Abstract
Children with type 1 diabetes (T1D) are at increased risk of celiac disease (CD). The replacement of insulin in T1D, and the exclusion of gluten in CD, are lifelong, burdensome treatments. Compliance to a gluten-free diet (GFD) in children with CD is reported to be high, while compliance in children with both diseases has scarcely been studied. To examine compliance to a GFD in children with both T1D and CD, we analyzed tissue transglutaminase IgA-antibodies (tTGA). Moreover, associations between compliance and age, sex, glycemic control, ketoacidosis (DKA), body mass index (BMI), and time of CD diagnosis were investigated. Of the 743 children diagnosed with T1D in southern Sweden between 2005 and 2012, 9% were also diagnosed with CD. Of these, 68% showed good compliance to a GFD, 18% showed intermediate compliance, and 14% were classified as non-compliant. Higher age, poorer HbA1c, and more DKAs were significantly (p < 0.05) associated with poorer compliance. In conclusion, we found that compliance to a GFD in children with T1D and CD is likely be lower than in children with CD only. Our results indicate that children with both T1D and CD could need intensified dietary support and that older children and children with poor metabolic control are especially vulnerable subgroups.
1. Introduction
Children with type 1 diabetes (T1D) have an increased risk of being diagnosed with celiac disease (CD) compared to the general population [1]. The estimated prevalence of CD in children is approximately 1% [2], while the prevalence of CD in children with T1D ranges between 2.4 and 16.3% [3], reflecting national and international differences in CD diagnostics and CD risk [1]. The prevalence of CD in Swedish children with T1D is around 10% [4,5]. T1D and CD are chronic diseases that share genetic risk factors such as HLA DQ2 and DQ8 [6]. The incidence of both diseases has increased during the last few decades [7,8,9], pointing towards possible environmental risk factors [10].
The necessary replacement of insulin in T1D and the total exclusion of dietary gluten in CD are lifelong, burdensome treatments, and the double burden for children with both diseases is reported to be difficult [11,12]. A diet with a low glycemic index is recommended for patients with T1D. However, GFD foods often have a high glycemic index [13,14] and, accordingly, children with CD have a higher dietary glycemic index in their diet [15,16]. For children with both T1D and CD, it may be difficult to find GFD foods that are low in sugar and high in fiber to keep a good metabolic control [11,17]. Even without T1D, a GFD can be hard to strictly follow. In one study, accidental gluten intake was observed in 86% of children with CD that aimed to keep a strict GFD [18].
In patients with untreated CD, the intestinal mucosa is damaged by an immunogenic reaction to gliadin. This reaction is measured by autoantibodies (tissue transglutaminase IgA antibodies (tTGA), endomysial antibodies (EMA), or deamidated gluten peptides (DGP)). A strict GFD usually resolves enteropathy and levels of tTGA are shown to be closely related to the degree of intestinal mucosa damage [19,20]. Thus, significant increases in tTGA levels can be used to detect a rise in CD activity [21]. The time needed to detect a healed mucosa through tTGA levels can vary, but for most GFD compliant CD patients, tTGA normalizes in around a year [22]. Interestingly, children with both CD and T1D on a GFD are reported to have a longer tTGA normalization period, ranging between 1 and 2 years or even longer [22,23].
In some children, CD is discovered because of typical symptoms like abdominal pain, failure to thrive, or diarrhea, but a large proportion seem to lack typical symptoms [24]. A Swedish screening study for CD in 12-year-olds from the general population showed that two-thirds of children with CD had an undiagnosed disease [25]. Likewise, in Swedish children with T1D, CD is often asymptomatic [5]. This is shown in another Swedish study on children with T1D that found 0.7% to be diagnosed with CD prior to T1D diagnosis, while 3% had a silent, screening-detected CD at T1D diagnosis, and annual screenings then led to a cumulative CD incidence that reached 10% after five years [4].
Around 90% of Swedish children with CD, including screening-detected cases, comply to a GFD [18,26], but there are few studies on compliance to a GFD in children with additional T1D. Most studies are small (including 9 to 35 children) and with somewhat conflicting results. The majority of these studies reported compliance to be relatively high, 63–100% [17,23,27,28,29], but two studies reported compliance to be only 30–36% [30,31]. In most studies, compliance was associated with better glycemic control [28,29] and/or growth [29,31]; only one found no association between compliance and growth [30]. We found one larger registry study on 608 double-diagnosed children which also reported good compliance to be associated with both better glycemic control and improved growth, but this study only found a good dietary adherence in 36% of the children [32]. Moreover a randomized controlled study comparing 15 double-diagnosed children on a GFD to 15 double-diagnosed children on a normal diet found children on a GFD to have better glycemic control after 12 months [33].
Because of the limited number of studies and small sample sizes, more knowledge on compliance to a GFD in children with T1D and CD is needed. Inconsistent results on whether or not compliance is associated with glycemic control and growth also show the need for more research. In this study, we examined compliance to a GFD in all children with T1D and CD from southern Sweden. All participants were screened for CD at T1D diagnosis and then screened annually for up to 10 years. Associations between compliance and a range of sociodemographic and clinical factors were examined.
2. Materials and Methods
2.1. Participants and Setting
This study was approved by the ethical committee in Lund in 2014 (Dnr:2014/476) with a complementary approval in 2020 (Dnr: 2020-04152). We collected data from medical records for all children, aged 0–18, in Skåne county, Sweden, diagnosed with T1D between 2005–2012. The diagnostic criteria used for T1D patients in Sweden subscribe to the diabetes classification of the American Diabetes Association [34], whereas all included children with a CD diagnosis were diagnosed by confirmed small intestinal biopsy. In general, all children diagnosed with T1D were screened for CD at T1D diagnosis and in most cases annually thereafter. A CD diagnosis either pre- or post-T1D diagnosis was an inclusion criterion for the study.
