Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine
Abstract
:1. Introduction
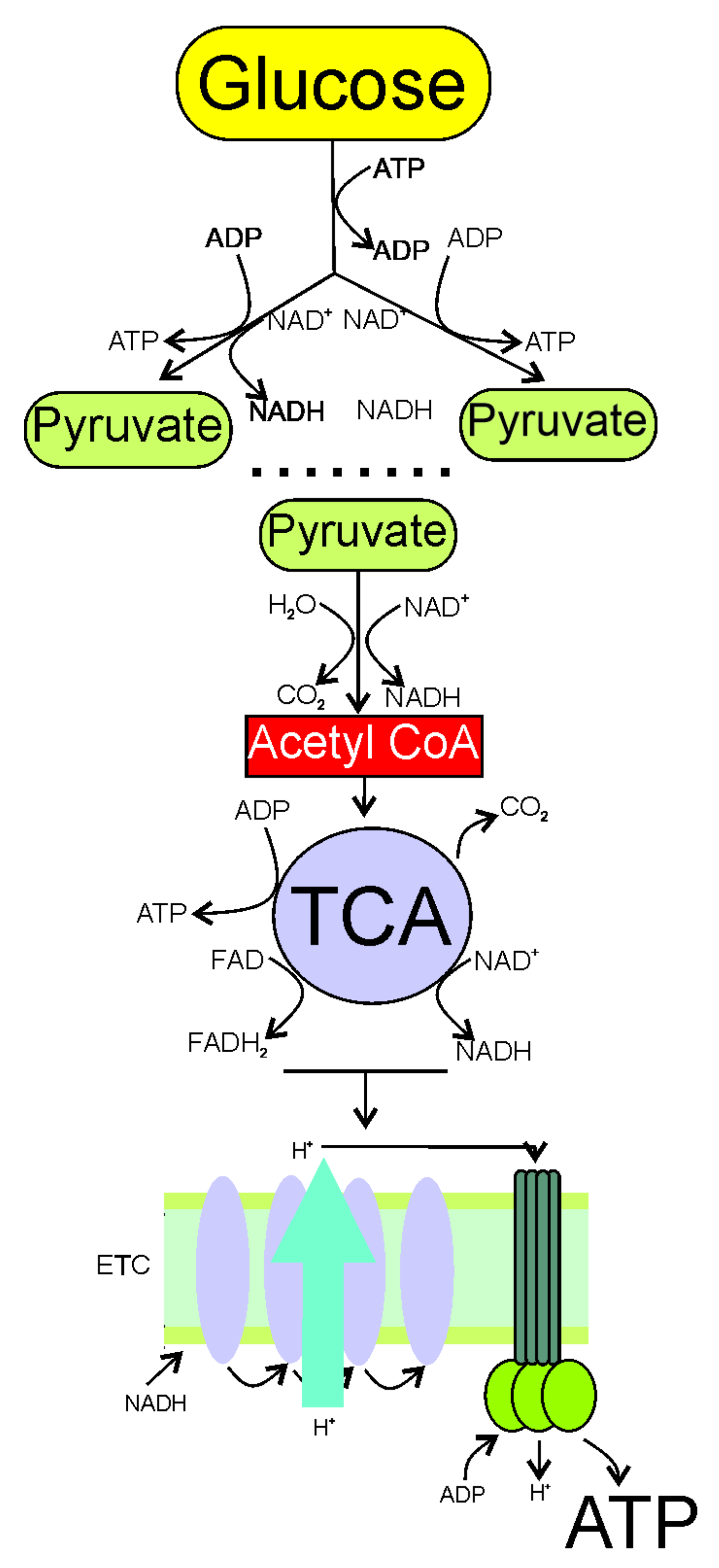
2. Increased Demand and Decreased Production of Energy in Migraine
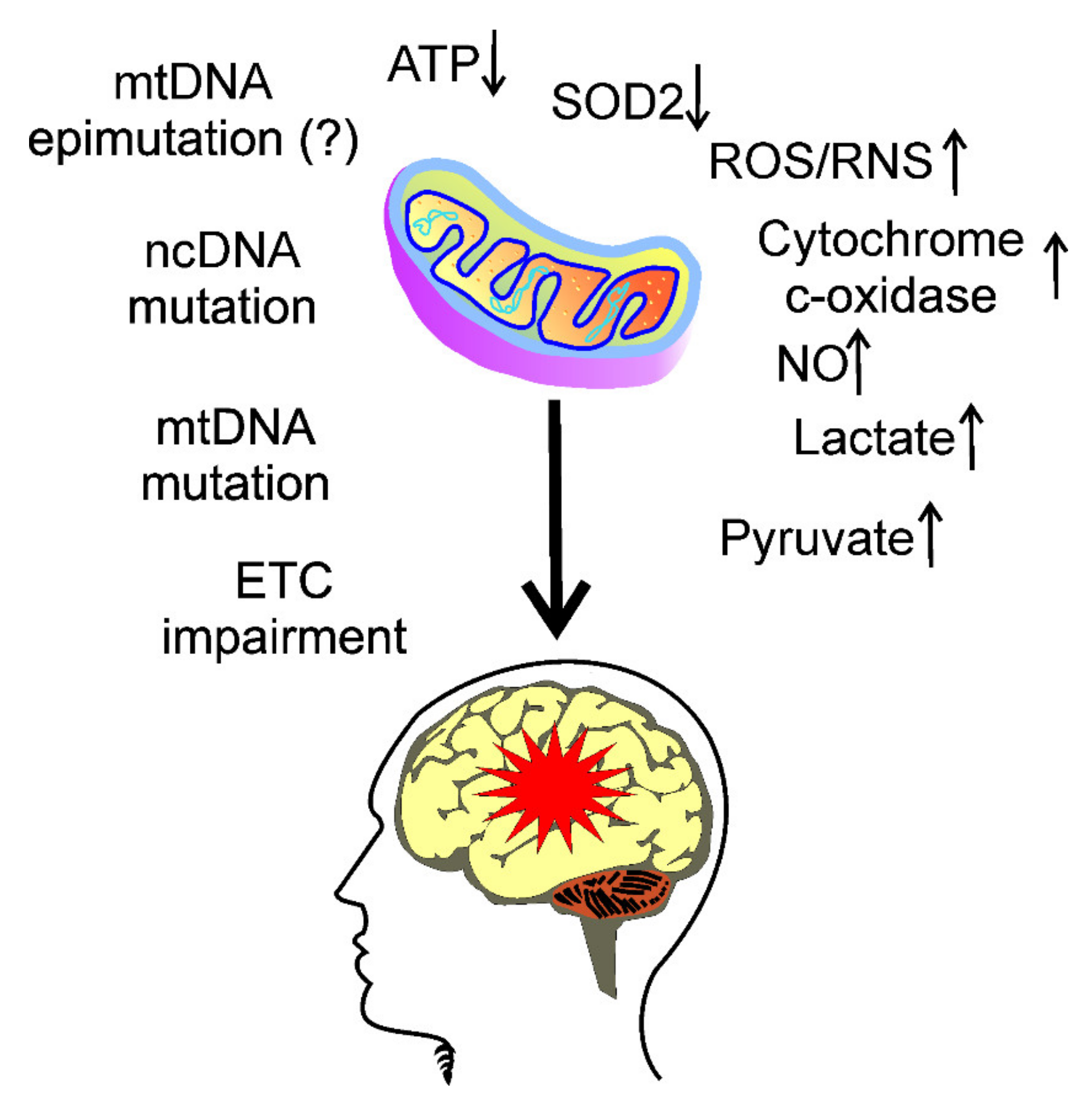
3. Mitochondria in Migraine Pathogenesis
4. Oxidative Stress in Migraine
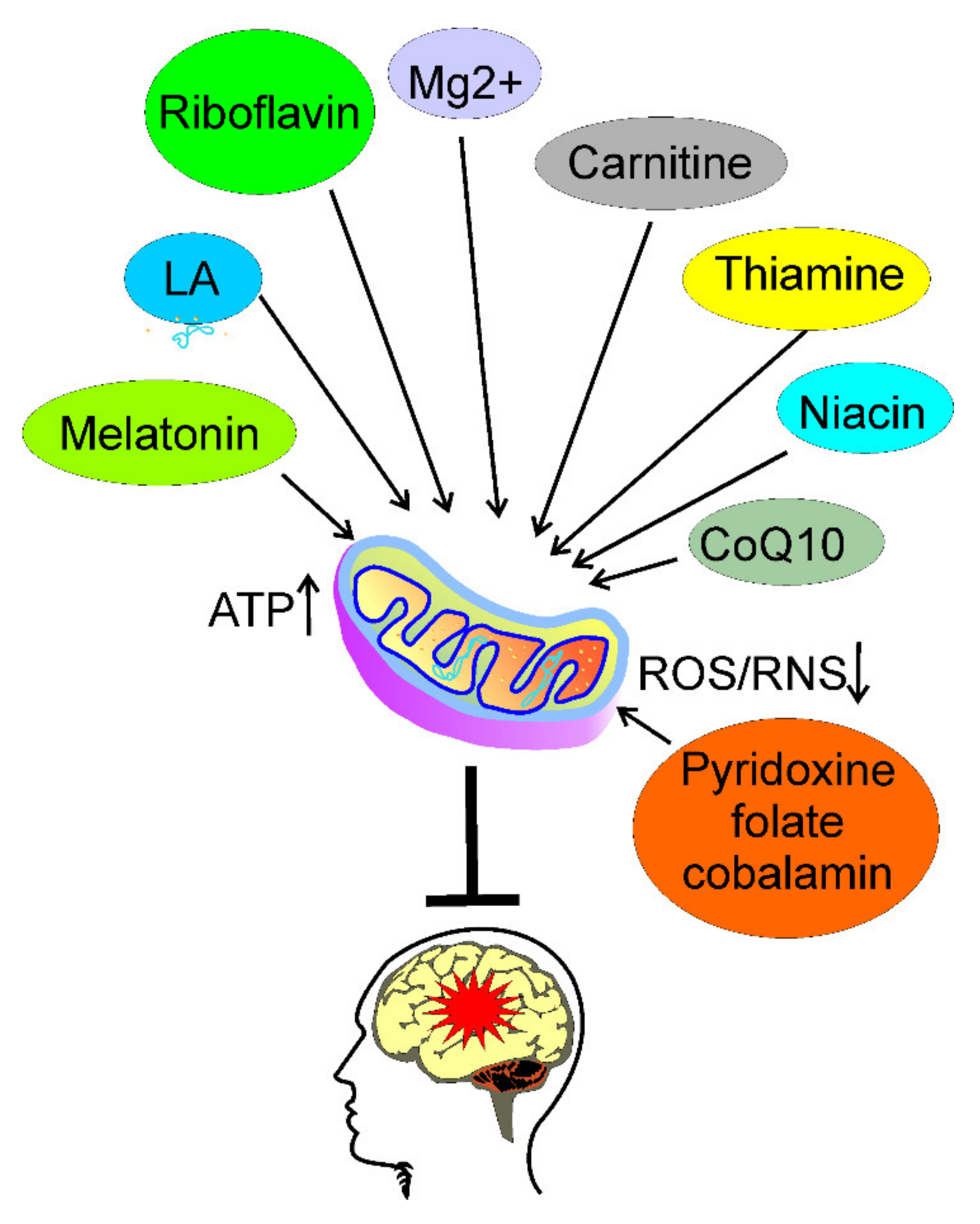
5. Usefulness of Nutrients Targeting Energy Production in Migraine Prevention
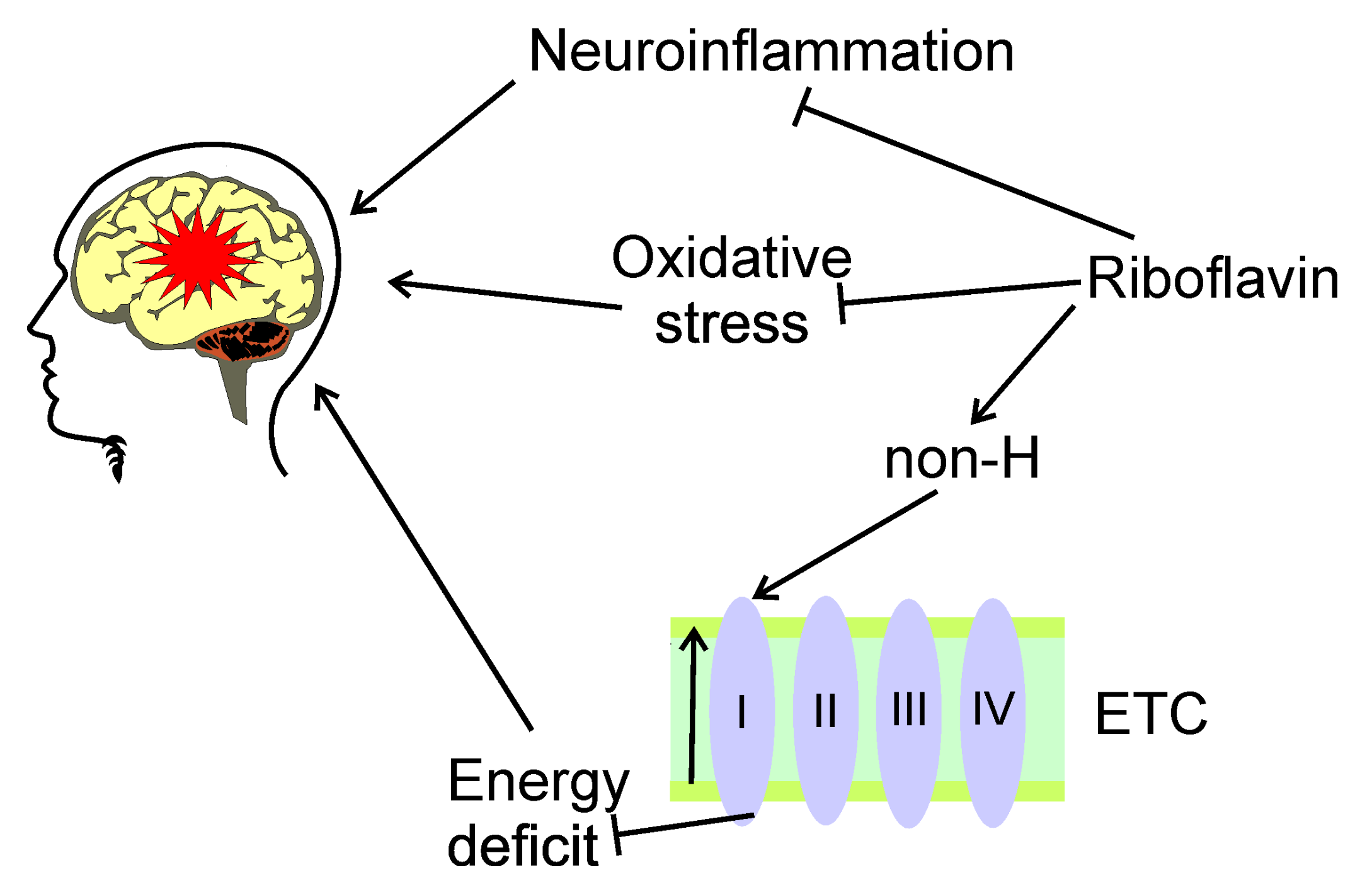
5.1. Riboflavin
5.2. Thiamine
5.3. Coenzyme Q10
5.4. Magnesium
5.5. Melatonin
5.6. Niacin
5.7. Carnitine
5.8. Lipoic Acid
5.9. Pyridoxine, Folate, and Cobalamin
5.10. Caffeine and Alcohol
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache. In StatPearls; StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W. The Ambiguous Role of Caffeine in Migraine Headache: From Trigger to Treatment. Nutrients 2020, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache 2016, 56, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. A link between migraine and prolactin: The way forward. Future Sci OA 2021, 7, Fso748. [Google Scholar] [CrossRef] [PubMed]
- Vollono, C.; Primiano, G.; Della Marca, G.; Losurdo, A.; Servidei, S. Migraine in mitochondrial disorders: Prevalence and characteristics. Cephalalgia Int. J. Headache 2018, 38, 1093–1106. [Google Scholar] [CrossRef]
