Diet and Dementia: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Dietary Intakes
2.3. Sociodemographic and Lifestyle Measurements as Covariates
2.4. Psychological Data Analyses
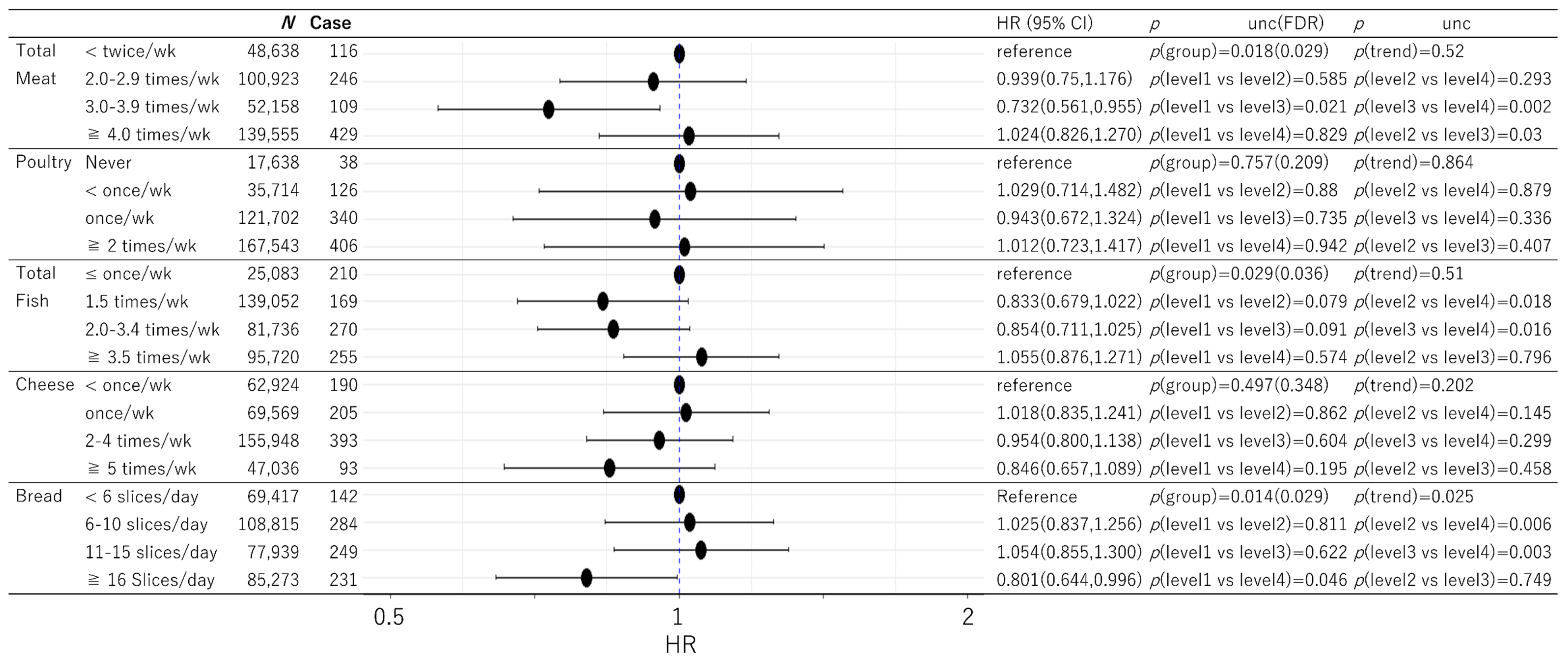
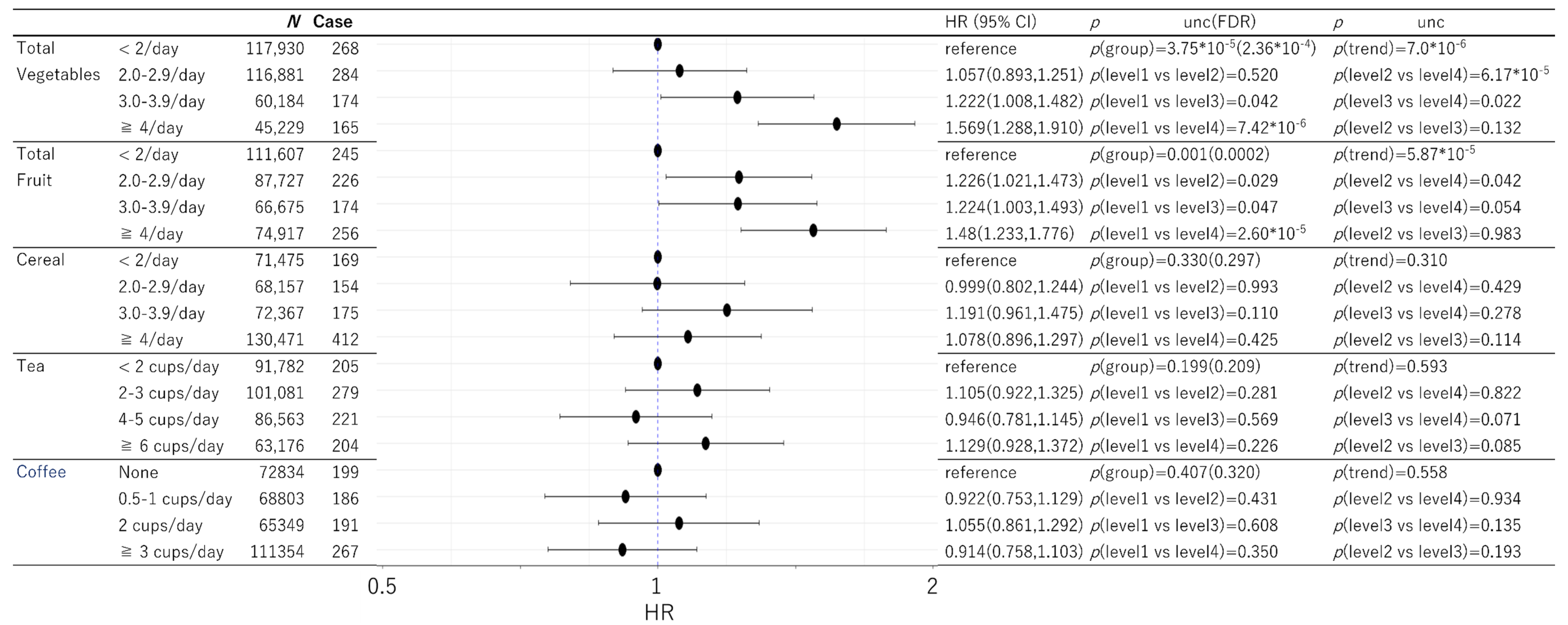
3. Results
3.1. Basic Baseline Data
3.2. Prospective Analysis of Dementia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Hou, J.; Mao, P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Qizilbash, N.; Gregson, J.; Johnson, M.E.; Pearce, N.; Douglas, I.; Wing, K.; Evans, S.J.; Pocock, S.J. BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 431–436. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, C.; Yang, H.; Li, S.; Xu, F.; Zhang, Y.; Wang, Y. Associations of BMI and serum urate with developing dementia: A prospective cohort study. J. Clin. Endocrinol. Metab. 2020, 105, e4688–e4698. [Google Scholar] [CrossRef] [PubMed]
- Albanese, E.; Launer, L.J.; Egger, M.; Prince, M.J.; Giannakopoulos, P.; Wolters, F.J.; Egan, K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 165–178. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Irish, M.; Kam, J.; Van Keizerswaard, J.; Bartley, L.; Samaras, K.; Hodges, J.R.; Piguet, O. Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurol. 2014, 71, 1540–1546. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.M.; Irish, M.; Piguet, O.; Halliday, G.M.; Ittner, L.M.; Farooqi, S.; Hodges, J.R.; Kiernan, M.C. Amyotrophic lateral sclerosis and frontotemporal dementia: Distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol. 2016, 15, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuźma, E.; Llewellyn, D.J. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Pike, N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouvari, M.; Tyrovolas, S.; Panagiotakos, D.B. Red meat consumption and healthy ageing: A review. Maturitas 2016, 84, 17–24. [Google Scholar] [CrossRef]