Between 2005 and 2012, 743 children in Skåne county were diagnosed with T1D. Of these, 64 (9%) had CD proven by a biopsy. The follow-up time ranged from 1 to 10 years. Information on date of birth, sex, and age at both T1D and CD diagnosis were collected from the medical records. Longitudinal data for HbA1c and BMI were collected from the national Swedish quality register, SWEDIABKIDS, complemented by data from medical journals at the included hospitals in Skåne. Information about the CD diagnoses was collected from the diagnostic registry by Statistics Sweden and confirmed by the medical journals of the children. Levels of antibodies (tTGA and EMA) were collected from medical journals at the included hospitals.
2.2. Compliance
2.2.1. tTGA
We used tTGA, a recommended serological measure for analyzing adherence to a GFD, for assessing compliance to a GFD [21]. tTGA was measured through blood samples and analyzed in a EliA Celikey IgA system from Thermo Fisher Scientific (Legal Manufacturer: Phadia AB, Uppsala, Sweden). An outcome equal to 10 U/mL or above was classified as positive [35]. However, EMA was still in use at the very beginning of the study and a few measurements were reported in EMA instead of tTGA. In accordance with a study that screened for CD in the general population using and evaluating both EMA and tTGA [36], as well as regional laboratory guidelines [37], a cut-off of <10 (1/10) was used for EMA as well.
2.2.2. Classifying Compliance
To evaluate adherence to a GFD, all patients with ≥3 serological results more than two years after CD diagnosis were divided into four compliance categories.
- Insufficient data (<2 serological results more than two years after CD diagnosis).
- Good compliance (all tTGA values < 10).
- Intermediate/varying compliance (≥1 tTGA value being positive and ≥1 tTGA value being negative).
- Non-compliance (all reported tTGA values ≥ 10).
A two-year period after the CD diagnosis was used because of prior reports that the antibody normalization period for children with T1D is longer [22,23].
All patients with ≥5 measurements that had just one deviating value (either positive or negative) were classified in accordance with the majority of values.
2.3. Metabolic Control and Growth
2.3.1. HbA1c and DKA
To evaluate metabolic control, we used glycemic control and ketoacidosis (DKA). To evaluate glycemic control, we used glycated hemoglobin (HbA1c) in blood, a measure of the blood glucose levels of the 2–3 preceding months. HbA1c was analyzed using the Capillarys 3 TERA Hemoglobin A1c Kit-program, accredited and used at Skanes uUniversity hospital, analysed at Labmedicin, “Laboratorium”, 105 02 Malmö. where separation is on the capillaries. Separation is optimized to eliminate interferences from variants of hemoglobin, pre-HbA1c and carbamylated hemoglobin. The instrument analyzes 68 samples per hour. Detection is at 415 nm.
DKA was defined as pH below 7.30 and we compared those that reexperienced DKAs with those that did not.
2.3.2. Growth
To evaluate growth, we used the standard deviation score of body mass index (SDS-BMI) [38].
