The Red Seaweed Grateloupia turuturu Prevents Epidermal Dysplasia in HPV16-Transgenic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. G. turuturu Samples
2.3. Diet Preparation
2.4. Experimental Design
2.5. Histological Analysis
2.6. Biochemical Analysis
2.7. DNA Integrity Assessment
2.8. Stastistical Analysis
3. Results
3.1. Organ Relative Weights and Blood Biochemistry
3.2. Histological Analysis
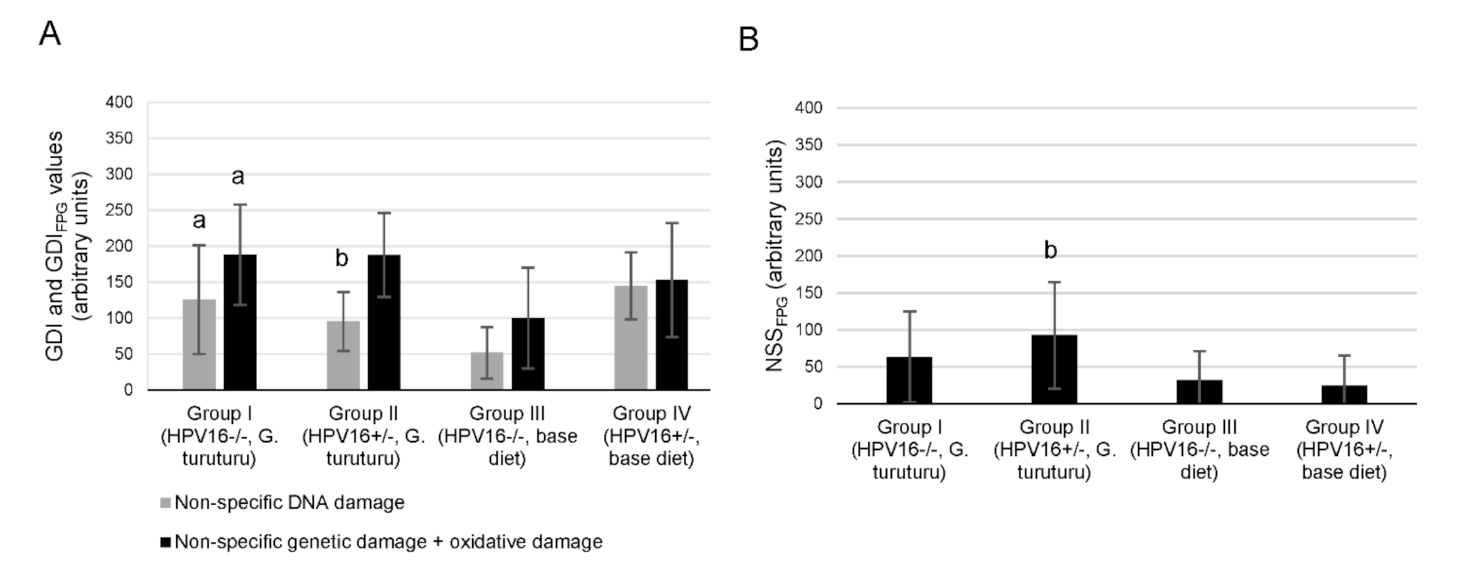
3.3. DNA Integrity Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faulkner, D.J. Marine Natural Products. Nat. Prod. Rep. 2001, 18, 1–49. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; Falcão, D.S.; Lima, D.M.; Gedson, R.; Montenegro, D.A.; Lira, N.S.; Athayde-Filho, D.; Petrônio, F.; Rodrigues, L.C.; De Souza, M.D.F.V.; et al. Bioactivities from Marine algae of the Genus Gracilaria. IJMS 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Rahelivao, M.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H.-J. Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Mar. Drugs 2015, 13, 4197–4216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and Human Health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World Cuisine of Seaweeds: Science Meets Gastronomy. Int. J. Gastron. Food Sci. 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Tokudome, S.; Kuriki, K.; Moore, M.A. Seaweed and Cancer Prevention. Jpn. J. Cancer Res. 2001, 92, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Moussavou, G.; Kwak, D.; Obiang-Obonou, B.; Maranguy, C.; Dinzouna-Boutamba, S.-D.; Lee, D.; Pissibanganga, O.; Ko, K.; Seo, J.; Choo, Y. Anticancer Effects of Different Seaweeds on Human Colon and Breast Cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the Chemical Composition of Edible Red Macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Kendel, M.; Couzinet-Mossion, A.; Viau, M.; Fleurence, J.; Barnathan, G.; Wielgosz-Collin, G. Seasonal Composition of Lipids, Fatty Acids, and Sterols in the Edible Red Alga Grateloupia turuturu. J. Appl. Phycol. 2013, 25, 425–432. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a Better Understanding of Medicinal Uses of the Brown Seaweed Sargassum in Traditional Chinese Medicine: A Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Jiang, Z.; Zheng, F.; Wang, H.; Zhang, Y.; Gao, F.; Chen, P.; Chen, Y.; Shi, G. Optimized Extraction of Polysaccharides from Grateloupia livida (Harv.) Yamada and Biological Activities. Molecules 2015, 20, 16817–16832. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, F.; Zhang, X.-W.; Wang, S.-C.; Li, M.-H.; Lin, L.-P.; Ding, J. Grateloupia longifolia Polysaccharide Inhibits Angiogenesis by Downregulating Tissue Factor Expression in HMEC-1 Endothelial Cells: GLP Inhibits Angiogenesis by Downregulating TF. Br. J. Pharmacol. 2006, 148, 741–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Yan, J.; Wang, S.; Ji, L.; Ding, K.; Vella, C.; Wang, Z.; Hu, Z. Antiangiogenic Effects of GFP08, an Agaran-Type Polysaccharide Isolated from Grateloupia filicina. Glycobiology 2012, 22, 1343–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masrour-Roudsari, J.; Ebrahimpour, S. Casual Role of Infectious Agents in Cancer: An Overview. Casp. J. Intern. Med. 2017, 8, 153–158. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type: Worldwide Burden of Cancer Attributable to HPV. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The Human Papillomavirus (HPV)-Related Cancer Biology: An Overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estêvão, D.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. Hallmarks of HPV Carcinogenesis: The Role of E6, E7 and E5 Oncoproteins in Cellular Malignancy. Biochim. Et Biophys. Acta (BBA) Gene Regul. Mech. 2019, 1862, 153–162. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Mestre, V.F.; Colaço, B.; Pires, M.J.; Martins, T.; Gil da Costa, R.M.; Neuparth, M.J.; Medeiros, R.; Moutinho, M.S.S.; Dias, M.I.; et al. Laurus nobilis (Laurel) Aqueous Leaf Extract’s Toxicological and Anti-Tumor Activities in HPV16-Transgenic Mice. Food Funct. 2018, 9, 4419–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.; Nascimento-Gonçalves, E.; Gil Da Costa, R.M.; Rosa, E.; Alexandra Oliveira, P. Therapeutic and Toxicological Effects of Natural Compounds: Data from HPV16-Transgenic and ICR Mice (Review). World Acad. Sci. J. 2020, 2. [Google Scholar] [CrossRef]
- Arbeit, J.M.; Münger, K.; Howley, P.M.; Hanahan, D. Progressive Squamous Epithelial Neoplasia in K14-Human Papillomavirus Type 16 Transgenic Mice. J. Virol. 1994, 68, 4358–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.; Vilanova, M.; Medeiros, R.; Gil da Costa, R.M. HPV-Transgenic Mouse Models: Tools for Studying the Cancer-Associated Immune Response. Virus Res. 2017, 235, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Gil da Costa, R.M.; Ribeiro, J.; Sousa, H.; Bastos, M.M.S.M.; Faustino-Rocha, A.; Lopes, C.; Oliveira, P.A.; Medeiros, R. MicroRNA-21 Expression and Susceptibility to HPV-Induced Carcinogenesis—Role of Microenvironment in K14-HPV16 Mice Model. Life Sci. 2015, 128, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia Turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Hartmann, A.; Martins-Gomes, C.; Nunes, F.M.; Souto, E.B.; Santos, D.L.; Abreu, H.; Pereira, R.; Pacheco, M.; Gaivão, I.; et al. Red Seaweeds Strengthening the Nexus between Nutrition and Health: Phytochemical Characterization and Bioactive Properties of Grateloupia Turuturu and Porphyra Umbilicalis Extracts. J. Appl. Phycol. 2021, 33, 3365–3381. [Google Scholar] [CrossRef]
- Ferreira, J.; Marques, A.; Abreu, H.; Pereira, R.; Rego, A.; Pacheco, M.; Gaivão, I. Red Seaweeds Porphyra Umbilicalis and Grateloupia turuturu Display Antigenotoxic and Longevity-Promoting Potential in Drosophila Melanogaster. Eur. J. Phycol. 2019, 54, 519–530. [Google Scholar] [CrossRef]
- Forbes, D.; Bloom, H.J.M.; Kostomitsopoulos, N.; Moore, G.; Perretta, G. Euroguide: On the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes; (Based on the Revised Appendix A of the European Convention ETS 123); FELASA: London, UK, 2007; ISBN 978-1-85315-751-6. [Google Scholar]
