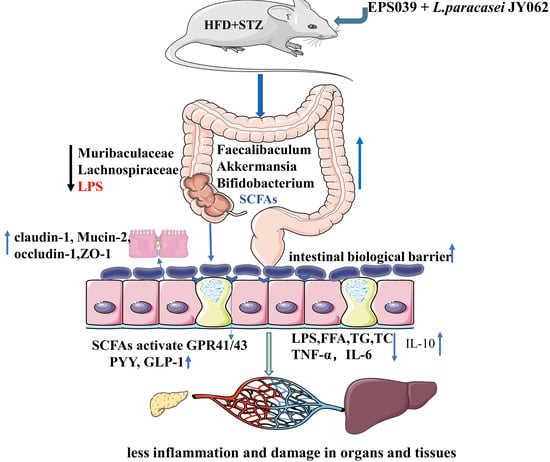
A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Isolation and Purification of the EPS
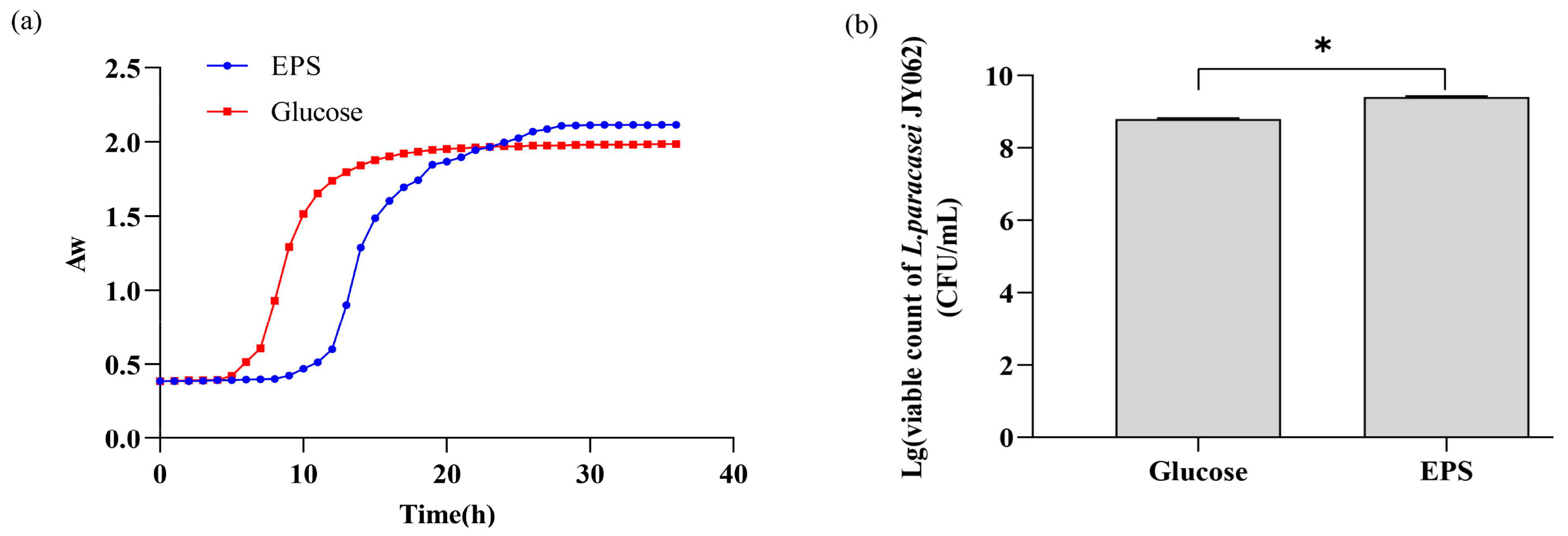
2.3. Monitoring the Growth Curve of L. paracasei JY062
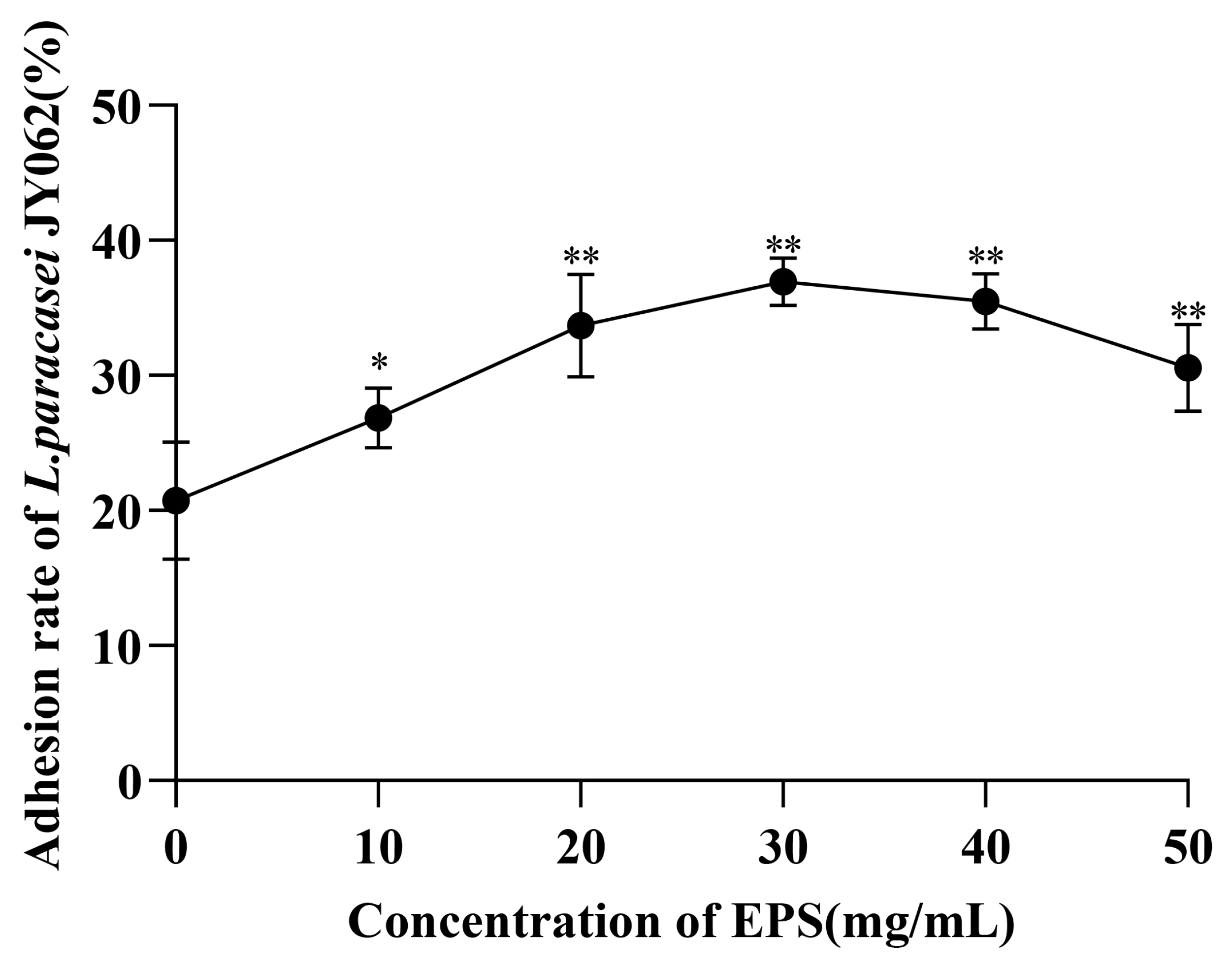
2.4. In Vitro Cell Adhesion Experiment
2.5. Animals and Treatments
2.6. Biochemical Parameter Analysis
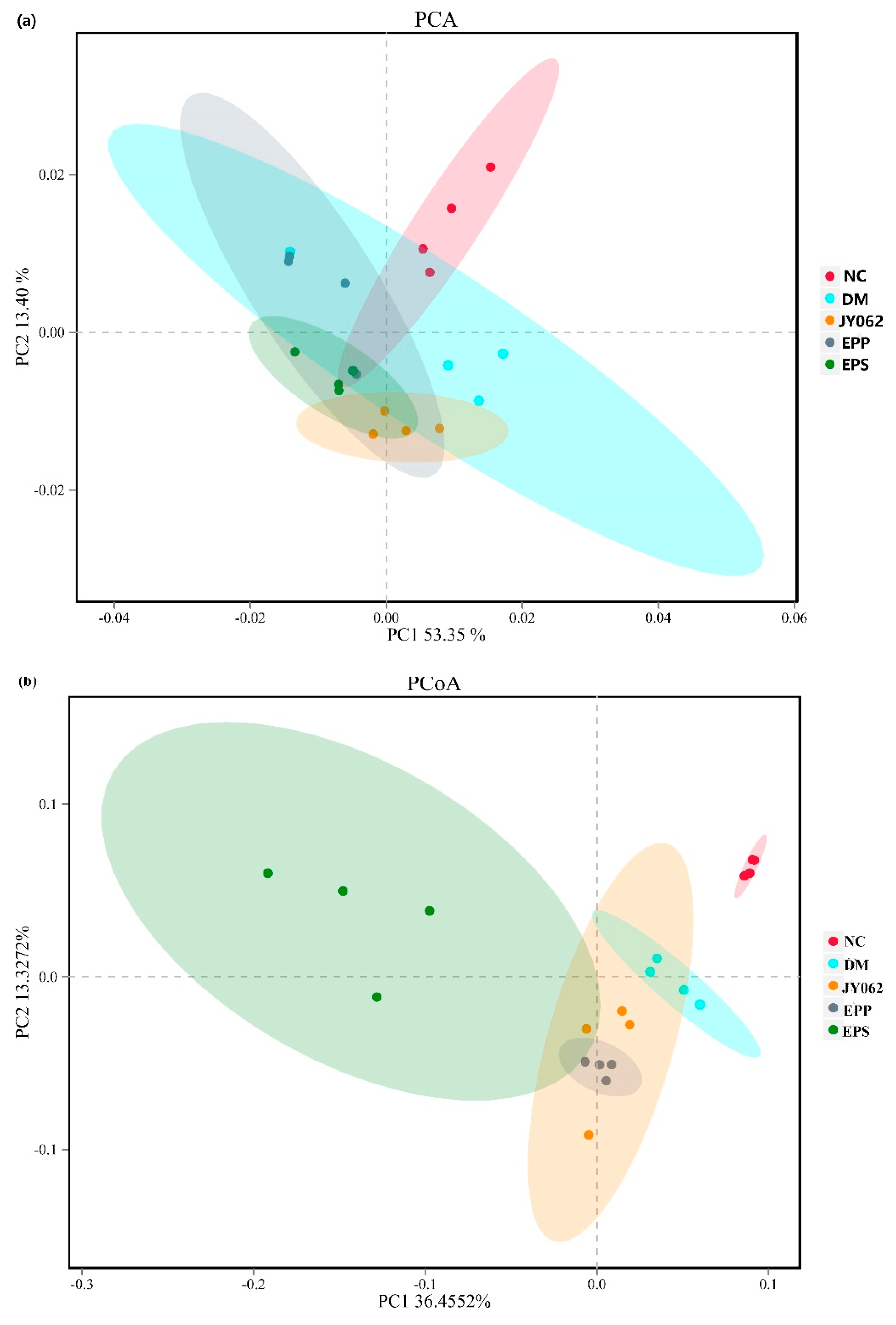
2.7. Intestinal Microbiota Analysis
2.8. Hematoxylin and Eosin (H&E) Staining and Immunohistochemistry
2.9. Quantitative Real-Time PCR (RT-PCR)
2.10. Statistical Analyses
3. Results
3.1. Effect of EPS on the Growth of L. paracasei JY062
3.2. Effect of EPS on the Intestinal Adhesion Capacity of L. paracasei JY062
3.3. Effects of Dietary Intervention on Blood Glucose and Lipid-Related Parameters
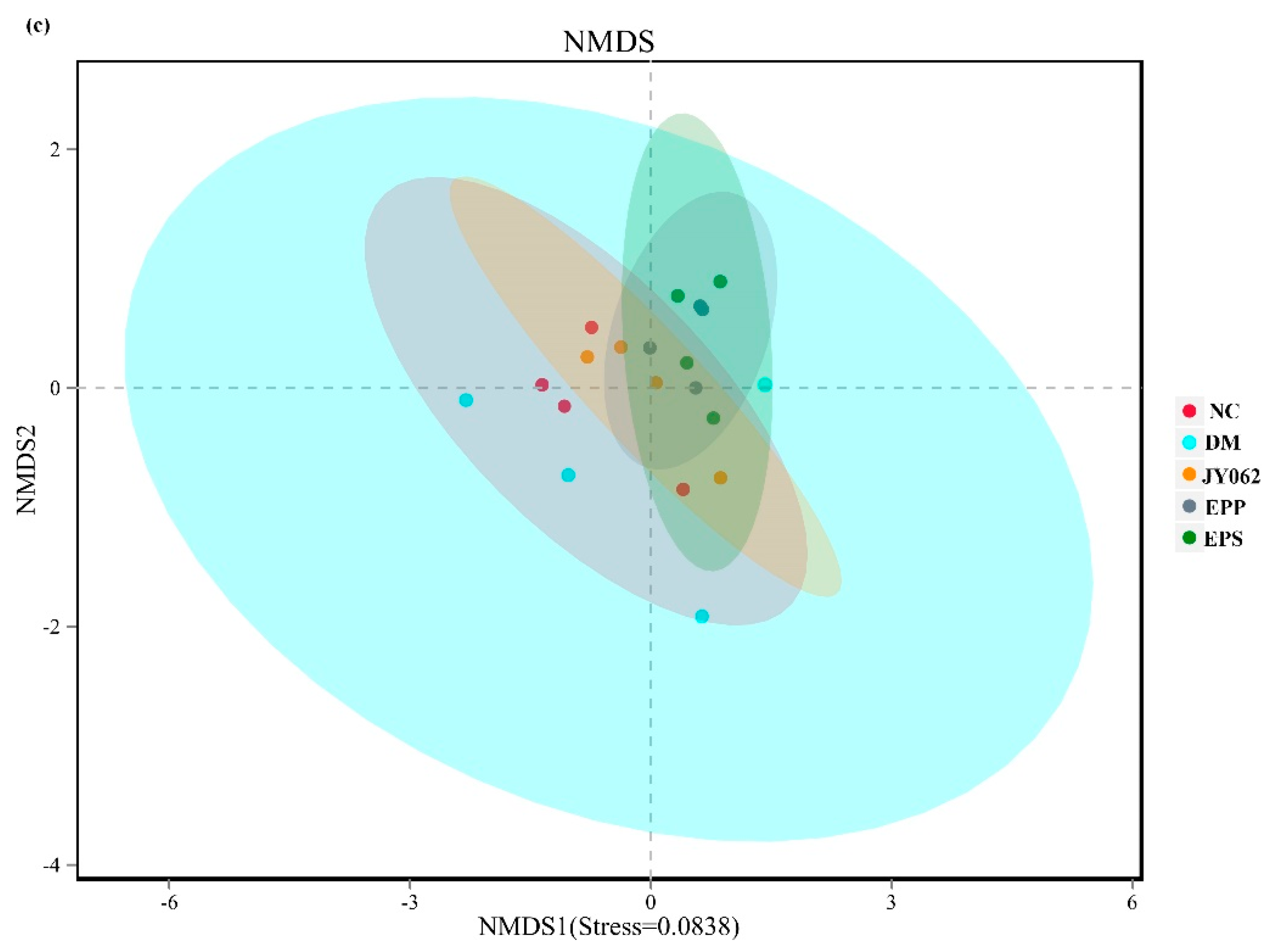
3.4. The Impact of Dietary Intervention on Intestinal Microbiota Structure
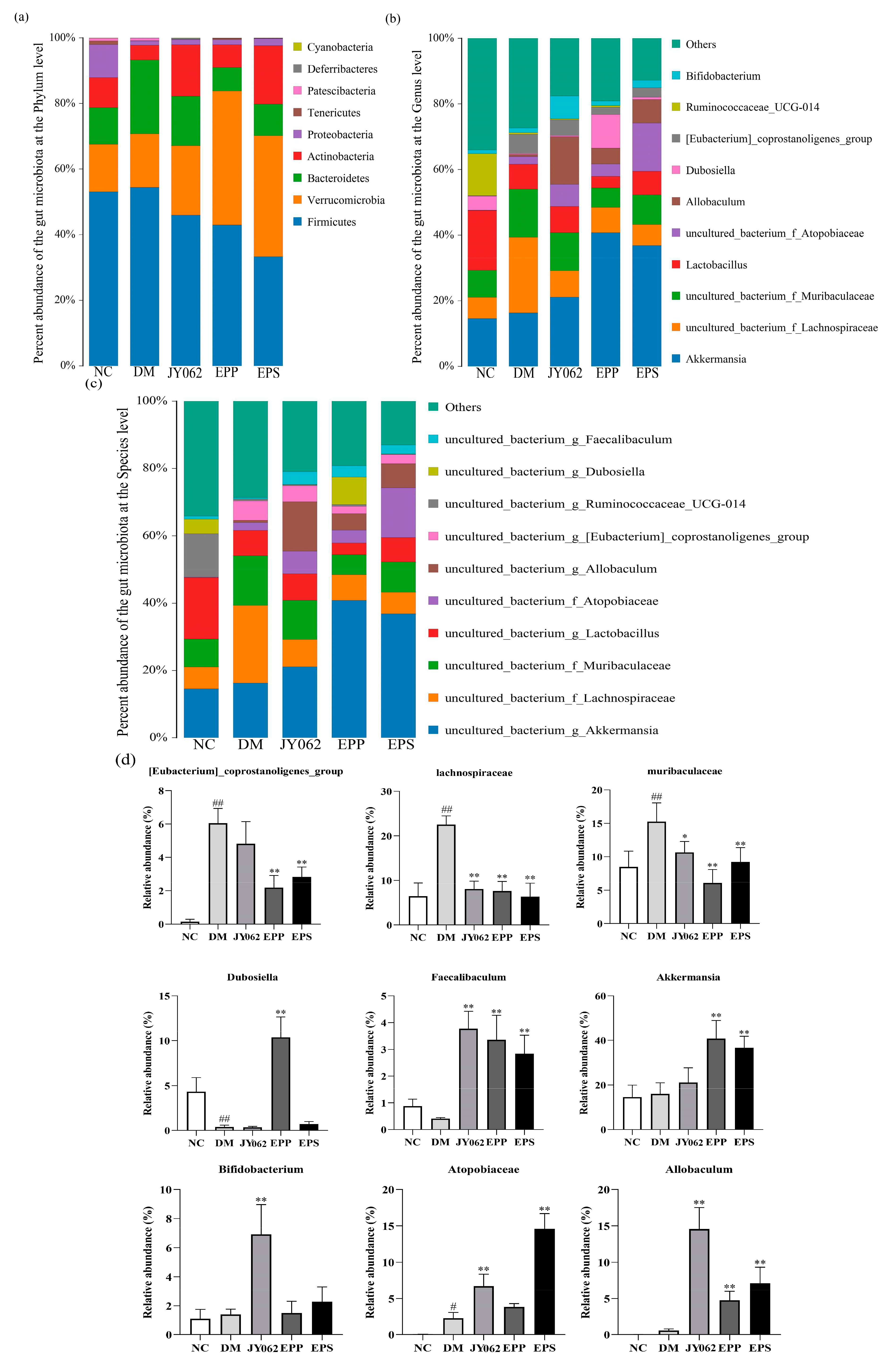
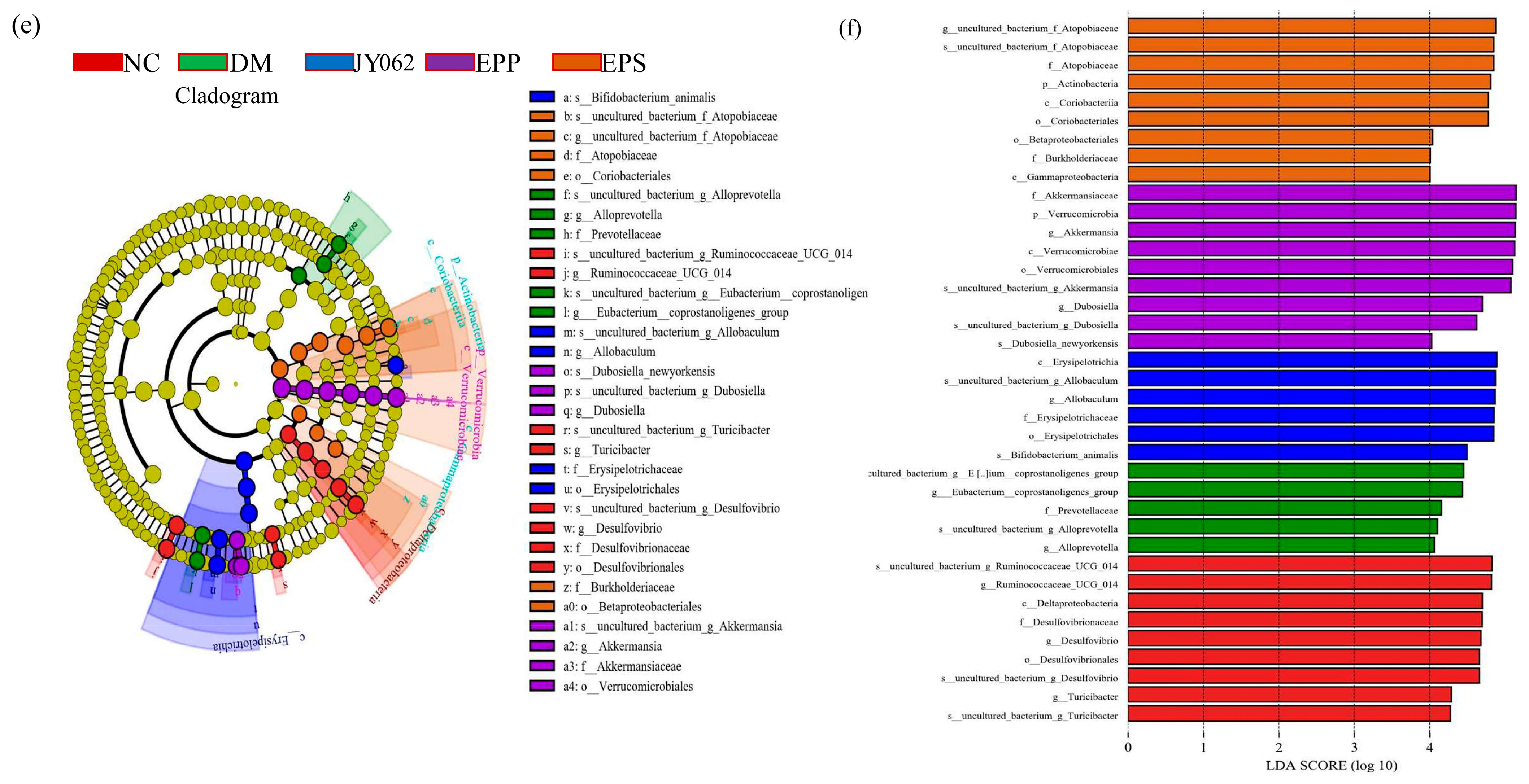
3.5. The Impact of Dietary Intervention on Intestinal Microbiota Composition
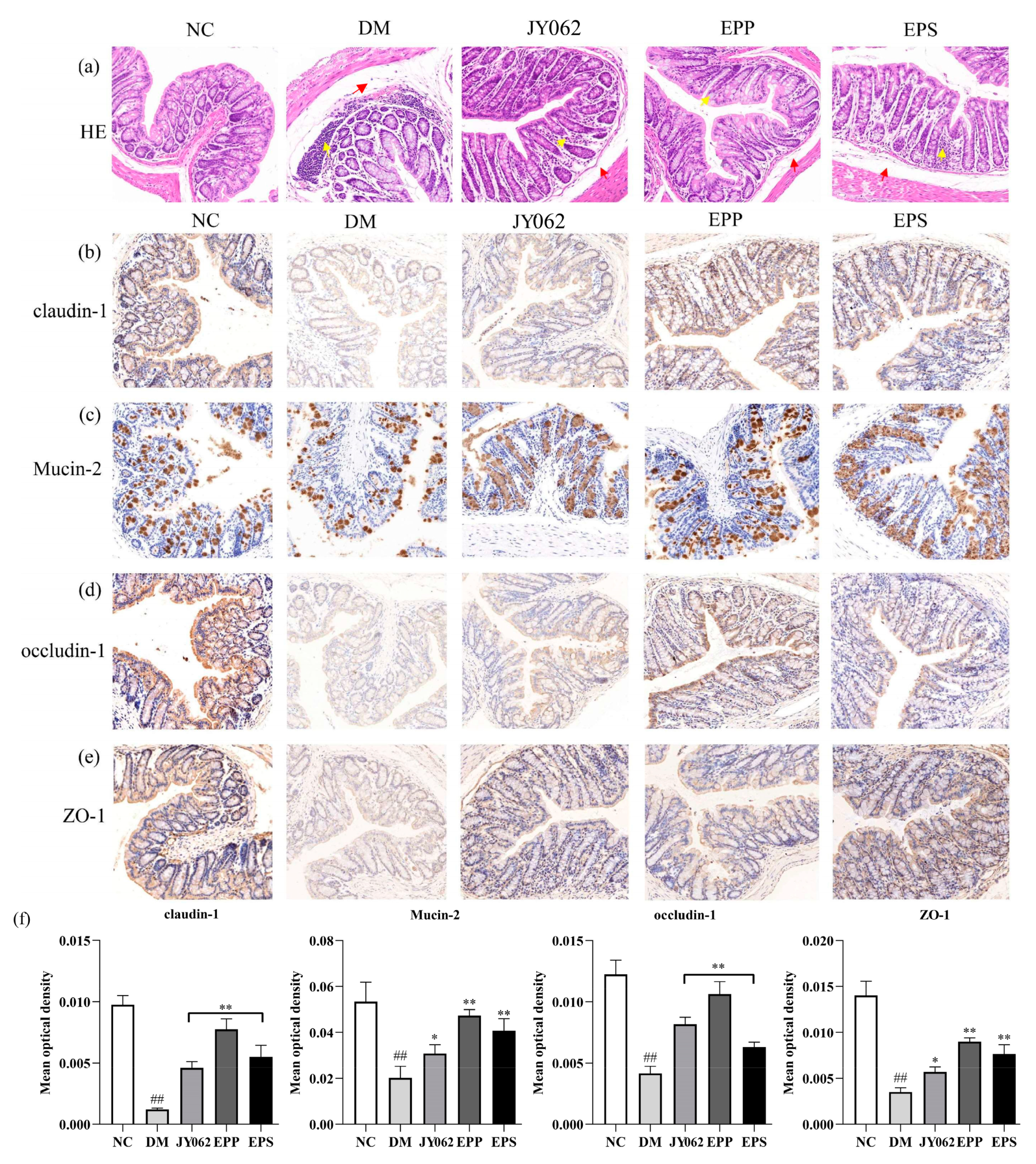
3.6. The Impact of Dietary Intervention on Intestinal Barrier Function
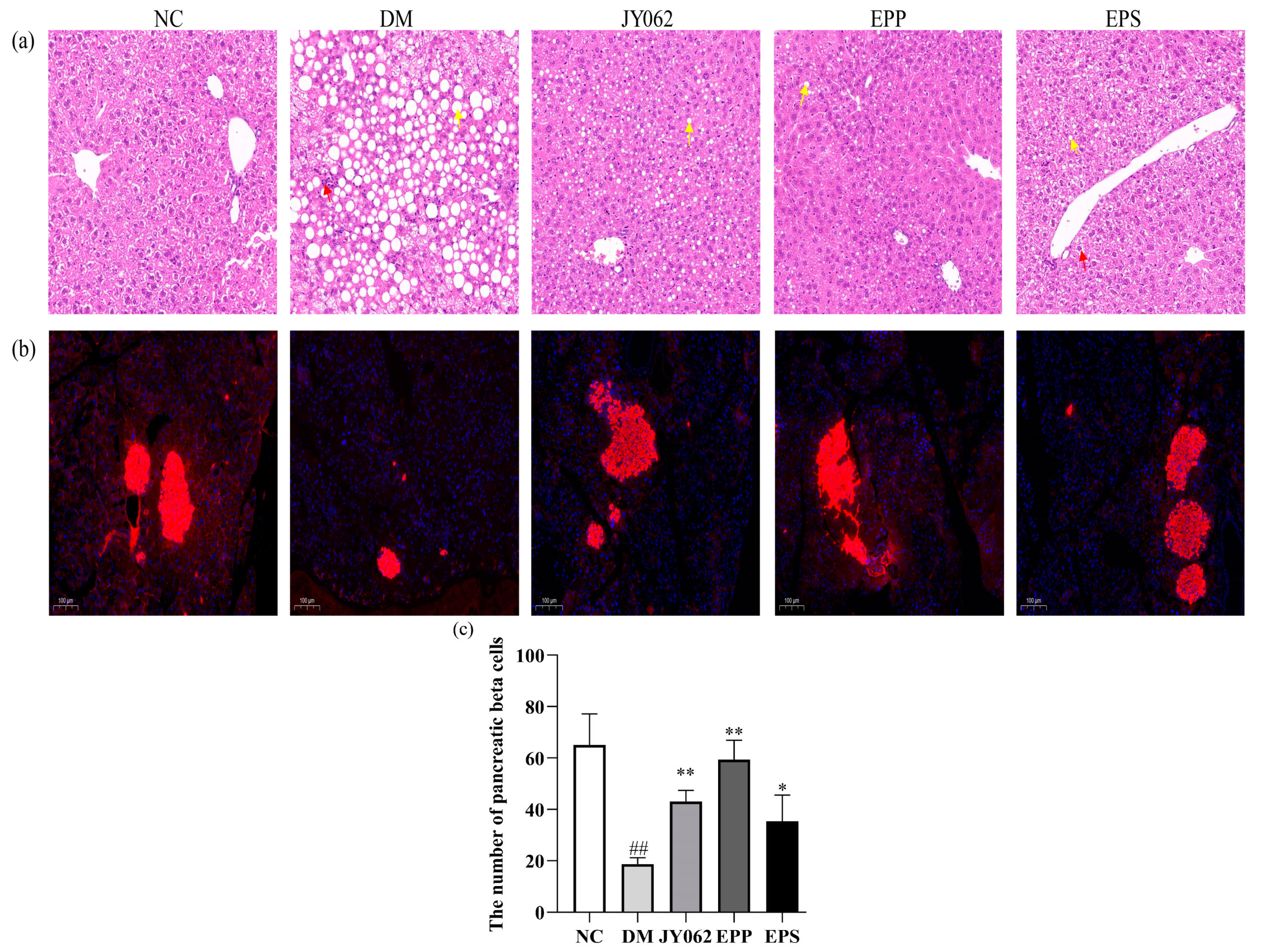
3.7. The Impact of Dietary Intervention on Liver and Pancreas Function
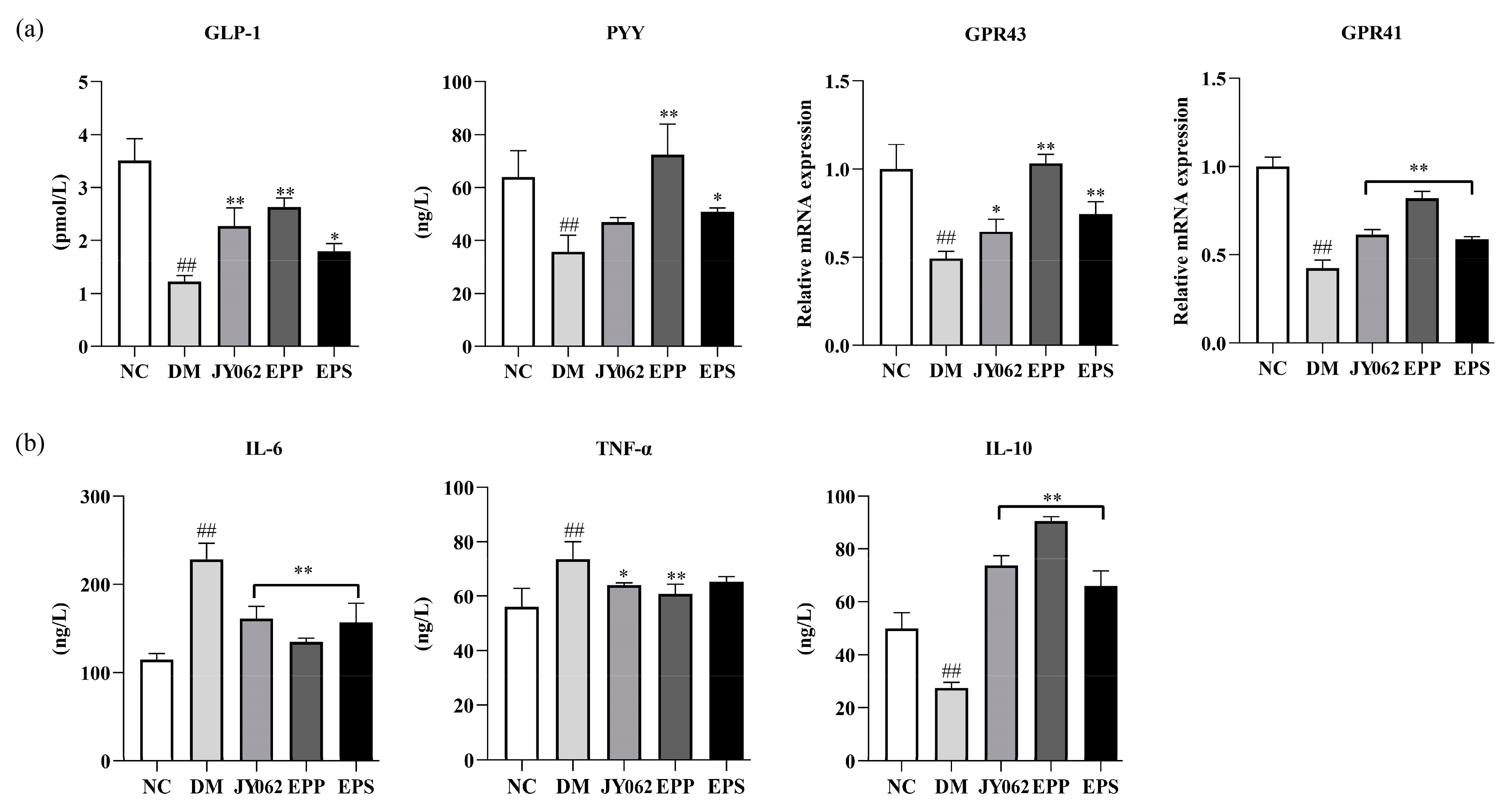
3.8. The Impact of Dietary Intervention on Gastrointestinal Hormones and Inflammatory Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebovitz, H.E.; Banerji, M.A. Ketosis-Prone Diabetes (Flatbush Diabetes): An Emerging Worldwide Clinically Important Entity. Curr. Diabetes Rep. 2018, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Teng, D.; Shi, X.G.; Qin, G.J.; Qin, Y.F.; Quan, H.B.; Shi, B.Y.; Sun, H.; Ba, J.M.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ-Br. Med. J. 2020, 369, m997. [Google Scholar] [CrossRef]