2.4. Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics version 25. To analyze the degree of compliance, we estimated the proportion of participants being classified into each compliance group (good, intermediate, non-compliant). To examine associations between compliance and metabolic control, growth, age, sex, DKA, and whether CD was diagnosed prior to or after T1D, we conducted a line of univariable ordinal regression models with compliance (good, intermediate, non-compliance) being the dependent variable and each of the above variables being entered as independent variables. Ordinal regression allowed us to respect the ordinal nature of the three compliance groups. An alpha level of 0.05 was used as an indicator of statistical significance. All variables that showed a statistically significant association with compliance were entered into a final ordinal regression model. In this model, we made an a priori decision to include age and sex as independent variables, as these were deemed to be possible confounders. Compliance was entered as the dependent variable in the analyses as we could not derive clear causal patterns from the data.
3. Results
3.1. Participant Characteristics and Degree of Compliance
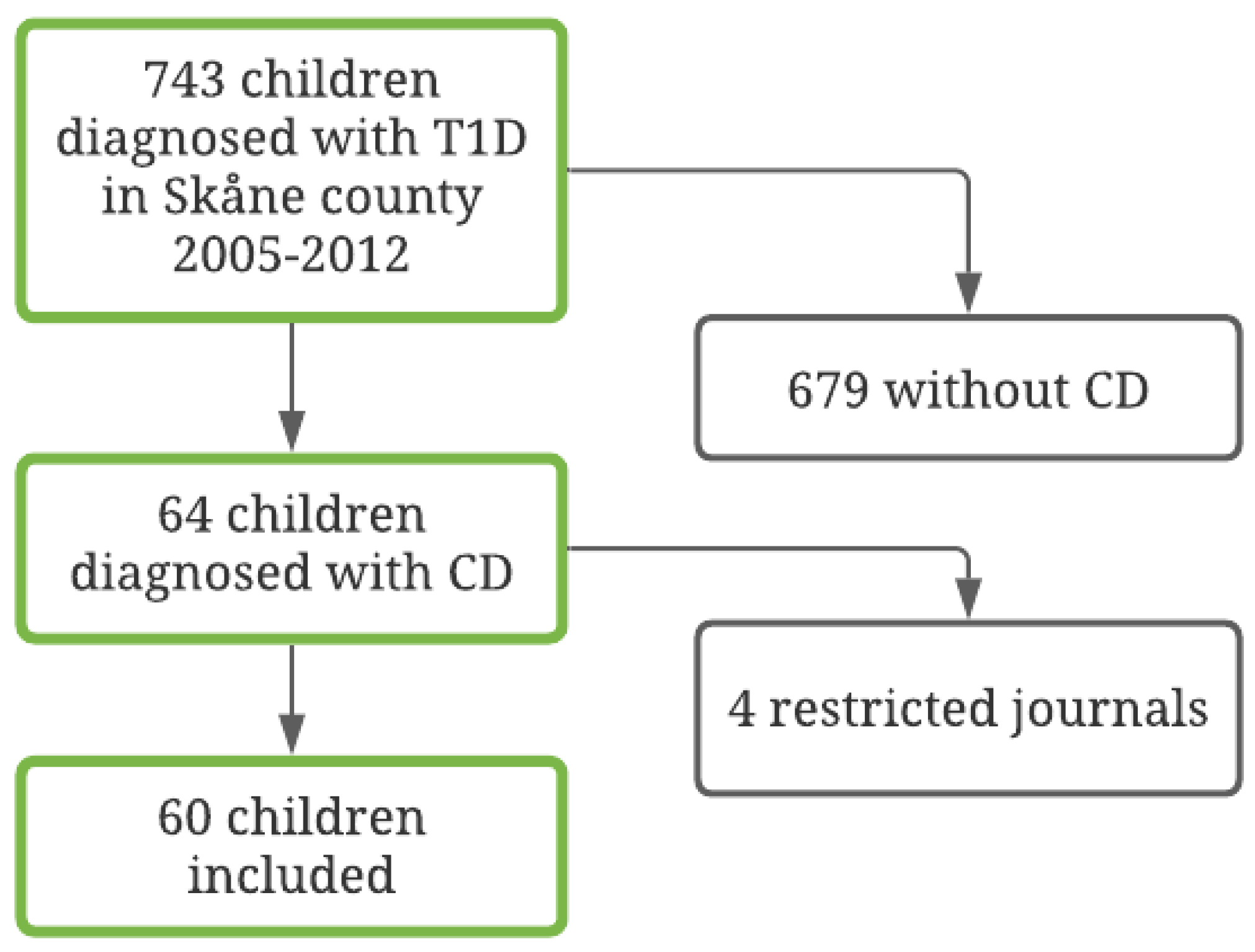
Of the 64 children that met the inclusion criteria, information on study variables could be retrieved for 60 children (94%). See Figure 1 for further information regarding patient inclusion. Medical records about HbA1c, BMI, and DKA were not available for the four patients that were excluded. Thus, a sample of 60 patients with T1D and CD was identified. Of the children, 32 (53%) were female and 28 (47%) were male. The mean age at the T1D diagnosis was 8.87 years (SD = 4.50; range = 1–17 years). Of these, 19 (32%) were diagnosed with CD prior to T1D and the rest had it detected at or after the T1D diagnosis. See Table 1 for full information on the demographic and clinical variables in the full sample and in the four compliance subsamples.
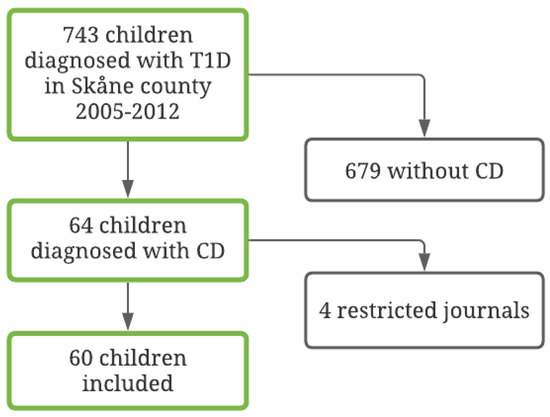
Figure 1.
Flow chart of the included diabetes population, 2005–2012.

Table 1.
Demographic and clinical variables in the full sample and across compliance subsamples.
Following the criteria used to classify degree of compliance, 10 patients (17%) had insufficient data due to too few follow-ups or because not enough time had elapsed after the CD diagnosis, resulting in 50 patients being included in the final analyses regarding compliance. The majority, 34 patients (68%), were classified as having good compliance, 9 patients (18%) as having intermediate compliance, and 7 patients (14%) as being non-compliant. Forty-four patients (73%) had tTGA values on at least five follow-up occasions. Thirty patients normalized their antibody levels: 23 (77%) within 1 year and another 6 (23%) within the second year.
3.2. Associations between Clinical/Demographic Variables and Compliance
We found no difference in compliance between those that received a CD diagnosis prior to T1D compared to those who received their CD diagnosis at or after T1D diagnosis (p = 0.91).