- Fila, M.; Pawłowska, E.; Blasiak, J. Mitochondria in migraine pathophysiology—does epigenetics play a role? Arch. Med. Sci. AMS 2019, 15, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, P.; Duarte, J.M.N.; Schuck, P.F.; Ferreira, G.C. Mitochondria and the Brain: Bioenergetics and Beyond. Neurotox Res 2019, 36, 219–238. [Google Scholar] [CrossRef]
- Gray, P.A.; Burtness, H.I. HYPOGLYCEMIC HEADACHE*. Endocrinology 1935, 19, 549–560. [Google Scholar] [CrossRef]
- Barbiroli, B.; Montagna, P.; Cortelli, P.; Funicello, R.; Iotti, S.; Monari, L.; Pierangeli, G.; Zaniol, P.; Lugaresi, E. Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 1992, 42, 1209–1214. [Google Scholar] [CrossRef]
- Lodi, R.; Tonon, C.; Testa, C.; Manners, D.; Barbiroli, B. Energy metabolism in migraine. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2006, 27 (Suppl. 2), S82–S85. [Google Scholar] [CrossRef]
- Montagna, P.; Cortelli, P.; Barbiroli, B. Magnetic resonance spectroscopy studies in migraine. Cephalalgia Int. J. Headache 1994, 14, 184–193. [Google Scholar] [CrossRef]
- Montagna, P.; Cortelli, P.; Lodi, R.; Barbiroli, B. Magnetic resonance spectroscopy of episodic ataxia type 2 and migraine. Ann. Neurol. 2000, 47, 838–839. [Google Scholar] [CrossRef]
- Sacquegna, T.; Lodi, R.; De Carolis, P.; Tinuper, P.; Cortelli, P.; Zaniol, P.; Funicello, R.; Montagna, P.; Barbiroli, B. Brain energy metabolism studied by 31P-MR spectroscopy in a case of migraine with prolonged aura. Acta Neurol. Scand. 1992, 86, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility. Neurochem. Res. 2021, 46, 1913–1932. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Ghorbani, Z.; Martelletti, P.; Lampl, C.; Togha, M.; On behalf of the School of Advanced Studies of the European Headache Federation. Association of diet and headache. J. Headache Pain 2019, 20, 106. [Google Scholar] [CrossRef] [Green Version]
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. (Edinb. Scotl.) 2019, 38, 982–995. [Google Scholar] [CrossRef] [Green Version]
- Rajapakse, T.; Pringsheim, T. Nutraceuticals in Migraine: A Summary of Existing Guidelines for Use. Headache 2016, 56, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- May, A.; Schulte, L.H. Chronic migraine: Risk factors, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 455–464. [Google Scholar] [CrossRef]
- Sekhon, S.; Sharma, R.; Cascella, M. Thunderclap Headache. In StatPearls; StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Thomsen, A.V.; Sørensen, M.T.; Ashina, M.; Hougaard, A. Symptomatic migraine: A systematic review to establish a clinically important diagnostic entity. Headache 2021, 61, 1180–1193. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kekelidze, T.; Khait, I.; Togliatti, A.; Benzecry, J.M.; Wieringa, B.; Holtzman, D. Altered brain phosphocreatine and ATP regulation when mitochondrial creatine kinase is absent. J. Neurosci. Res. 2001, 66, 866–872. [Google Scholar] [CrossRef]