- Smith-McCune, K.; Zhu, Y.H.; Hanahan, D.; Arbeit, J. Cross-Species Comparison of Angiogenesis during the Premalignant Stages of Squamous Carcinogenesis in the Human Cervix and K14-HPV16 Transgenic Mice. Cancer Res. 1997, 57, 1294–1300. [Google Scholar] [PubMed]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotech 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Arbeit, J.M.; Howley, P.M.; Hanahan, D. Chronic Estrogen-Induced Cervical and Vaginal Squamous Carcinogenesis in Human Papillomavirus Type 16 Transgenic Mice. Proc. Natl. Acad. Sci. USA 1996, 93, 2930–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelzer, M.K.; Pitot, H.C.; Liem, A.; Schweizer, J.; Mahoney, C.; Lambert, P.F. A Mouse Model for Human Anal Cancer. Cancer Prev. Res. 2010, 3, 1534–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestre, V.F.; Medeiros-Fonseca, B.; Estêvão, D.; Casaca, F.; Silva, S.; Félix, A.; Silva, F.; Colaço, B.; Seixas, F.; Bastos, M.M.; et al. HPV16 Is Sufficient to Induce Squamous Cell Carcinoma Specifically in the Tongue Base in Transgenic Mice. J. Pathol. 2020, 251, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Neto, T.; Ferreirinha, P.; Sousa, H.; Ribeiro, J.; Bastos, M.M.S.M.; Faustino-Rocha, A.I.; Oliveira, P.A.; Medeiros, R.; Vilanova, M.; et al. Celecoxib Promotes Degranulation of CD8+ T Cells in HPV-Induced Lesions of K14-HPV16 Transgenic Mice. Life Sci. 2016, 157, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil da Costa, R.M.; Aragão, S.; Moutinho, M.; Alvarado, A.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Ferreira, R.; et al. HPV16 Induces a Wasting Syndrome in Transgenic Mice: Amelioration by Dietary Polyphenols via NF-ΚB Inhibition. Life Sci. 2017, 169, 11–19. [Google Scholar] [CrossRef]
- Santos, C.; Ferreirinha, P.; Sousa, H.; Ribeiro, J.; Bastos, M.M.S.M.; Neto, T.; Oliveira, P.A.; Medeiros, R.; Vilanova, M.; Gil da Costa, R.M. Ptaquiloside from Bracken (Pteridium Spp.) Inhibits Tumour-Infiltrating CD8+ T Cells in HPV-16 Transgenic Mice. Food Chem. Toxicol. 2016, 97, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Gil da Costa, R.M.; Neto, T.; Estêvão, D.; Moutinho, M.; Félix, A.; Medeiros, R.; Lopes, C.; Bastos, M.M.S.M.; Oliveira, P.A. Ptaquiloside from Bracken (Pteridium Spp.) Promotes Oral Carcinogenesis Initiated by HPV16 in Transgenic Mice. Food Funct. 2020, 11, 3298–3305. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Fonseca, B.; Mestre, V.F.; Estêvão, D.; Sánchez, D.F.; Cañete-Portillo, S.; Fernández-Nestosa, M.J.; Casaca, F.; Silva, S.; Brito, H.; Félix, A.; et al. HPV16 Induces Penile Intraepithelial Neoplasia and Squamous Cell Carcinoma in Transgenic Mice: First Mouse Model for HPV-related Penile Cancer. J. Pathol. 2020, 251, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ferreira, T.; Almeida, J.; Pires, M.J.; Colaço, A.; Lemos, S.; Gil da Costa, R.M.; Medeiros, R.; Bastos, M.M.S.M.; Neuparth, M.J.; et al. Dietary Supplementation with the Red Seaweed Porphyra Umbilicalis Protects against DNA Damage and Pre-Malignant Dysplastic Skin Lesions in HPV-Transgenic Mice. Mar. Drugs 2019, 17, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-C.; Hou, M.-F.; Huang, H.-W.; Chang, F.-R.; Yeh, C.-C.; Tang, J.-Y.; Chang, H.-W. Marine Algal Natural Products with Anti-Oxidative, Anti-Inflammatory, and Anti-Cancer Properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, T.; Campos, S.; Silva, M.G.; Ribeiro, R.; Santos, S.; Almeida, J.; Pires, M.J.; Gil da Costa, R.M.; Córdova, C.; Nogueira, A.; et al. The Cyclooxigenase-2 Inhibitor Parecoxib Prevents Epidermal Dysplasia in HPV16-Transgenic Mice: Efficacy and Safety Observations. IJMS 2019, 20, 3902. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.M.O.; Moreira-Pais, A.; Neto, T.; Peixoto da Silva, S.; Oliveira, P.A.; Ferreira, R.; Mendes, J.; Bastos, M.M.S.M.; Lopes, C.; Casaca, F.; et al. Dimethylaminoparthenolide Reduces the Incidence of Dysplasia and Ameliorates a Wasting Syndrome in HPV16-transgenic Mice. Drug Dev. Res. 2019, 80, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Nascimento-Gonçalves, E.; Macedo, S.; Borges, I.; Gama, A.; Gil da Costa, R.; Neuparth, M.J.; Lanzarin, G.; Venâncio, C.; Félix, L.; et al. Toxicological and Anti-Tumor Effects of a Linden Extract (Tilia Platyphyllos Scop.) in a HPV16-Transgenic Mouse Model. Food Funct. 2021, 12, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.N.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the Comet and Micronucleus Assays for Genotoxicity Studies: A Review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, Y.; Iwasaki, K.; Kinae, N.; Tsuda, S.; Murakami, M.; Tanaka, M.; Sasaki, Y.F. Detection of DNA Lesions Induced by Chemical Mutagens Using the Single-Cell Gel Electrophoresis (Comet) Assay. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 1997, 393, 107–113. [Google Scholar] [CrossRef]
- Marques, A.; Ferreira, J.; Abreu, H.; Pereira, R.; Rego, A.; Serôdio, J.; Christa, G.; Gaivão, I.; Pacheco, M. Searching for Antigenotoxic Properties of Marine Macroalgae Dietary Supplementation against Endogenous and Exogenous Challenges. J. Toxicol. Environ. Health Part A 2018, 81, 939–956. [Google Scholar] [CrossRef]
- Pereira, V.; Marques, A.; Gaivão, I.; Rego, A.; Abreu, H.; Pereira, R.; Santos, M.A.; Guilherme, S.; Pacheco, M. Marine Macroalgae as a Dietary Source of Genoprotection in Gilthead Seabream (Sparus aurata) against Endogenous and Exogenous Challenges. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Oyeyemi, I.T.; Yekeen, O.M.; Odusina, P.O.; Ologun, T.M.; Ogbaide, O.M.; Olaleye, O.I.; Bakare, A.A. Genotoxicity and Antigenotoxicity Study of Aqueous and Hydro-Methanol Extracts of Spondias mombin L., Nymphaea lotus L. and Luffa cylindrical L. Using Animal Bioassays. Interdiscip. Toxicol. 2015, 8, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The Antioxidant and Pro-Oxidant Activities of Green Tea Polyphenols: A Role in Cancer Prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forester, S.C.; Lambert, J.D. The Role of Antioxidant versus Pro-Oxidant Effects of Green Tea Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Ferreira, J.; Abreu, H.; Pereira, R.; Pinto, D.; Silva, A.; Gaivão, I.; Pacheco, M. Comparative Genoprotection Ability of Wild-Harvested vs Aqua-Cultured Ulva Rigida Coupled with Phytochemical Profiling. Eur. J. Phycol. 2021, 56, 105–118. [Google Scholar] [CrossRef]

| Grateloupia turuturu | Standard Diet | |||
|---|---|---|---|---|
| Group I (HPV16−/−) | Group II (HPV16+/−) | Group III (HPV16−/−) | Group IV (HPV16+/−) | |
| Liver | 0.0609 ± 0.0013 | 0.0688 ± 0.0049 | 0.0574 ± 0.0012 | 0.0717 ± 0.0185 |
| Right Kidney | 0.0063 ± 0.0001 | 0.0069 ± 0.0002 | 0.0057 ± 0.0002 | 0.0069 ± 0.0002 |
| Left Kidney | 0.0067 ± 0.0001 | 0.0070 ± 0.0003 | 0.0062 ± 0.0002 | 0.0068 ± 0.0002 |