3.2.1. Compliance and HbA1c
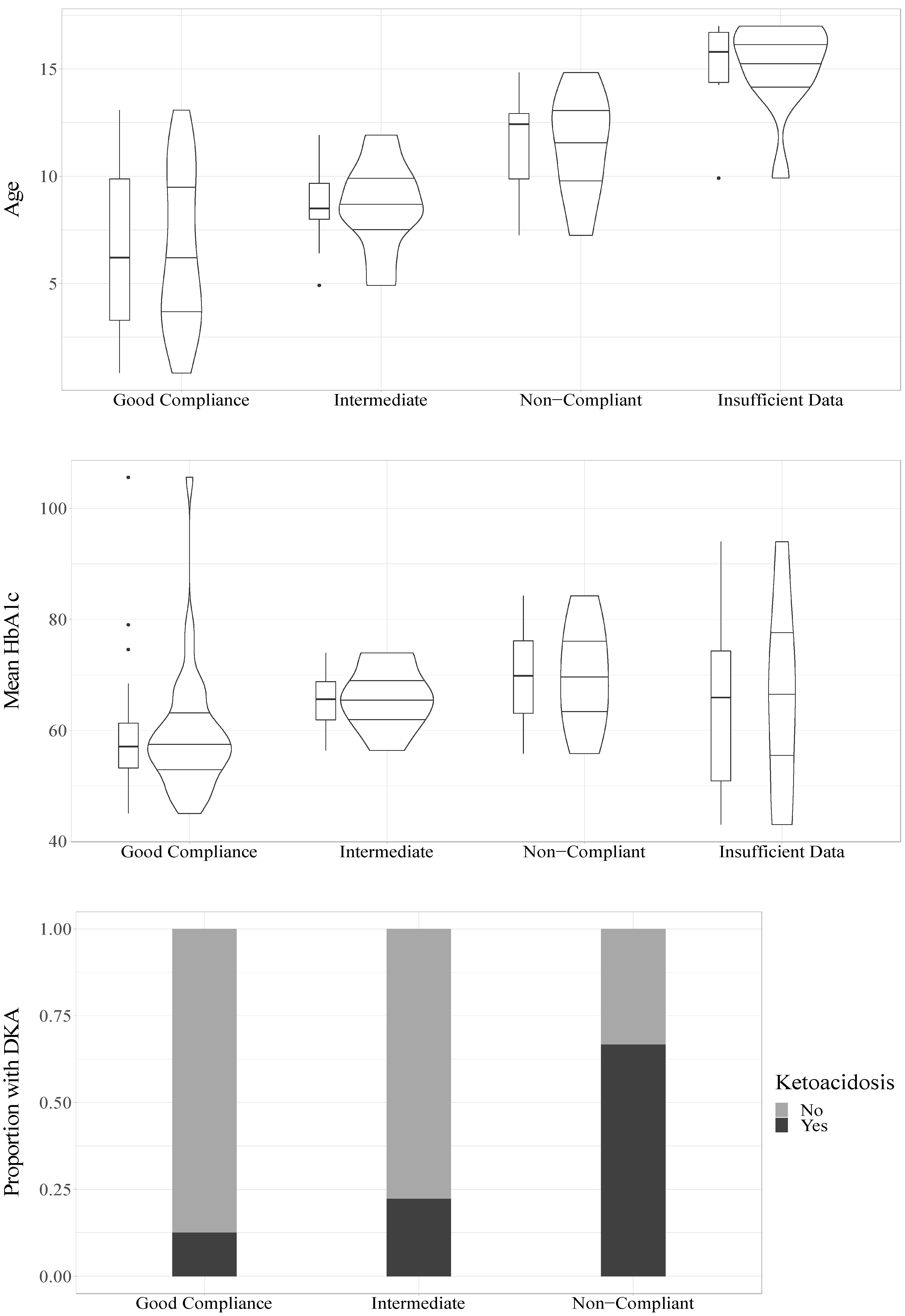
The mean HbA1c value during the follow-up study period had a significant association with compliance (OR = 1.09, CI95% = 1.02–1.16, p = 0.008) such that higher mean HbA1c values increased the odds of being classified as having poorer compliance, see Figure 2.
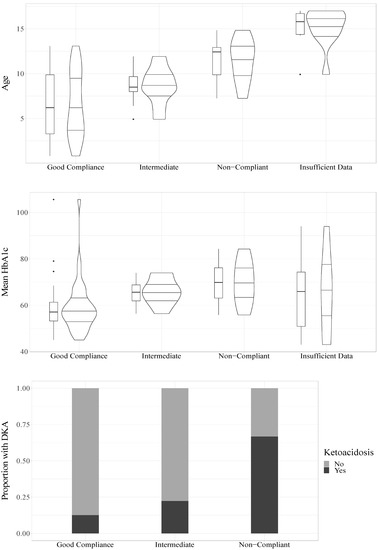
Figure 2.
Relations of age, HbA1c and DKA to compliance.
3.2.2. Compliance and Age
In a univariable model, age was significantly associated with compliance (OR = 1.41, CI95% = 1.14–1.75, p = 0.002) such that older age was associated with increased odds of being classified as having poorer compliance, see Figure 2. In a univariable model, sex was not statistically significantly associated with compliance (OR = 3.57, CI95% = 0.96–13.22, p = 0.057), but it trended towards significance with girls showing increased odds of being classified as having poorer compliance.
3.2.3. Compliance and BMI
Median SDS-BMI for the whole group was below −0.5 at baseline and changed to +0.4 at year one and remained positive (0.5–0.7) throughout the ten-year follow-up period (see Table 1). Mean SDS-BMI was not significantly associated with compliance (p = 0.36) but there was a trend towards a higher SDS-BMI with higher compliance.
3.2.4. Compliance and DKA
To analyze whether there was an association between DKA and compliance, we divided participants into those who experienced at least one DKA during the follow-up period (n = 10) and those who did not (n = 37). For three participants with a compliance classification, no information about DKA was available. The univariable ordinal regression indicated a significant association between DKA and compliance (OR = 6.22, CI95% = 1.53–25.33, p = 0.011, n = 47), indicating that those who had a DKA had increased odds of being classified as having poorer compliance, see Figure 1. DKA was also significantly associated with poorer compliance (OR = 7.39, CI95% = 1.44–37.83, p = 0.016, n = 47) when accounting for age and sex.
3.2.5. Full Model
In line with our statistical plan, we conducted a model that included age, sex, and HbA1c as independent variables and compliance as the dependent variable. Results showed that age and HbA1c were significantly associated with compliance, and the results fully aligned with those reported above: age (OR = 1.36, CI95% = 1.07–1.73, p = 0.011); sex (OR = 4.08, CI95% = 0.85–19.73, p = 0.080); and HbA1c (OR = 1.10, CI95% = 1.02–1.17, p = 0.011). The pseudo R2 (Nagelkerke) of this model was 0.420, indicating that 42% of the variance in compliance could be explained by variance in the independent variables. Lastly, we added DKA to the above model. DKA was not entered in the previous model because of missing data for three participants. In this model (n = 47), only age (OR = 1.48, CI95% = 1.13–1.93, p = 0.004) was statistically significantly associated with compliance, while HbA1c (p = 0.60), DKA (p = 0.06), and sex (p = 0.11) were not. The model explained 46% (Nagelkerke) of the variance in compliance.