- Sonnay, S.; Gruetter, R.; Duarte, J.M.N. How Energy Metabolism Supports Cerebral Function: Insights from (13)C Magnetic Resonance Studies In vivo. Front. Neurosci. 2017, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younis, S.; Hougaard, A.; Vestergaard, M.B.; Larsson, H.B.W.; Ashina, M. Migraine and magnetic resonance spectroscopy: A systematic review. Curr. Opin. Neurol. 2017, 30, 246–262. [Google Scholar] [CrossRef]
- Lodi, R.; Iotti, S.; Cortelli, P.; Pierangeli, G.; Cevoli, S.; Clementi, V.; Soriani, S.; Montagna, P.; Barbiroli, B. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res. Bull. 2001, 54, 437–441. [Google Scholar] [CrossRef]
- Reyngoudt, H.; Paemeleire, K.; Descamps, B.; De Deene, Y.; Achten, E. 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia Int. J. Headache 2011, 31, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Reyngoudt, H.; Achten, E.; Paemeleire, K. Magnetic resonance spectroscopy in migraine: What have we learned so far? Cephalalgia Int. J. Headache 2012, 32, 845–859. [Google Scholar] [CrossRef] [Green Version]
- Auffenberg, E.; Hedrich, U.B.; Barbieri, R.; Miely, D.; Groschup, B.; Wuttke, T.V.; Vogel, N.; Lührs, P.; Zanardi, I.; Bertelli, S.; et al. Hyperexcitable interneurons trigger cortical spreading depression in an Scn1a migraine model. J. Clin. Investig. 2021, 131, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lisicki, M.; D’Ostilio, K.; Coppola, G.; Maertens de Noordhout, A.; Parisi, V.; Schoenen, J.; Magis, D. Brain Correlates of Single Trial Visual Evoked Potentials in Migraine: More Than Meets the Eye. Front Neurol 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; Magis, D.; Schoenen, J. Migraine--clinical neurophysiology. Handb. Clin. Neurol. 