embolization and Chemoembolization in a Rabbit Tumor Model. PLoS ONE 2013, 8, e70757. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.O.; Kim, G.-B.; Lee, S.-J.; Lee, H.-R.; Kim, S.-J.; Kang, H.-S.; Kim, J.-S. Long-term oral intake of Panax ginseng improves hypomagnesemia, hyperlactatemia, base deficit, and metabolic acidosis in an alloxan-induced rabbit model. Iran. J. Basic Med. Sci. 2019, 22, 703–709. [Google Scholar] [PubMed]
- Aulbach, A.; Amuzie, C. Biomarkers in Nonclinical Drug Development; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 447–471. [Google Scholar]
- Blair, P.M.R.; Thompson, M.B.; Wilson, R.E.; Esber, H.H. Correlation of changes in serum analytes and hepatic histopathology in rats exposed to carbon tetrachloride. Toxicol. Lett. 1991, 55, 149–159. [Google Scholar] [CrossRef]
- Fraser, A.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. The associations between birthweight and adult markers of liver damage and function. Paediatr. Périnat. Epidemiol. 2007, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Campisano, S.E.; Echarte, S.; Podaza, E.; Chisari, A.N. Protein malnutrition during fetal programming induces fatty liver in adult male offspring rats. J. Physiol. Biochem. 2017, 73, 275–285. [Google Scholar] [CrossRef]

| Control (n = 7) | R021 (n = 7) | Ptime > f | PMFR > f | PtimexMFR > f | |||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | ||||
| LBW (g) | 3990 ± 122.0 | 4692 ± 122.0 | 4049 ± 86.6 | 4608 ± 86.6 | 0.0001 | 0.9288 | 0.1989 |
| Water (%) | 58.8 ± 1.75 | 88.0 ± 1.75 | 58.8 ± 1.24 | 86.8 ± 1.24 | 0.0001 | 0.6609 | 0.7218 |
| Ash (%) | 3.30 ± 0.05 | 3.06 ± 0.05 | 3.30 ± 0.03 | 3.08 ± 0.03 | 0.0003 | 0.8481 | 0.8036 |
| Lipids (%) | 15.4 ± 1.78 | 11.9 ± 1.78 | 15.3 ± 1.26 | 12.1 ± 1.26 | 0.0835 | 0.9780 | 0.9454 |
| Proteins (%) | 19.36 ± 0.13 | 17.6 ± 0.13 | 19.38 ± 0.09 | 17.7 ± 0.09 | 0.0001 | 0.7102 | 0.7530 |
| Energy (MJ/kg) | 147.0 ± 80.6 | 955.7 ± 80.6 | 1144.0 ± 57.0 | 966.04 ± 57.0 | 0.0439 | 0.9518 | 0.9350 |
| Control (n = 7) | R021 (n = 7) | p > f | |
|---|---|---|---|
| Glucose (mg/dL) | 119.4 ± 8.29 | 102.3 ± 8.29 | 0.1715 |
| Insulin (mU/L) | 8.37 ± 2.10 | 12.34 ± 2.10 | 0.2078 |
| Ratio glucose/insulin | 18.1 ± 6.05 | 16.2 ± 2.10 | 0.8308 |
| HOMA IR index 1 | 2.46 ± 0.52 | 2.90 ± 0.52 | 0.5602 |
| TG (mg/dL) | 78.6 ± 12.9 | 88.2 ± 12.9 | 0.6096 |
| Total cholesterol (mg/dL) | 31.6 ± 4.62 | 23.9 ± 4.62 | 0.2597 |
| Ratio TG/cholesterol | 2.97 ± 0.72 | 4.06 ± 0.72 | 0.3074 |
| AST(U/L) | 58.4 ± 20.3 | 103 ± 20.3 | 0.1461 |
| ALT (U/L) | 9.14 ± 6.11 | 23.6 ± 6.11 | 0.1194 |
| Ratio AST/ALT | 6.77 ± 1.32 | 5.64 ± 1.32 | 0.5553 |
| IGF1 (ng/mL) | 267.9 ± 26.1 | 296.4 ± 26.1 | 0.4621 |
| Control (n = 7) | R021 (n = 7) | p > f | |
|---|---|---|---|
| Total placenta weight (g) | 4.99 ± 0.14 | 5.08 ± 0.12 | 0.6785 |
| Total placenta efficiency (%) | 7.84 ± 0.19 | 7.35 ± 0.17 | 0.0751 |
| Decidual zone | |||
| Weight (g) | 1.44 ± 0.05 | 1.42 ± 0.05 | 0.8493 |
| Length (mm) | 40.9 ± 0.65 | 35.8 ± 0.56 | 0.0001 |
| Thickness (mm) | 3.25 ± 0.12 | 3.28 ± 0.10 | 0.8545 |
| Maternal placenta efficiency | 28.2±1.14 | 27.3±1.00 | 0.5907 |
