Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Data Collection
2.2. Dietary Assessment and Estimation of AGE Intake
2.3. Cancer Ascertainment and Follow-Up
2.4. Vital Status Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Dietary Intakes of AGEs and Mortality among CRC Patients
3.3. Sensitivity Analyses
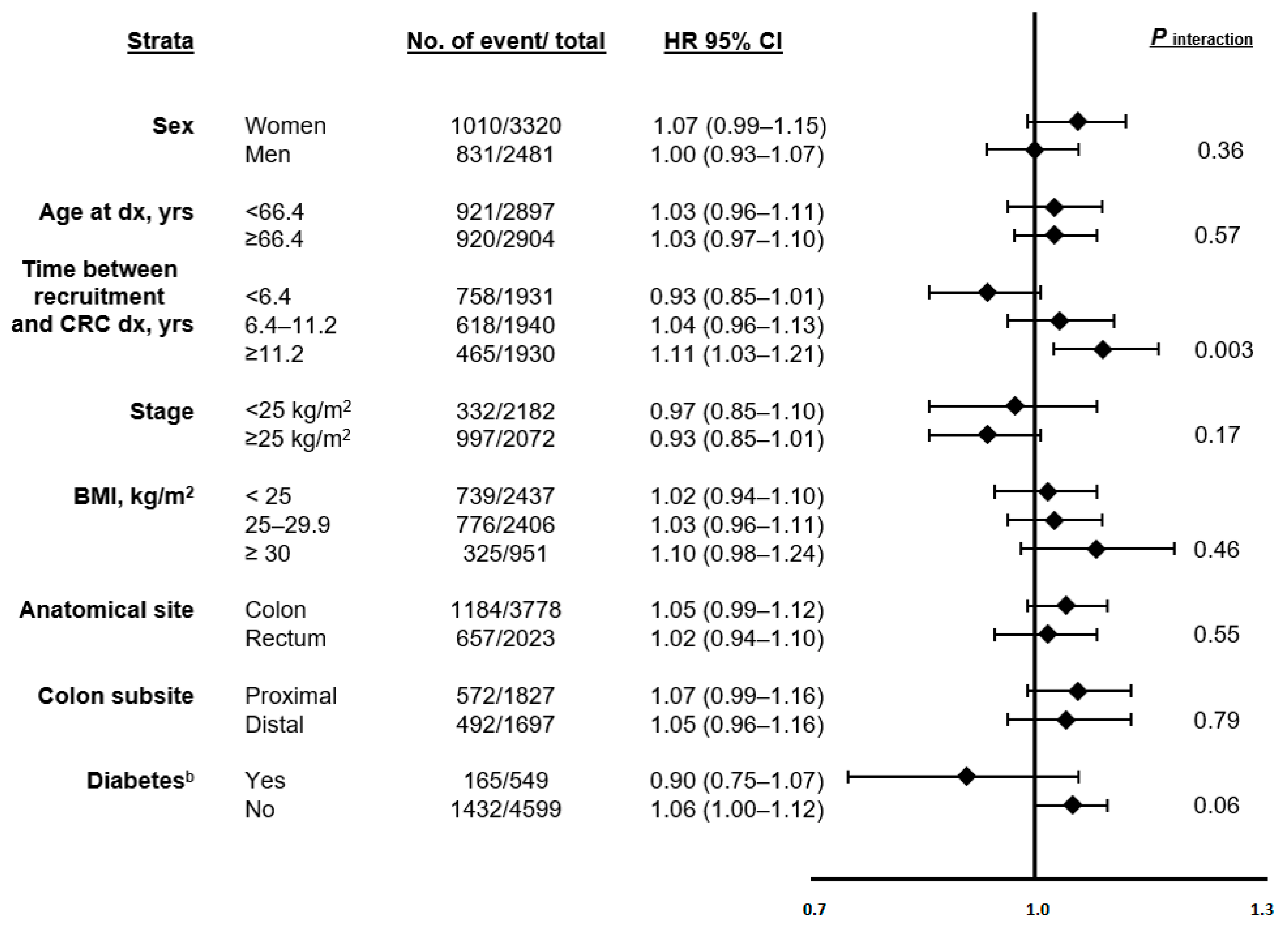
3.4. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 23 September 2021).