To further examine the associations between DKA, HbA1c, compliance, and whether collinearity problems were present (i.e., that two independent variables were highly associated), we ran an independent samples t-test which showed that there was a clear and statistically significant association between having experienced a DKA and having poorer mean HbA1c (DKA, M = 72.71 (SD = 12.72); no DKA, M = 58.94 (SD = 7.90); t(45) = 4.26, p < 0.001). Thus, both HbA1c and DKA were significantly associated with compliance when accounting for age and sex, while neither HbA1c nor DKA were significantly associated with compliance when they were included in the same model, which indicates that HbA1c and DKA are associated and may suppress each other when they are entered in tandem in a statistical model.
4. Discussion
In this study, we found compliance to a GFD in children with both CD and T1D to be at 68%, which is considerably lower than the 85–96% that has been found in large samples of Swedish children with CD only [18,26] We also found poor compliance to be associated with being older, having poorer glycemic control, and a higher probability of experiencing DKA. Whether CD diagnosis was present before T1D or detected at or after T1D diagnosis was not associated with the level of compliance.
Our compliance rate (68% good compliance) is similar but somewhat lower compared to estimates from other studies in children with T1D and CD, where compliance, assessed by antibodies, dieticians’ assessment, and/or questionnaires, was found to be around 80% [17,23,27,28,29]. Although smaller (between 9 and 35 children) and with fewer longitudinal follow-up assessments, these studies, conducted between 2002–2016, provide estimates in line with ours. Taken together, this indicates that a majority of children with T1D and CD show a fairly good compliance to a GFD.
In contrast, two small studies with 9 and 20 children [30,31], conducted in 2008 and 1999, respectively, and one large register study with 608 children [32], conducted in 2019, found substantially lower compliance: between 30–44%. Reasons for these differences are unclear but could be explained by older data, different methods to establish compliance, and representativity of the analyzed samples. In 1995, GFD foods were less available and less variable, making it harder to follow a GFD. Two of the studies were old and also quite small. In the larger and newer study, data collection started as early as 1995. Also, a multicenter design where antibody testing was performed by different laboratories with different types of tTGA tests was used, which makes a direct comparison difficult [21]. Another limitation with the registry study is that the majority (62.7%) of the registered children with T1D were excluded. We also speculate that screening to detect CD in these T1D populations might have been scarce, since only 1.36% of the children with T1D in their material were found to have a double diagnosis, leaving a less representative sample.
To understand whether the presence of T1D in addition to CD affects compliance, it is important to compare the compliance of children with a double diagnosis to the compliance of children with CD only. In Sweden, all patients with CD—symptomatic or asymptomatic—are asked to keep a strict GFD. A Swedish study from 2014 showed that 96.8% of children with CD adhered to a strict GFD [18] and another Swedish study from 2015 showed that normalized tTGA levels were present in 85% after one year [26], although the true compliance rate was probably higher since one year may be a short follow-up time for complete tTGA normalization [26]. Thus, although children with T1D and CD in our study and many others show fairly good compliance, compliance seems to be lower than in children with CD only. This is supported by a Canadian study from 2017 on 487 pediatric patients diagnosed with CD, which found compliance to a GFD to be significantly different in patients with T1D: 77% compared to 89% in patients without T1D. Additionally they found a significantly longer time to tTGA normalization in CD children with T1D: 1204 days compared to 403 days in patients without T1D [22]. In our study, 77% of the children that normalized their tTGA levels reached normalization within one year, while 23% needed two years. Thus, if normalized tTGA levels one year after the CD diagnosis had been required to be classified as having good compliance, compliance levels would have been markedly lower. That many children with T1D and CD seem to need more than one year to normalize tTGA is supported by previous research [22,39] and may be important to consider when examining compliance to a GFD in children with T1D. Whether this difference depends on the burden of management and control of two heavy treatments, or on the physiological ability to neutralize tTGA antibodies in children with T1D, remains to be answered.
Our results with a lower compliance in children with T1D and CD compared to children with CD only is important as it indicates that children with both diseases could need intensified support. This support could, for example, be more frequent visits with a dietician who specializes in the specific challenges that comes with monitoring both T1D and CD simultaneously. These results may also suggest children with both T1D and CD as a group suitable for trying promising new treatments like transglutaminase 2 inhibitors [40].
In our study worse glycemic control and the risk of experiencing DKAs was associated with poorer compliance. That better compliance is associated with a better glycemic control is in line with previous studies [28,29,32], and the only, to our knowledge, randomized controlled study (RCT) on the topic to date also found that a GFD resulted in better glycemic control [33]. In contrast with our results, the only other study on DKAs we found described no association between compliance and DKA [32].
The associations between poorer compliance and poorer glycemic control and more DKAs could be related to a possibility of active CD negatively influencing metabolic control [41]. Another, and perhaps more plausible explanation, is that individual differences in overall compliance to healthcare treatments and advice may explain these associations. Children who tend not to adhere to a GFD perhaps are less likely to adhere to their T1D regimen, resulting in both poorer compliance to a GFD, poorer metabolic control, and more DKAs.
We also found higher age to be associated with poorer compliance which has been reported previously [32]. Several studies have reported older children with T1D to have worse metabolic control than younger children [42,43]. This makes age a tempting explanation for the association of worse metabolic control to poor compliance. However, we found both age and metabolic control to be independently associated with compliance, indicating that age alone does not explain the link between compliance and glycemic control.
We did not find any significant association between compliance and BMI, although there was a trend towards higher BMI being associated with better compliance. The only other study on BMI and GFD compliance in T1D patients [31] reported the same trend. Other studies [23,27,44] have reported an increased growth velocity in children after being diagnosed with CD and introduced to a GFD, but without a clear association with compliance.