- Zimmet, P.; Shi, Z.; El-Osta, A.; Ji, L. Epidemic T2DM, early development and epigenetics: Implications of the Chinese Famine. Nat. Rev. Endocrinol. 2018, 14, 738–746. [Google Scholar] [CrossRef]
- Demirci, M.; Bahar Tokman, H.; Taner, Z.; Keskin, F.E.; Çağatay, P.; Ozturk Bakar, Y.; Özyazar, M.; Kiraz, N.; Kocazeybek, B.S. Bacteroidetes and Firmicutes levels in gut microbiota and effects of hosts TLR2/TLR4 gene expression levels in adult type 1 diabetes patients in Istanbul, Turkey. J. Diabetes Complicat. 2020, 34, 107449. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Zhang, F.; Lin, Y.; Ma, Y.; Zhao, S.; Chen, C.; Wang, X.; Liu, J. Preventive effect of pressed degreased walnut meal extracts on T2DM rats by regulating glucolipid metabolism and modulating gut bacteria flora. J. Funct. Foods 2020, 64, 103694. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 2021, 39, 708–724.e711. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, M.; Peschard, S.; Lestavel, S.; Staels, B. Intestine-liver crosstalk in Type 2 Diabetes and non-alcoholic fatty liver disease. Metabolism 2021, 123, 154844. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Park, S.Y.; Seo, G.S. Fecal Microbiota Transplantation: Is It Safe? Clin. Endosc. 2021, 54, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.S.; Koefoed, A.K.; Jakobsen, R.R.; Deng, L.; Castro-Mejia, J.L.; Brunse, A.; Neve, H.; Vogensen, F.K.; Nielsen, D.S. Bacteriophage-mediated manipulation of the gut microbiome-promises and presents limitations. FEMS Microbiol. Rev. 2020, 44, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ge, Y.; Du, H.; Li, Q.; Xu, X.; Yi, H.; Wu, X.; Kuang, T.; Fan, G.; Zhang, Y. Berberis kansuensis extract alleviates type 2 diabetes in rats by regulating gut microbiota composition. J. Ethnopharmacol. 2021, 273, 113995. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chien, Y.-W.; Yang, S.-C. The alteration of gut microbiota in newly diagnosed type 2 diabetic patients. Nutrition 2019, 63–64, 51–56. [Google Scholar] [CrossRef]