| Thymus | 0.0012 ± 0.0001 | 0.0012 ± 0.0001 | 0.0012 ± 0.0002 | 0.0014 ± 0.0001 |
| Heart | 0.0048 ± 0.0001 | 0.0049 ± 0.0002 | 0.0048 ± 0.0002 | 0.0051 ± 0.0002 |
| Lungs | 0.0065 ± 0.0003 | 0.0060 ± 0.0004 | 0.0063 ± 0.0003 | 0.0071 ± 0.0002 |
| Bladder | 0.0086 ± 0.0070 | 0.0009 ± 0.0001 | 0.0003 ± 0.0002 | 0.0008 ± 0.0001 |
| Spleen | 0.0052 ± 0.0003 | 0.0066 ± 0.0005 | 0.0047 ± 0.0002 | 0.0083 ± 0.0010 |
| Grateloupia turuturu | Standard Diet | |||
|---|---|---|---|---|
| Group I (HPV16 −/−) | Group II (HPV16 +/−) | Group III (HPV16 −/−) | Group IV (HPV16 +/−) | |
| Albumin (g/L) | 31.48 ± 0.69 | 30.64 ± 0.75 | 29.78 ± 1.71 | 30.37 ± 0.96 |
| Total proteins (g/L) | 50.11 ± 2.04 | 53.65 ± 1.63 | 51.34 ± 4.07 | 49.62 ± 1.12 |
| Glucose (mg/dL) | 234.96 ± 17.92 | 185.75 ± 9.06 | 195.70 ± 15.99 | 198.07 ± 13.36 |
| Alanine aminotransferase (U/L) | 30.77 ± 5.32 | 36.59 ± 4.27 | 37.28 ± 4.70 | 41.87 ± 3.54 |
| Aspartate aminotransferase (U/L) | 64.96 ± 8.31 | 67.03 ± 8.45 | 44.74 ± 3.76 | 51.82 ± 3.70 |
| Gamma-glutamyl transferase (U/L) | 33.39 ± 3.70 | 36.75 ± 4.00 | 48.61 ± 6.11 | 60.78 ± 8.35 |
| G. turuturu Supplementation | Standard Diet | ||||
|---|---|---|---|---|---|
| Group I (HPV16−/−) | Group II (HPV16+/−) | Group III (HPV16−/−) | Group IV (HPV16+/−) | ||
| Chest skin affected mice/n (%) | Normal | 11/11 (100.0%) | 0/10 (0.0%) | 11/11 (100.0%) | 0/11 (0.0%) |
| Epidermal Hyperplasia | 0/11 (0.0%) | 8/10 (80.0%) | 0/11 (0.0%) | 4/11 (36.4%) | |
| Epidermal Dysplasia | 0/11 (0.0%) | 2/10 (20.0%) | 0/11 (0.0%) | 7/11 (63.6%) | |
| Ear affected mice/n (%) | Normal | 11/11 (100.0%) | 0/10 (0.0%) | 11/11 (100.0%) | 0/11 (0.0%) |
| Epidermal Hyperplasia | 0/11 (0.0%) | 9/10 (90.0%) a | 0/11 (0.0%) | 4/11 (36.4%) | |
| Epidermal Dysplasia | 0/11 (0.0%) | 1/10 (10.0%) a | 0/11 (0.0%) | 7/11 (63.6%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, J.; Ferreira, T.; Santos, S.; Pires, M.J.; da Costa, R.M.G.; Medeiros, R.; Bastos, M.M.S.M.; Neuparth, M.J.; Faustino-Rocha, A.I.; Abreu, H.; et al. The Red Seaweed Grateloupia turuturu Prevents Epidermal Dysplasia in HPV16-Transgenic Mice. Nutrients 2021, 13, 4529. https://doi.org/10.3390/nu13124529
Almeida J, Ferreira T, Santos S, Pires MJ, da Costa RMG, Medeiros R, Bastos MMSM, Neuparth MJ, Faustino-Rocha AI, Abreu H, et al. The Red Seaweed Grateloupia turuturu Prevents Epidermal Dysplasia in HPV16-Transgenic Mice. Nutrients. 2021; 13(12):4529. https://doi.org/10.3390/nu13124529
Chicago/Turabian StyleAlmeida, José, Tiago Ferreira, Susana Santos, Maria J. Pires, Rui M. Gil da Costa, Rui Medeiros, Margarida M.S.M. Bastos, Maria J. Neuparth, Ana I. Faustino-Rocha, Helena Abreu, and et al. 2021. "The Red Seaweed Grateloupia turuturu Prevents Epidermal Dysplasia in HPV16-Transgenic Mice" Nutrients 13, no. 12: 4529. https://doi.org/10.3390/nu13124529
APA StyleAlmeida, J., Ferreira, T., Santos, S., Pires, M. J., da Costa, R. M. G., Medeiros, R., Bastos, M. M. S. M., Neuparth, M. J., Faustino-Rocha, A. I., Abreu, H., Pereira, R., Pacheco, M., Gaivão, I., Rosa, E., & Oliveira, P. A. (2021). The Red Seaweed Grateloupia turuturu Prevents Epidermal Dysplasia in HPV16-Transgenic Mice. Nutrients, 13(12), 4529. https://doi.org/10.3390/nu13124529