A major contribution of the present study is the representative sample, in which all children with T1D in our region were screened for CD at T1D diagnosis and followed up with up to ten years after T1D diagnosis, but several limitations also merit mentioning. First, there are no clear recommendations from the Food and Drug Administration (FDA) on how to best measure compliance, and previous studies have used different methods. Outcome measures could be based on histology, different serology, and clinical outcome assessment (including patient-reported outcomes) All these methods have advantages and shortcomings [21]. Newer measures, such as immunological tools [45] and gluten immunogenic peptides [46,47], have been reported to show a higher reliability. It would have been an advantage to also measure GIP in our study since it has been shown to be more reliable than tTGA in monitoring response to a GFD [46]. Unfortunately, this method was not yet established in clinical practice during the timespan of our study. tTGA is an indirect measure and has been shown to correlate well with mucosal damage of the intestine [19,20,48] and with the severity of the disease [49]. However, a recent meta-analysis found that tTGA often underestimates the degree of mucosal damage [50] and that normalized, complete histological recovery may not be obtained [51]. Another problem with tTGA is a partly individual response. Different patients digesting the same amount of gluten produce different amounts of tTGA [52], and at different speeds [53]. A small group of patients even became immune to gluten after longer GFD treatment and then did not produce tTGA at all [54]. Nevertheless, tTGA is recommended as a key outcome measure by the Tampere recommendations in 2018 [21]. Questionnaires assessing self-reported compliance in combination with tTGA are used by some and could have benefited our study. Another limitation is the absence of an age- and sex-matched group of children with CD only, which would have strengthened the comparison of compliance to a GFD in T1D children with CD compared to children with CD only. However, we have good estimates on compliance to a GFD from screened children with CD in Sweden [18,26], making our indirect comparisons valid.
5. Conclusions
We report a fairly high compliance to a GFD in Swedish children with T1D and CD, although compliance levels may be somewhat lower than in children with CD only. Moreover, we found older age and poorer metabolic control to be associated with poorer compliance. Our results indicate that children diagnosed with both T1D and CD could need intensified dietary support, especially the subgroups with older children and children with poorer metabolic control.
Author Contributions
Conceptualization, H.S. and A.C.; methodology, H.S., M.C. and A.C.; formal analysis, J.R. and M.C.; investigation, J.R.; resources, A.C.; data curation, C.A.; writing—original draft preparation, H.S.; writing—review and editing, A.C., M.C. and M.C.B.; visualization, H.S. and A.C.; supervision, A.C.; project administration, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from FoU, funding number 20180612 The local Research and Development Council of Region Skåne.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Committee in Lund in 2014 (Dnr:2014/476) with a complementary approval in 2020 (Dnr: 2020-04152).
Informed Consent Statement
Patient consent was waived due to deidentified data.
Data Availability Statement
Data can be found in SWEDIABKIDS (https://ndr.nu, accessed last on 31 December 2020) and The National Patient Register—Socialstyrelsen (https://www.socialstyrelsen.se, accessed last on 31 December 2020).
Acknowledgments
We would like to give thanks to Qefsere Brahimi and Marie Lindgren.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| CD | celiac disease |
| T1D | type 1 diabetes |
| GFD | gluten-free diet |
| DKA | ketoacidosis |
| BMI | body mass index |
| tTGA | tissue transglutaminase IgA-antibodies |
| EMA | endomysial antibodies |
| DGP | deamidated gluten peptides |
| AGA | gliadin antibodies |
References
- Craig, M.E.; Prinz, N.; Boyle, C.T.; Campbell, F.M.; Jones, T.W.; Hofer, S.E.; Simmons, J.H.; Holman, N.; Tham, E.; Froehlich-Reiterer, E.; et al. Prevalence of Celiac Disease in 52,721 Youth with Type 1 Diabetes: International Comparison Across Three Continents. Diabetes Care 2017, 40, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef]
- Elfström, P.; Sundström, J.; Ludvigsson, J.F. Systematic review with meta-analysis: Associations between coeliac disease and type 1 diabetes. Aliment. Pharmacol. Ther. 2014, 40, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Carlsson, A.; Cederwall, E.; Jönsson, B.; Neiderud, J.; Jonsson, B.; Lernmark, Å.; Ivarsson, S.A.; Group, S.S. Annual screening detects celiac disease in children with type 1 diabetes. Pediatr. Diabetes 2008, 9, 354–359. [Google Scholar] [CrossRef]
- Bybrant, M.C.; Örtqvist, E.; Lantz, S.; Grahnquist, L. High prevalence of celiac disease in Swedish children and adolescents with type 1 diabetes and the relation to the Swedish epidemic of celiac disease: A cohort study. Scand. J. Gastroenterol. 2013, 49, 52–58. [Google Scholar] [CrossRef]
- Alshiekh, S.; Geraghty, D.E.; Agardh, D. High-resolution genotyping of HLA class I loci in children with type 1 diabetes and celiac disease. HLA 2021, 97, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef]
- Tuomilehto, J. The emerging global epidemic of type 1 diabetes. Curr. Diabetes Rep. 2013, 13, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Kyle, R.A.; Kaplan, E.L.; Johnson, D.R.; Page, W.; Erdtmann, F.; Brantner, T.L.; Kim, W.R.; Phelps, T.K.; Lahr, B.D.; et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009, 137, 88–93. [Google Scholar] [CrossRef]