- Sroka-Oleksiak, A.; Mlodzinska, A.; Bulanda, M.; Salamon, D.; Major, P.; Stanek, M.; Gosiewski, T. Metagenomic Analysis of Duodenal Microbiota Reveals a Potential Biomarker of Dysbiosis in the Course of Obesity and Type 2 Diabetes: A Pilot Study. J. Clin. Med. 2020, 9, 369. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Sailike, J.; Sun, X.; Abuduwaili, N.; Tuoliuhan, H.; Yusufu, M.; Nabi, X.-H. Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol. Res. 2020, 161, 105150. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Neyrinck, A.M.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Azmal Ali, S.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Mining Lactobacillus and Bifidobacterium for organisms with long-term gut colonization potential. Clin. Nutr. 2020, 39, 1315–1323. [Google Scholar] [CrossRef]
- Harimawan, A.; Ting, Y.P. Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtillis and their role in bacterial adhesion. Colloids Surf. B-Biointerfaces 2016, 146, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shao, A.; Feng, S.; Ding, M.; Luo, G. Physicochemical characterization and gastrointestinal adhesion of S-layer proteins-coating liposomes. Int. J. Pharm. 2017, 529, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, C.; Zhou, X.; Qi, R.; Liu, L.; Lv, F.; Li, Z.; Wang, S. Cationic conjugated polymers for enhancing beneficial bacteria adhesion and biofilm formation in gut microbiota. Colloids Surf. B Biointerfaces 2020, 188, 110815. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Mendoza, D.; Kosmerl, E.; Miyagusuku-Cruzado, G.; Giusti, M.M.; Jimenez-Flores, R.; Garcia-Cano, I. Growth of lactic acid bacteria in milk phospholipids enhances their adhesion to Caco-2 cells. J. Dairy Sci. 2020, 103, 7707–7718. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Flórez, A.B. Lactic Acid Bacteria: Lactobacillus plantarum. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 206–217. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Lopez, P.; Capozzi, V.; Fernandez de Palencia, P.; Teresa Duenas, M.; Spano, G.; Fiocco, D. Beta-Glucans Improve Growth, Viability and Colonization of Probiotic Microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef] [Green Version]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm Formation by Lactic Acid Bacteria and Resistance to Environmental Stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef]
- Cai, G.; Wu, D.; Li, X.; Lu, J. Levan from Bacillus amyloliquefaciens JN4 acts as a prebiotic for enhancing the intestinal adhesion capacity of Lactobacillus reuteri JN101. Int. J. Biol. Macromol. 2020, 146, 482–487. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Olesen, S.V.; Prehn, K.; Lahtinen, S.J.; Brix, S.; Abou Hachem, M.; Svensson, B. Mucin- and carbohydrate-stimulated adhesion and subproteome changes of the probiotic bacterium Lactobacillus acidophilus NCFM. J. Proteom. 2017, 163, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Liu, F.; Chen, A.M.; Yang, P.-F.; Peng, Y.; Gong, J.-P.; Li, Z.; Zhong, G.-C. Type 2 diabetes prevention diet and the risk of pancreatic cancer: A large prospective multicenter study. Clin. Nutr. 2021, 40, 5595–5604. [Google Scholar] [CrossRef]
- Koh, J.H.; Kim, N.; Hwang, D.; Lim, Y.-H. Effect of water-soluble fraction of cherry tomatoes on the adhesion of probiotics and Salmonella to intestinal epithelial cells. J. Sci. Food Agric. 2013, 93, 3897–3900. [Google Scholar] [CrossRef]