- Potter, J.D.; Slattery, M.L.; Bostick, R.M.; Gapstur, S.M. Colon Cancer: A Review of the Epidemiology. Epidemiol. Rev. 1993, 15, 499–545. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Uribarri, J.; Del Castillo, M.D.; De La Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Bastos, D.H.M.; Medrano, A.; Menini, T.; et al. Dietary Advanced Glycation End Products and Their Role in Health and Disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Morrissey, P.A.; Ames, J.M. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 211–248. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Cai, W.; Gao, Q.-D.; Zhu, L.; Peppa, M.; He, C.; Vlassara, H. Oxidative Stress-Inducing Carbonyl Compounds From Common Foods: Novel Mediators of Cellular Dysfunction. Mol. Med. 2002, 8, 337–346. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Crandall, J.; Goldberg, T.; Oberstein, R.; Dardaine, V.; Peppa, M.; Rayfield, E.J. Nonlinear partial differential equations and applications: Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 15596–15601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, M. Serum Levels of Toxic AGEs (TAGE) May Be a Promising Novel Biomarker for the Onset/Progression of Lifestyle-Related Diseases. Diagnostics 2016, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against Loss of Innate Defenses in Adulthood by Low Advanced Glycation End Products (AGE) Intake: Role of the Antiinflammatory AGE Receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Greenwald, S. Ageing of the conduit arteries. J. Pathol. 2007, 211, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsov, S.; Graaff, R.; Van Oeveren, W.; Stegmayr, B.; Sikole, A.; Rakhorst, G.; Smit, A.J. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: A review. Clin. Chem. Lab. Med. 2014, 52, 11–20. [Google Scholar] [CrossRef]
- Hartog, J.W.L.; Voors, A.A.; Bakker, S.J.L.; Smit, A.J.; Van Veldhuisen, D.J. Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. Eur. J. Heart Fail. 2007, 9, 1146–1155. [Google Scholar] [CrossRef]
- Kizer, J.R.; Benkeser, D.; Arnold, A.M.; Ix, J.H.; Mukamal, K.J.; Djousse, L.; Tracy, R.P.; Siscovick, D.S.; Psaty, B.M.; Zieman, S.J. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis 2014, 235, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pino, A.; Currenti, W.; Urbano, F.; Scicali, R.; Piro, S.; Purrello, F.; Rabuazzo, A.M. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Mayén, A.; Aglago, E.K.; Knaze, V.; Cordova, R.; Schalkwijk, C.G.; Wagner, K.; Aleksandrova, K.; Fedirko, V.; Keski-Rahkonen, P.; Leitzmann, M.F.; et al. Dietary intake of advanced glycation endproducts and risk of hepatobiliary cancers: A multinational cohort study. Int. J. Cancer 2021, 149, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.L.; Omofuma, O.; Turner, D.P.; Merchant, A.; Zhang, J.; Neuhouser, M.L.; Snetselaar, L.; Caan, B.J.; Shadyab, A.H.; Saquib, N.; et al. Dietary advanced glycation end products (AGEs) and breast cancer mortality in the women’s health initiative (WHI). J. Clin. Oncol. 2020, 38, 1570-1570. [Google Scholar] [CrossRef]