- Hardman, R.J.; Kennedy, G.; Macpherson, H.; Scholey, A.B.; Pipingas, A. Adherence to a Mediterranean-style diet and effects on cognition in adults: A qualitative evaluation and systematic review of longitudinal and prospective trials. Front. Nutr. 2016, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O’Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimer’s Dis. 2012, 32, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Huang, Y.; Cheng, H. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J. Nutr. Health Aging 2012, 16, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Bakre, A.T.; Chen, R.; Khutan, R.; Wei, L.; Smith, T.; Qin, G.; Danat, I.M.; Zhou, W.; Schofield, P.; Clifford, A. Association between fish consumption and risk of dementia: A new study from China and a systematic literature review and meta-analysis. Public Health Nutr. 2018, 21, 1921–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Brockman, J.; Schneider, J.A.; Wang, Y.; Bennett, D.A.; Tangney, C.C.; van de Rest, O. Association of seafood consumption, brain mercury level, and APOE ε4 status with brain neuropathology in older adults. JAMA 2016, 315, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Item | No. (%) | Mean (SD) | Range |
|---|---|---|---|
| Sex | |||
| Female | 273,382 (54.4) | ||
| Male | 229,122 (45.6) | ||
| Missing | 1 | ||
| Age, years | 56.5 (8.0) | 37–73 | |
| Missing | 1 (0.0) | ||
| BMI | 27.4 (4.8) | 12–75 | |
| Underweight (18.5 ≥ BMI) | 2626 (0.5) | ||
| Normal (25 ≥ BMI > 18.5) | 162,523 (32.3) | ||
| Overweight (30 ≥ BMI > 25) | 212,097 (42.2) | ||
| Obesity (30 > BMI) | 122,153 (24.3) | ||
| Missing | 3107 (0.6) | ||
| Average total household income before tax | |||
| < £18,000 | 97,198 (19.3) | ||
| £18,000 to £30,999 | 108,177 (21.5) | ||
| £31,000 to £51,999 | 110,772 (22.0) | ||