- Iraporda, C.; Rubel, I.A.; Manrique, G.D.; Abraham, A.G. Influence of inulin rich carbohydrates from Jerusalem artichoke (Helianthus tuberosus L.) tubers on probiotic properties of Lactobacillus strains. Lwt-Food Sci. Technol. 2019, 101, 738–746. [Google Scholar] [CrossRef]
- Kadlec, R.; Jakubec, M. The effect of prebiotics on adherence of probiotics. J. Dairy Sci. 2014, 97, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, P.-B.; Ren, Z.-Q.; Zhou, F.; Hu, H.-H.; Zhang, H.; Xue, K.-K.; Xu, P.; Shao, X.-Q. Changes of serum lipopolysaccharide, inflammatory factors, and cecal microbiota in obese rats with type 2 diabetes induced by Roux-en-Y gastric bypass. Nutrition 2019, 67–68, 110565. [Google Scholar] [CrossRef]
- Joshi, M.B.; Ahamed, R.; Hegde, M.; Nair, A.S.; Ramachandra, L.; Satyamoorthy, K. Glucose induces metabolic reprogramming in neutrophils during type 2 diabetes to form constitutive extracellular traps and decreased responsiveness to lipopolysaccharides. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165940. [Google Scholar] [CrossRef]
- Ying, W.; Lee, Y.S.; Dong, Y.; Seidman, J.S.; Yang, M.; Isaac, R.; Seo, J.B.; Yang, B.-H.; Wollam, J.; Riopel, M.; et al. Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting beta Cell Proliferation and Function in Obesity. Cell Metab. 2019, 29, 457–474.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Shen, X.-H.; Feng, W.-M.; Ye, G.-f.; Qiu, W.; Li, B. Analysis of Inflammatory Mediators in Prediabetes and Newly Diagnosed Type 2 Diabetes Patients. J. Diabetes Res. 2016, 2016, 7965317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, D.T.; Morcos, M.; Samarasekera, T.; Zraika, S.; Hull, R.L.; Kahn, S.E. Islet amyloid formation is an important determinant for inducing islet inflammation in high-fat-fed human IAPP transgenic mice. Diabetologia 2014, 57, 1884–1888. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive Effect and Molecular Mechanism of Lactobacillus rhamnosus JL1 on Food-Borne Obesity in Mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef] [PubMed]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, H.; Jing, Y.; Dong, C. Association between blood microbiome and type 2 diabetes mellitus: A nested case-control study. J. Clin. Lab. Anal. 2019, 33, e22842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Barcena, C.; Valdes-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodriguez, F.; Teresa Fernandez-Garcia, M.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Zhao, C.; Qu, Q.; Yang, F.; Li, Z.; Yang, P.; Han, L.; Shi, X. Monascus ruber fermented Panax ginseng ameliorates lipid metabolism disorders and modulate gut microbiota in rats fed a high-fat diet. J. Ethnopharmacol. 2021, 278, 114300. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Ishaq, S.L.; Zhao, F.-Q.; Wright, A.-D.G. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J. Nutr. Biochem. 2016, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Nobel, Y.R.; Cox, L.M.; Kirigin, F.F.; Bokulich, N.A.; Yamanishi, S.; Teitler, I.; Chung, J.; Sohn, J.; Barber, C.M.; Goldfarb, D.S.; et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015, 6, 7486. [Google Scholar] [CrossRef]
- Wan, F.; Han, H.; Zhong, R.; Wang, M.; Tang, S.; Zhang, S.; Hou, F.; Yi, B.; Zhang, H. Dihydroquercetin supplement alleviates colonic inflammation potentially through improved gut microbiota community in mice. Food Funct. 2021, 12, 11420–11434. [Google Scholar] [CrossRef]
- Inglis, G.D.; Wright, B.D.; Sheppard, S.A.; Abbott, D.W.; Oryschak, M.A.; Montina, T. Expeller-Pressed Canola (Brassica napus) Meal Modulates the Structure and Function of the Cecal Microbiota, and Alters the Metabolome of the Pancreas, Liver, and Breast Muscle of Broiler Chickens. Animals 2021, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Anonye, B.O. Commentary: Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and attenuate High-Fat Diet-Induced metabolic Syndrome. Front. Immunol. 