- Ebert, H.; Lacruz, M.E.; Kluttig, A.; Simm, A.; Greiser, K.H.; Tiller, D.; Kartschmit, N.; Mikolajczyk, R. Association between advanced glycation end products, their soluble receptor, and mortality in the general population: Results from the CARLA study. Exp. Gerontol. 2019, 131, 110815. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J.M.; Fried, L.P. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin. Exp. Res. 2009, 21, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.A.; Thomas, M.C.; Fernando, D.; Macmillan, N.; Power, D.A.; Ierino, F.L. Low molecular weight advanced glycation end products predict mortality in asymptomatic patients receiving chronic haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1611–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.-H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher Plasma Levels of Advanced Glycation End Products Are Associated With Incident Cardiovascular Disease and All-Cause Mortality in Type 1 Diabetes: A 12-year follow-up study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Ronnemaa, T.; Torjesen, P.A.; Birkeland, K.I.; Berg, T.J.; Hanssen, K.F.; Laakso, M. High Serum Levels of Advanced Glycation End Products Predict Increased Coronary Heart Disease Mortality in Nondiabetic Women but not in Nondiabetic Men. Arter. Thromb. Vasc. Biol. 2005, 25, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Nagata, C.; Wada, K.; Yamakawa, M.; Nakashima, Y.; Koda, S.; Uji, T.; Oba, S. Dietary Intake of Nε-carboxymethyl-lysine, a Major Advanced Glycation End Product, is Not Associated with Increased Risk of Mortality in Japanese Adults in the Takayama Study. J. Nutr. 2020, 150, 2799–2805. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferraria, P.; Norata, T.; Fahey, M.; Charrondierea, U.R.; Hemona, B.; Casagrandea, C.; Vignata, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.; Riboli, E. Diet and cancer — the European Prospective Investigation into Cancer and Nutrition. Nat. Rev. Cancer 2004, 4, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Slimani, N.; Deharveng, G.; Unwin, I.; Southgate, D.A.T.; Vignat, J.; Skeie, G.; Salvini, S.; Parpinel, M.; Møller, A.; Ireland, J.; et al. The EPIC nutrient database project (ENDB): A first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur. J. Clin. Nutr. 2007, 61, 1037–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aglago, E.K.; Mayén, A.-L.; Knaze, V.; Freisling, H.; Fedirko, V.; Hughes, D.J.; Jiao, L.; Eriksen, A.K.; Tjønneland, A.; Boutron-Ruault, M.-C.; et al. Dietary Advanced Glycation End-Products and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Nutrients 2021, 13, 3132. [Google Scholar] [CrossRef]
- Cordova, R.; Knaze, V.; Viallon, V.; Rust, P.; Schalkwijk, C.G.; Weiderpass, E.; Wagner, K.-H.; Mayen-Chacon, A.-L.; Aglago, E.K.; Dahm, C.; et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur. J. Nutr. 2019, 59, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Govindarajulu, U.S.; Spiegelman, D.; Thurston, S.W.; Ganguli, B.; Eisen, E.A. Comparing smoothing techniques in Cox models for exposure–response relationships. Stat. Med. 2007, 26, 3735–3752. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hertzmark, E.; Louie, M.; Chen, L.; Spiegelman, D. The SAS LGTPHCURV9 Macro; Channing Laboratory: Boston, MA, USA, 2011. [Google Scholar]