| £52,000 to £100,000 | 86,266 (17.2) | ||
| >£100,000 | 22,929 (4.6) | ||
| Missing | 77,164 (15.4) | ||
| Townsend index of material deprivation | −1.3 (3.1) | −6.25–11.00 | |
| Missing | 624 (0.1) | ||
| Employment status | |||
| In paid employment or self-employed | 287,149 (57.1) | ||
| Not in paid employment or self-employed | 212,404 (42.3) | ||
| Missing | 2952 (0.6) | ||
| Highest education qualification (years) | 13.95 (5.1) | 7–20 | |
| Missing | 10,113 (2.0) |
| Estimated Fibre Content/Portion (g) [14] | p Value | HR (95% CI) | |
|---|---|---|---|
| Main type of bread | |||
| white | 0.68 | p(group difference) = 0.596 | reference |
| brown | 1.26 | p(type1 vs. type2) = 0.473 | 1.082(0.873,1.341) |
| wholemeal or wholegrain | 1.8 | p(type1 vs. type3) = 0.491 | 0.946(0.807,1.109) |
| other type | 1.25 | p(type1 vs. type4) = 0.751 | 0.943(0.658,1.353) |
| Main type of cereal | |||
| Bran cereal | 7.16 | p(group difference) = 0.928 | reference |
| Biscuit cereal | 2.92 | p(type1 vs. type2) = 0.945 | 1.008(0.792,1.285) |
| Oat cereal | 1.92 | p(type1 vs. type3) = 0.980 | 0.997(0.797,1.247) |
| Muesli | 4.18 | p(type1 vs. type4) = 0.637 | 1.060(0.832,1.351) |
| Other (e.g., cornflakes, frosties) | 0.54 | p(type1 vs. type5) = 0.663 | 0.947(0.741,1.210) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, H.; Kawashima, R. Diet and Dementia: A Prospective Study. Nutrients 2021, 13, 4500. https://doi.org/10.3390/nu13124500
Takeuchi H, Kawashima R. Diet and Dementia: A Prospective Study. Nutrients. 2021; 13(12):4500. https://doi.org/10.3390/nu13124500
Chicago/Turabian StyleTakeuchi, Hikaru, and Ryuta Kawashima. 2021. "Diet and Dementia: A Prospective Study" Nutrients 13, no. 12: 4500. https://doi.org/10.3390/nu13124500
APA StyleTakeuchi, H., & Kawashima, R. (2021). Diet and Dementia: A Prospective Study. Nutrients, 13(12), 4500. https://doi.org/10.3390/nu13124500