2010, 97, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, A.R.; Sandor, P.S.; Fritschy, J.; Turner, R.; Goadsby, P.J.; Kaube, H. Sensory information processing may be neuroenergetically more demanding in migraine patients. Neuroreport 2013, 24, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Kraya, T.; Deschauer, M.; Joshi, P.R.; Zierz, S.; Gaul, C. Prevalence of Headache in Patients With Mitochondrial Disease: A Cross-Sectional Study. Headache 2018, 58, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Almuqbil, M.; Rivkin, M.J.; Takeoka, M.; Yang, E.; Rodan, L.H. Transient regional cerebral hypoperfusion during a paroxysmal hemiplegic event in GLUT1 deficiency syndrome. Eur. J. Paediatr. Neurol. 2018, 22, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Arngrim, N.; Schytz, H.W.; Britze, J.; Amin, F.M.; Vestergaard, M.B.; Hougaard, A.; Wolfram, F.; de Koning, P.J.; Olsen, K.S.; Secher, N.H.; et al. Migraine induced by hypoxia: An MRI spectroscopy and angiography study. Brain 2016, 139, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Roberts, H.J. Migraine and related vascular headaches due to diabetogenic hyperinsulinism. Observations on pathogenesis and rational treatment in 421 patients. Headache 1967, 7, 41–62. [Google Scholar] [CrossRef]