2017, 8, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Groene, H.-J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Lafferty, R.A.; Flatt, P.R.; Irwin, N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides 2018, 100, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Lam, A.; Kanoski, S.E. Hippocampal GLP-1 Receptors Influence Food Intake, Meal Size, and Effort-Based Responding for Food through Volume Transmission. Neuropsychopharmacology 2015, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]

| Group | Treatment (Week 1–12) |
|---|---|
| NC | A normal chow diet |
| DM | High-fat diet (HFD) |
| JY062 | HFD and 0.2 mL of L. paracasei JY062, daily |
| EPP(EPS + JY062) | HFD and 0.2 mL of the mixture (EPS and L. paracasei JY062), daily |
| EPS | HFD and 0.2 mL of EPS solution, daily |
| Genes | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| β-actin | CTACCTCATGAAGATCCTGACC | CACAGCTTCTCTTTGATGTCAC |
| GPR41 | CCACATGCTCATCTTCTTCGTCTG | ACGGACTCTCAGGCTGACATAG |
| GPR43 | CTGTATGGATGATCGCTGCTCTG | CTGCTCTTGGGTGAAGTTCTCGTAG |
| Time (week) | NC (mmol/L) | DM (mmol/L) | JY062 (mmol/L) | EPP (mmol/L) | EPS (mmol/L) |
|---|---|---|---|---|---|
| Week4 | 4.72 ± 0.25 a | 5.80 ± 0.43 b | 4.13 ± 0.40 ac | 3.53 ± 0.19 c | 4.73 ± 0.58 a |
| Week5 | 4.70 ± 0.59 a | 13.27 ± 0.49 b | 9.83 ± 0.26 c | 7.83 ± 0.62 d | 12.10 ± 0.37 e |
| Week6 | 4.53 ± 0.59 a | 14.50 ± 0.88 b | 8.73 ± 0.62 c | 7.20 ± 0.29 d | 11.30 ± 0.37 e |
| Week7 | 4.97 ± 0.45 a | 15.13 ± 0.79 b | 8.20 ± 0.78 c | 6.97 ± 0.54 c | 10.10 ± 0.62 d |
| Week8 | 5.53 ± 0.31 a | 15.80 ± 0.43 b | 7.93 ± 0.48 c | 6.23 ± 0.45 a | 10.80 ± 0.37 d |
| Week9 | 4.77 ± 0.39 a | 16.83 ± 0.29 b | 7.63 ± 0.40 c | 6.57 ± 0.53 d | 9.67 ± 0.65 e |