| Labyrinth zone | |||
| Weight (g) | 3.53 ± 0.12 | 3.57 ± 0.11 | 0.8384 |
| Length (mm) | 38.8 ± 0.63 | 34.3 ± 0.55 | 0.0001 |
| Thickness (mm) | 4.91 ± 0.13 | 5.23 ± 0.14 | 0.1727 |
| Fetal placenta efficiency | 11.4 ± 0.30 | 10.6 ± 0.26 | 0.0684 |
| Control (n = 7) | R021 (n = 7) | p > f | |
|---|---|---|---|
| Morphometric measurements | |||
| Biparietal diameter (mm) | 19.4 ± 0.15 | 19.1 ± 0.13 | 0.2291 |
| Crown-rump length (mm) | 100.7 ± 0.76 | 99.3 ± 0.68 | 0.1998 |
| Thoracic diameter (mm) | 20.9 ± 0.30 | 20.6 ± 0.27 | 0.4534 |
| Fetus weights | |||
| Total (g) | 39.4 ± 0.74 | 38.2 ± 0.67 | 0.2733 |
| Head (g) | 9.45 ± 0.15 | 9.23 ± 0.13 | 0.3245 |
| Trunk (g) | 28.5 ± 0.59 | 27.8 ± 0.52 | 0.4302 |
| Liver (g) | 2.52 ± 0.10 | 2.40 ± 0.08 | 0.3940 |
| Gut (g) | 1.89 ± 0.06 | 1.90 ± 0.05 | 0.8697 |
| Brain (g) | 0.91 ± 0.02 | 0.93 ± 0.01 | 0.4239 |
| Weight ratios | |||
| Brain ratio (%) | 2.36 ± 0.06 | 2.48 ± 0.05 | 0.1385 |
| Liver ratio (%) | 6.40 ± 0.17 | 6.49 ± 0.14 | 0.7288 |
| Brain: Liver ratio (%) | 37.9 ± 1.98 | 39.9 ± 1.66 | 0.4546 |
| Control (n = 30) | R021 (n = 30) | p > f | |
|---|---|---|---|
| Glucose (mg/dL) | 46.26 ± 3.16 | 49.70 ± 3.99 | 0.5028 |
| Insulin (mU/L) | 3.80 ± 0.77 | 7.28 ± 0.87 | 0.0063 |
| Ratio insulin/glucose | 0.10 ± 0.18 | 0.15 ± 0.02 | 0.0600 |
| HOMA IR index 1 | 0.40 ± 0.09 | 0.87 ± 0.07 | 0.0001 |
| TG (mg/dL) | 95.00 ± 4.98 | 112.44 ± 6.07 | 0.0347 |
| Total cholesterol (mg/dL) | 112.39 ± 4.24 | 105.91 ± 5.38 | 0.3500 |
| Ratio TG/cholesterol | 0.85 ± 0.05 | 0.98 ± 0.06 | 0.0940 |
| AST (U/L) | 33.27 ± 2.55 | 33.28 ± 2.84 | 0.9977 |
| IGF1 (ng/mL) | 123.88 ± 7.45 | 140.82 ± 8.92 | 0.1564 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, R.M.; Arias-Álvarez, M.; Millán, P.; Rodríguez, M.; Sánchez-Rodríguez, A.; Lorenzo, P.L.; Rebollar, P.G. Gestation Food Restriction and Refeeding Compensate Maternal Energy Status and Alleviate Metabolic Consequences in Juvenile Offspring in a Rabbit Model. Nutrients 2021, 13, 310. https://doi.org/10.3390/nu13020310
García-García RM, Arias-Álvarez M, Millán P, Rodríguez M, Sánchez-Rodríguez A, Lorenzo PL, Rebollar PG. Gestation Food Restriction and Refeeding Compensate Maternal Energy Status and Alleviate Metabolic Consequences in Juvenile Offspring in a Rabbit Model. Nutrients. 2021; 13(2):310. https://doi.org/10.3390/nu13020310
Chicago/Turabian StyleGarcía-García, Rosa M., María Arias-Álvarez, Pilar Millán, María Rodríguez, Ana Sánchez-Rodríguez, Pedro L. Lorenzo, and Pilar G. Rebollar. 2021. "Gestation Food Restriction and Refeeding Compensate Maternal Energy Status and Alleviate Metabolic Consequences in Juvenile Offspring in a Rabbit Model" Nutrients 13, no. 2: 310. https://doi.org/10.3390/nu13020310
APA StyleGarcía-García, R. M., Arias-Álvarez, M., Millán, P., Rodríguez, M., Sánchez-Rodríguez, A., Lorenzo, P. L., & Rebollar, P. G. (2021). Gestation Food Restriction and Refeeding Compensate Maternal Energy Status and Alleviate Metabolic Consequences in Juvenile Offspring in a Rabbit Model. Nutrients, 13(2), 310. https://doi.org/10.3390/nu13020310