- Zhang, S.; Li, F.; Younes, M.; Liu, H.; Chen, C.; Yao, Q. Reduced Selenium-Binding Protein 1 in Breast Cancer Correlates with Poor Survival and Resistance to the Anti-Proliferative Effects of Selenium. PLoS ONE 2013, 8, e63702. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, W.L.; Krishnan, K.; E Campbell, S.; E Palau, V. The role of antioxidants and pro-oxidants in colon cancer. World J. Gastrointest. Oncol. 2014, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pitmon, E.; Wang, K. Microbiome, inflammation and colorectal cancer. Semin. Immunol. 2017, 32, 43–53. [Google Scholar] [CrossRef]
- Levental, K.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Peppa, M.; Cai, W.; Goldberg, T.; Lu, M.; He, C.; Vlassara, H. Restriction of Dietary Glycotoxins Reduces Excessive Advanced Glycation End Products in Renal Failure Patients. J. Am. Soc. Nephrol. 2003, 14, 728–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, K.; Ford, M.E.; Gregoski, M.J.; Kramer, R.M.; Knight, K.D.; Spruill, L.; Nogueira, L.M.; Krisanits, B.A.; Phan, V.; La Rue, A.C.; et al. Advanced glycation end products are elevated in estrogen receptor-positive breast cancer patients, alter response to therapy, and can be targeted by lifestyle intervention. Breast Cancer Res. Treat. 2018, 173, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Nass, N.; Ignatov, A.; Andreas, L.; Weißenborn, C.; Kalinski, T.; Sel, S. Accumulation of the advanced glycation end product carboxymethyl lysine in breast cancer is positively associated with estrogen receptor expression and unfavorable prognosis in estrogen receptor-negative cases. Histochem. Cell Biol. 2016, 147, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Wu, C.; Lu, C.; Hsia, S.; Yen, G. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: An emerging biological factor in cancer onset and progression. Mol. Nutr. Food Res. 2016, 60, 1850–1864. [Google Scholar] [CrossRef]
- Hellwig, M.; Humpf, H.-U.; Hengstler, J.G.; Mally, A.; Vieths, S.; Henle, T. Quality Criteria for Studies on Dietary Glycation Compounds and Human Health. J. Agric. Food Chem. 2019, 67, 11307–11311. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Combined a Dietary AGEs (mg/d) | |||||
|---|---|---|---|---|---|---|
| Quintile 1: <19.79 (N = 1160) | Quintile 2: 19.79–23.20 (N = 1160) | Quintile 3: 23.21–26.80 (N = 1161) | Quintile 4: 26.81–32.26 (N = 1160) | Quintile 5: >32.26 (N = 1160) | p-Value | |
| Age at diagnosis, mean (SD), y | 66.04 (7.68) | 65.69 (8.63) | 66.01 (8.65) | 66.49 (8.70) | 67.25 (8.93) | 0.39 |
| Women, N (%) | 870 (75) | 709 (61) | 680 (59) | 570 (49) | 491 (42) | <0.001 |
| Year at dx, median (min-max) | 2004 (1993–2013) | 2005 (1993–2013) | 2005 (1993–2013) | 2005 (1993–2013) | 2005 (1992–2013) | 0.89 |
| Stage of disease, N (%) c | <0.001 | |||||
| I | 225 (19) | 217 (19) | 228 (20) | 259 (22) | 226 (19) | |
| II | 241 (21) | 224 (19) | 177 (15) | 213 (18) | 172 (15) | |
| III | 286 (25) | 304 (26) | 325 (28) | 258 (22) | 250 (22) | |
| IV | 165 (14) | 142 (12) | 128 (11) | 116 (10) | 98 (8) | |