| Week10 | 4.67 ± 0.33 a | 16.33 ± 0.42 b | 7.93 ± 0.53 c | 6.70 ± 0.42 d | 9.70 ± 0.49 e |
| Week11 | 4.87 ± 0.31 a | 16.67 ± 0.31 b | 7.80 ± 0.22 c | 6.93 ± 0.56 c | 9.60 ± 0.51 d |
| Week12 | 5.70 ± 0.41 a | 16.80 ± 0.75 b | 7.87 ± 0.12 c | 6.30 ± 0.45 a | 9.13 ± 0.59 d |
| Group | FFA (mmol/L) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | Fasting Insulin (mIU/L) |
|---|---|---|---|---|---|---|
| NC | 0.19 ± 0.03 a | 3.94 ± 0.25 a | 1.58 ± 0.07 a | 6.44 ± 0.76 a | 0.26 ± 0.14 a | 8.3 ± 1.1 a |
| DM | 0.52 ± 0.02 b | 6.13 ± 0.31 b | 2.89 ± 0.17 b | 3.55 ± 0.76 b | 1.18 ± 0.12 b | 17.0 ± 1.5 b |
| JY062 | 0.30 ± 0.03 c | 4.66 ± 0.40 ac | 0.97 ± 0.20 c | 4.00 ± 0.33 b | 0.64 ± 0.10 d | 10.5 ± 0.9 ac |
| EPP | 0.21 ± 0.02 a | 4.42 ± 0.32 ac | 0.69 ± 0.10 c | 6.11 ± 0.40 a | 0.39 ± 0.04 ac | 9.1 ± 1.3 a |
| EPS | 0.35 ± 0.02 d | 4.80 ± 0.44 c | 1.07 ± 0.30 c | 4.79 ± 0.17 b | 0.59 ± 0.05 cd | 12.2 ± 1.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, L.; Cheng, S.; Zhang, Y.; Yang, M.; Fang, R.; Li, H.; Man, C.; Jiang, Y. A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. https://doi.org/10.3390/nu14020377
Zhao J, Wang L, Cheng S, Zhang Y, Yang M, Fang R, Li H, Man C, Jiang Y. A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039. Nutrients. 2022; 14(2):377. https://doi.org/10.3390/nu14020377
Chicago/Turabian StyleZhao, Jiayuan, Lihan Wang, Shasha Cheng, Yu Zhang, Mo Yang, Ruxue Fang, Hongxuan Li, Chaoxin Man, and Yujun Jiang. 2022. "A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039" Nutrients 14, no. 2: 377. https://doi.org/10.3390/nu14020377
APA StyleZhao, J., Wang, L., Cheng, S., Zhang, Y., Yang, M., Fang, R., Li, H., Man, C., & Jiang, Y. (2022). A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039. Nutrients, 14(2), 377. https://doi.org/10.3390/nu14020377