- Goodwin, G. Type 1 diabetes mellitus and celiac disease: Distinct autoimmune disorders that share common pathogenic mechanisms. Horm. Res. Paediatr. 2019, 92, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Bhadada, S.K.; Minz, R.W.; Dayal, D.; Kochhar, R. Interplay between type 1 diabetes mellitus and celiac disease: Implications in treatment. Dig. Dis. 2018, 36, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.; Cureton, P.; Fasano, A. Managing coeliac disease in patients with diabetes. Diabetes Obes. Metab. 2015, 17, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: An approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.E.M.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of nutrients in I talian children with celiac disease and the role of commercially available gluten-free products. J. Hum. Nutr. Diet. 2013, 26, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Alzaben, A.S.; Turner, J.; Shirton, L.; Samuel, T.M.; Persad, R.; Mager, D. Assessing nutritional quality and adherence to the gluten-free diet in children and adolescents with celiac disease. Can. J. Diet. Pract. Res. 2015, 76, 56–63. [Google Scholar] [CrossRef]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Garnett, S.; Craig, M.E. Quality of life in type 1 diabetes and celiac disease: Role of the gluten-free diet. J. Pediatr. 2016, 179, 131–138.e1. [Google Scholar] [CrossRef] [PubMed]
- Tapsas, D.; Fälth-Magnusson, K.; Högberg, L.; Hammersjö, J.-Å.; Hollén, E. Swedish children with celiac disease comply well with a gluten-free diet, and most include oats without reporting any adverse effects: A long-term follow-up study. Nutr. Res. 2014, 34, 436–441. [Google Scholar] [CrossRef]
- Taavela, J.; Kurppa, K.; Collin, P.; Lähdeaho, M.L.; Salmi, T.; Saavalainen, P.; Haimila, K.; Huhtala, H.; Laurila, K.; Sievänen, H.; et al. Degree of damage to the small bowel and serum antibody titers correlate with clinical presentation of patients with celiac disease. Clin. Gastroenterol. Hepatol. 2013, 11, 166–171.e1. [Google Scholar] [CrossRef]
- Singh, P.; Kurray, L.; Agnihotri, A.; Das, P.; Verma, A.K.; Sreenivas, V.; Dattagupta, S.; Makharia, G.K. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J. Clin. Gastroenterol. 2015, 49, 212–217. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Ciacci, C.; Green, P.H.; Kaukinen, K.; Korponay-Szabo, I.R.; Kurppa, K.; Murray, J.A.; Lundin, K.E.A.; Maki, M.J.; Popp, A.; et al. Outcome measures in coeliac disease trials: The Tampere recommendations. Gut 2018, 67, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Isaac, D.M.; Rajani, S.; Yaskina, M.; Huynh, H.Q.; Turner, J.M. Antitissue transglutaminase normalization postdiagnosis in children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 195–199. [Google Scholar] [CrossRef]
- Hansen, D.; Brock-Jacobsen, B.; Lund, E.; Bjørn, C.; Hansen, L.P.; Nielsen, C.; Fenger, C.; Lillevang, S.T.; Husby, S. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: A population-based screening study with 2 years’ follow-up. Diabetes Care 2006, 29, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, I.A.; Reilly, N.R.; Rubio-Tapia, A. Celiac disease: Clinical features and diagnosis. Gastroenterol. Clin. 2019, 48, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Myléus, A.; Ivarsson, A.; Webb, C.; Danielsson, L.; Hernell, O.; Högberg, L.; Karlsson, E.; Lagerqvist, C.; Norström, F.; Rosén, A.; et al. Celiac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 170–176. [Google Scholar] [CrossRef]
- Webb, C.; Myléus, A.; Norström, F.; Hammarroth, S.; Högberg, L.; Lagerqvist, C.; Rosén, A.; Sandström, O.; Stenhammar, L.; Ivarsson, A.; et al. High adherence to a gluten-free diet in adolescents with screening-detected celiac disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 54–59. [Google Scholar] [CrossRef]
- Saadah, O.; Zacharin, M.; O’Callaghan, A.; Oliver, M.; Catto-Smith, A. Effect of gluten-free diet and adherence on growth and diabetic control in diabetics with coeliac disease. Arch. Dis. Child. 2004, 89, 871–876. [Google Scholar] [CrossRef]
- Amin, R.; Murphy, N.; Edge, J.; Ahmed, M.L.; Acerini, C.L.; Dunger, D.B. A longitudinal study of the effects of a gluten-free diet on glycemic control and weight gain in subjects with type 1 diabetes and celiac disease. Diabetes Care 2002, 25, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Albisua, I.; Wolf, J.; Neu, A.; Geiger, H.; Wäscher, I.; Stern, M. Coeliac disease in children with type 1 diabetes mellitus: The effect of the gluten-free diet. Diabet. Med. 2005, 22, 1079–1082. [Google Scholar] [CrossRef]
- Westman, E.; Ambler, G.R.; Royle, M.; Peat, J.; Chan, A. Children with coeliac disease and insulin dependent diabetes mellitus-growth, diabetes control and dietary intake. J. Pediatr. Endocrinol. Metab. 1999, 12, 433–442. [Google Scholar] [CrossRef]
- Artz, E.; Warren-Ulanch, J.; Becker, D.; Greenspan, S.; Freemark, M. Seropositivity to celiac antigens in asymptomatic children with type 1 diabetes mellitus: Association with weight, height, and bone mineralization. Pediatr. Diabetes 2008, 9, 277–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagl, K.; Bollow, E.; Liptay, S.; Rosenbauer, J.; Koletzko, S.; Pappa, A.; Näke, A.; Fröhlich-Reiterer, E.; Döring, C.; Wolf, J.; et al. Lower HbA1c in patients with type 1 diabetes and celiac disease who reached celiac-specific antibody-negativity—A multicenter DPV analysis. Pediatr. Diabetes 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Kaur, P.; Agarwala, A.; Makharia, G.; Bhatnagar, S.; Tandon, N. Effect of gluten-free diet on metabolic control and anthropometric parameters in type 1 diabetes with subclinical celiac disease: A randomized controlled trial. Endocr. Pract. 2020, 26, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Bulow, E. Vavnadstransglutaminas-Antikroppar (IgA/IgG), a-tTG (IgA/IgG). Available online: http://analysportalen-labmedicin.skane.se/pics/Labmedicin/analysportalen/KIT/Vävnadstransglutaminas-antikroppar%20(IgA_IgG),%20a-tTG%20(IgA_IgG)(200207).pdf2020 (accessed on 1 July 2021).