| Location of primary tumor, N (%) | 0.75 | |||||
| Colon | 762 (66) | 748 (65) | 766 (66) | 750 (65) | 752 (65) | |
| Rectum | 398 (34) | 412 (35) | 395 (34) | 410 (35) | 408 (35) | |
| Smoking status, N (%) c | <0.001 | |||||
| Never | 462 (40) | 482 (42) | 462 (40) | 471 (41) | 494 (43) | |
| Former | 364 (31) | 365 (31) | 403 (35) | 410 (35) | 422 (36) | |
| Current | 326 (28) | 301 (26) | 276 (24) | 259 (22) | 224 (19) | |
| BMI, mean (SD), kg/m2 | 26.14 (4.33) | 26.29 (4.21) | 26.34 (4.16) | 26.54 (4.16) | 26.17 (4.26) | 0.15 |
| Physical activity d, N (%) c | <0.001 | |||||
| Inactive | 197 (17) | 182 (16) | 187 (16) | 181 (16) | 128 (11) | |
| Moderately inactive | 383 (33) | 375 (32) | 351 (30) | 320 (28) | 347 (30) | |
| Moderately active | 446 (38) | 450 (39) | 458 (40) | 458 (39) | 438 (38) | |
| Active | 85 (7) | 105 (9) | 99 (9) | 103 (9) | 123 (11) | |
| Diabetes e, N (%) c | <0.001 | |||||
| No | 952 (82) | 943 (81) | 922 (80) | 901 (78) | 881 (76) | |
| Yes | 105 (9) | 106 (9) | 122 (11) | 111 (10) | 105 (9) | |
| Daily dietary intakes | ||||||
| Total energy, mean (SD), kcal | 2019.4 (584.6) | 2098.2 (609.6) | 2115.0 (602.4) | 2158.6 (628.8) | 2155.3 (639.2) | <0.001 |
| Fiber, mean (SD), g | 19.8 (6.9) | 21.6 (6.8) | 22.8 (7.1) | 23.9 (7.7) | 25.4 (8.7) | <0.001 |
| Dietary calcium, mean (SD), mg | 980.4 (440.7) | 976.7 (408.5) | 969.8 (378.6) | 989.4 (375.1) | 1003.4 (394.1) | 0.30 |
| Fruits, mean (SD), g | 220.6 (191.4) | 223.3 (184.7) | 225.2 (183.0) | 222.1 (161.4) | 202.9 (155.0) | 0.02 |
| Vegetables, mean (SD), g | 179.9 (123.3) | 178.7 (115.9) | 187.0 (115.0) | 193.7 (125.2) | 191.7 (132.1) | 0.007 |
| Red meat, mean (SD), g | 50.9 (35.3) | 52.7 (38.0) | 52.7 (41.1) | 46.5 (37.6) | 41.9 (37.4) | <0.001 |
| Processed meat, mean (SD), g | 30.9 (26.7) | 34.5 (28.8) | 34.6 (31.3) | 37.8 (30.5) | 37.2 (36.1) | <0.001 |
| AGEs a | Cut-Offs | N | All-Cause Mortality | CRC-Specific Mortality | ||||
|---|---|---|---|---|---|---|---|---|
| Event | HR (95% CI) b,c | HR (95% CI) b,d | Event | HR (95% CI) b,c | HR (95% CI) b,d | |||
| CML, mg/d | ||||||||
| Quintile 1 | <2.3 | 1160 | 447 | 1.00 (ref) | 1.00 (ref) | 348 | 1.00 (ref) | 1.00 (ref) |
| Quintile 2 | [2.3–2.7) | 1160 | 489 | 1.11 (0.97–1.26) | 1.14 (1.00–1.30) | 384 | 1.13 (0.98–1.31) | 1.13 (0.98–1.32) |
| Quintile 3 | [2.7–3.1) | 1161 | 498 | 1.11 (0.97–1.27) | 1.13 (0.99–1.30) | 368 | 1.08 (0.93–1.26) | 1.10 (0.94–1.28) |
| Quintile 4 | [3.1–3.7) | 1160 | 474 | 0.99 (0.86–1.14) | 1.02 (0.89–1.18) | 361 | 1.03 (0.88–1.21) | 1.04 (0.89–1.22) |
| Quintile 5 | ≥3.7 | 1160 | 513 | 1.08 (0.93–1.25) | 1.13 (0.98–1.30) | 380 | 1.14 (0.97–1.34) | 1.16 (0.98–1.36) |
| ptrende | 0.72 | 0.36 | 0.31 | 0.23 | ||||
| Per 1.01 mg/d | 1.00 (0.96, 1.04) | 1.02 (0.98–1.06) | 1.03 (0.98–1.08) | 1.03 (0.98, 1.08) | ||||
| CEL, mg/d | ||||||||
| Quintile 1 | <1.6 | 1160 | 463 | 1.00 (ref) | 1.00 (ref) | 354 | 1.00 (ref) | 1.00 (ref) |
| Quintile 2 | [1.6–1.9) | 1160 | 435 | 0.90 (0.79–1.03) | 0.92 (0.80–1.05) | 335 | 0.92 (0.79–1.07) | 0.93 (0.80–1.09) |
| Quintile 3 | [1.9–2.2) | 1161 | 492 | 1.01 (0.88–1.15) | 1.01 (0.89–1.15) | 378 | 1.04 (0.89–1.21) | 1.04 (0.89–1.21) |
| Quintile 4 | [2.2–2.6) | 1160 | 496 | 0.92 (0.80–1.06) | 0.92 (0.80–1.06) | 368 | 0.96 (0.82–1.13) | 0.96 (0.82–1.13) |
| Quintile 5 | ≥2.6 | 1160 | 535 | 1.03 (0.89–1.18) | 1.03 (0.89–1.19) | 406 | 1.10 (0.93–1.29) | 1.11 (0.94–1.31) |
| ptrende | 0.45 | 0.47 | 0.14 | 0.13 | ||||
| Per 0.73 mg/d | 1.01 (0.97–1.06) | 1.01 (0.97–1.06) | 1.02 (0.97–1.08) | 1.02 (0.97–1.08) | ||||
| MG-H1, mg/d | ||||||||
| Quintile 1 | <15.5 | 1160 | 457 | 1.00 (ref) | 1.00 (ref) | 360 | 1.00 (ref) | 1.00 (ref) |
| Quintile 2 | [15.5–18.4) | 1160 | 471 | 1.07 (0.93–1.22) | 1.07 (0.94–1.22) | 370 | 1.07 (0.93–1.25) | 1.08 (0.93–1.26) |
| Quintile 3 | [18.4–21.4) | 1161 | 460 | 0.99 (0.87–1.14) | 1.00 (0.88–1.15) | 335 | 0.94 (0.80–1.09) | 0.95 (0.81–1.11) |
| Quintile 4 | [21.4–26.1) | 1160 | 506 | 1.08 (0.94–1.23) | 1.09 (0.95–1.24) | 393 | 1.07 (0.92–1.25) | 1.09 (0.93–1.27) |
| Quintile 5 | ≥26.1 | 1160 | 527 | 1.07 (0.93–1.22) | 1.09 (0.95–1.25) | 383 | 1.08 (0.92–1.26) | 1.10 (0.94–1.28) |
| ptrende | 0.37 | 0.23 | 0.35 | 0.24 | ||||
| Per 8.47 mg/d | 1.01 (0.97–1.05) | 1.02 (0.98–1.05) | 1.02 (0.98–1.07) | 1.03 (0.98–1.08) | ||||
| Combined AGEs f, mg/d | ||||||||
| Quintile 1 | <19.8 | 1160 | 453 | 1.00 (ref) | 1.00 (ref) | 356 | 1.00 (ref) | 1.00 (ref) |
| Quintile 2 | [19.8–23.2) | 1160 | 467 | 1.08 (0.94–1.23) | 1.09 (0.95–1.24) | 367 | 1.08 (0.93–1.26) | 1.10 (0.95–1.28) |
| Quintile 3 | [23.2–26.8) | 1161 | 463 | 1.01 (0.88–1.15) | 1.02 (0.89–1.17) | 336 | 0.96 (0.82–1.12) | 0.97 (0.83–1.13) |
| Quintile 4 | [26.8–32.3) | 1160 | 520 | 1.09 (0.95–1.24) | 1.10 (0.96–1.26) | 405 | 1.10 (0.95–1.28) | 1.11 (0.96–1.30) |
| Quintile 5 | ≥32.3 | 1160 | 518 | 1.06 (0.92–1.21) | 1.08 (0.94–1.24) | 377 | 1.07 (0.91–1.25) | 1.09 (0.93–1.28) |
| ptrende | 0.50 | 0.33 | 0.42 | 0.29 | ||||
| Per 9.83 mg/d | 1.01 (0.97–1.05) | 1.02 (0.98–1.05) | 1.02 (0.98–1.08) | 1.03 (0.98–1.08) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Aglago, E.K.; Zhao, Z.; Schalkwijk, C.; Jiao, L.; Freisling, H.; Weiderpass, E.; Hughes, D.J.; Eriksen, A.K.; Tjønneland, A.; et al. Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer. Nutrients 2021, 13, 4435. https://doi.org/10.3390/nu13124435
Mao Z, Aglago EK, Zhao Z, Schalkwijk C, Jiao L, Freisling H, Weiderpass E, Hughes DJ, Eriksen AK, Tjønneland A, et al. Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer. Nutrients. 2021; 13(12):4435. https://doi.org/10.3390/nu13124435
Chicago/Turabian StyleMao, Ziling, Elom K. Aglago, Zhiwei Zhao, Casper Schalkwijk, Li Jiao, Heinz Freisling, Elisabete Weiderpass, David J. Hughes, Anne Kirstine Eriksen, Anne Tjønneland, and et al. 2021. "Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer" Nutrients 13, no. 12: 4435. https://doi.org/10.3390/nu13124435
APA StyleMao, Z., Aglago, E. K., Zhao, Z., Schalkwijk, C., Jiao, L., Freisling, H., Weiderpass, E., Hughes, D. J., Eriksen, A. K., Tjønneland, A., Severi, G., Rothwell, J., Boutron-Ruault, M.-C., Katzke, V., Kaaks, R., Schulze, M. B., Birukov, A., Krogh, V., Panico, S., ... Fedirko, V. (2021). Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer. Nutrients, 13(12), 4435. https://doi.org/10.3390/nu13124435