- Webb, C.; Norström, F.; Myléus, A.; Ivarsson, A.; Halvarsson, B.; Högberg, L.; Lagerqvist, C.; Rosén, A.; Sandström, O.; Stenhammar, L.; et al. Celiac disease can be predicted by high levels of anti-tissue transglutaminase antibodies in population-based screening. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 787–791. [Google Scholar] [CrossRef]
- Bulow, E. Endomysium-antikroppar (IgA), EmA (IgA). 2020. Available online: http://analysportalen-labmedicin.skane.se/pics/Labmedicin/analysportalen/KIT/ndomyseum-antikroppar%20(IgA)200207).pdf (accessed on 1 July 2021).
- Cole, T. A method for assessing age-standardized weight-for-height in children seen cross-sectionally. Ann. Hum. Biol. 1979, 6, 249–268. [Google Scholar] [CrossRef]
- Esch, C.E.H.; Wolters, V.M.; Gerritsen, S.A.; Putter, H.; von Blomberg, B.M.; van Hoogstraten, I.M.; Houwen, R.H.J.; van der Lely, N.; Mearin, M.L. Specific celiac disease antibodies in children on a gluten-free diet. Pediatrics 2011, 128, 547–552. [Google Scholar] [CrossRef]
- Schuppan, D.; Mäki, M.; Lundin, K.E.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, Ø. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Korkmaz, H.; Sozen, M.; Kebapcilar, L. Increased arterial stiffness and its relationship with inflammation, insulin, and insulin resistance in celiac disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, V.S.; Honcharuk, E.; Becker, D.; Escobar, O.; Siminerio, L. A focus on blood glucose monitoring: Relation to glycemic control and determinants of frequency. Pediatr. Diabetes 2011, 12, 25–30. [Google Scholar] [CrossRef]
- Rausch, J.R.; Hood, K.K.; Delamater, A.; Pendley, J.S.; Rohan, J.M.; Reeves, G.; Dolan, L.; Drotar, D. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care 2012, 35, 1219–1224. [Google Scholar] [CrossRef]
- Rami, B.; Sumnik, Z.; Schober, E.; Waldhör, T.; Battelino, T.; Bratanic, N.; Kürti, K.; Lebl, J.; Limbert, C.; Madacsy, L.; et al. Screening detected celiac disease in children with type 1 diabetes mellitus: Effect on the clinical course (a case control study). J. Pediatr. Gastroenterol. Nutr. 2005, 41, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Tye-Din, J.; Hardy, M.; Anderson, R. Ex-vivo whole blood secretion of interferon (IFN)-γ and IFN-γ-inducible protein-10 measured by enzyme-linked immunosorbent assay are as sensitive as IFN-γ enzyme-linked immunospot for the detection of gluten-reactive T cells in human leucocyte antigen (HLA)-DQ 2·5+-associated coeliac disease. Clin. Exp. Immunol. 2014, 175, 305–315. [Google Scholar]
- Comino, I.; Fernández-Bañares, F.; Esteve, M.; Ortigosa, L.; Castillejo, G.; Fambuena, B.; Ribes-Koninckx, C.; Sierra, C.; Rodríguez-Herrera, A.; Salazar, J.C. Fecal gluten peptides reveal limitations of serological tests and food questionnaires for monitoring gluten-free diet in celiac disease patients. Am. J. Gastroenterol. 2016, 111, 1456. [Google Scholar] [CrossRef]
- De Lourdes Moreno, M.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef]
- Alessio, M.G.; Tonutti, E.; Brusca, I.; Radice, A.; Licini, L.; Sonzogni, A.; Florena, A.; Schiaffino, E.; Marus, W.; Sulfaro, S.; et al. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 44–49. [Google Scholar] [CrossRef]
- Pekki, H.; Kurppa, K.; Mäki, M.; Huhtala, H.; Sievänen, H.; Laurila, K.; Collin, P.; Kaukinen, K. Predictors and significance of incomplete mucosal recovery in celiac disease after 1 year on a gluten-free diet. Off. J. Am. Coll. Gastroenterol. 2015, 110, 1078–1085. [Google Scholar] [CrossRef]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, D.R. Tests for serum transglutaminase and endomysial antibodies do not detect most patients with celiac disease and persistent villous atrophy on gluten-free diets: A meta-analysis. Gastroenterology 2017, 153, 689–701.e1. [Google Scholar] [CrossRef] [PubMed]
- Daveson, A.J.M.; Popp, A.; Taavela, J.; Goldstein, K.E.; Isola, J.; Truitt, K.E.; Mäki, M.; Anderson, R.P.; Group, R.C.S.; Adams, A. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep 2020, 2, 22–30. [Google Scholar] [CrossRef]
- Lähdeaho, M.-L.; Mäki, M.; Laurila, K.; Huhtala, H.; Kaukinen, K. Small-bowel mucosal changes and antibody responses after low-and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.; Schuppan, D.; Pallav, K.; Najarian, R.; Goldsmith, J.D.; Hansen, J.; Kabbani, T.; Dennis, M.; Kelly, C.P. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013, 62, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Budnik, T.; Malamut, G.; de Serre, N.P.-M.; Grosdidier, E.; Seguier, S.; Brousse, N.; Caillat-Zucman, S.; Cerf-Bensussan, N.; Schmitz, J.; Cellier, C. Long-term follow-up of 61 coeliac patients diagnosed in childhood: Evolution toward latency is possible on a normal diet. Gut 2007, 56, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).