- Kozai, D.; Ogawa, N.; Mori, Y. Redox regulation of transient receptor potential channels. Antioxid. Redox Signal. 2014, 21, 971–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals (Basel) 2019, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Henriques, B.J.; Katrine Jentoft Olsen, R.; Gomes, C.M.; Bross, P. Electron transfer flavoprotein and its role in mitochondrial energy metabolism in health and disease. Gene 2021, 776, 145407. [Google Scholar] [CrossRef]
- Yamanaka, G.; Suzuki, S.; Morishita, N.; Takeshita, M.; Kanou, K.; Takamatsu, T.; Morichi, S.; Ishida, Y.; Watanabe, Y.; Go, S.; et al. Experimental and Clinical Evidence of the Effectiveness of Riboflavin on Migraines. Nutrients 2021, 13, 2612. [Google Scholar] [CrossRef]
- Bron, C.; Sutherland, H.G.; Griffiths, L.R. Exploring the Hereditary Nature of Migraine. Neuropsychiatr. Dis. Treat. 2021, 17, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Yorns, W.R., Jr.; Hardison, H.H. Mitochondrial dysfunction in migraine. Semin. Pediatr. Neurol. 2013, 20, 188–193. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [Green Version]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.K. Hypoxia. 3. Hypoxia and neurotransmitter synthesis. Am. J. Physiol. Cell Physiol. 2011, 300, C743–C751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, E.C.; Putananickal, N.; Orsini, A.L.; Vogt, D.R.; Sandor, P.S.; Schoenen, J.; Fischer, D. Mitochondrial function and oxidative stress markers in higher-frequency episodic migraine. Sci. Rep. 2021, 11, 4543. [Google Scholar] [CrossRef]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef]
- Caminha, M.C.; Moreira, A.B.; Matheus, F.C.; Rieger, D.K.; Moreira, J.D.; Dalmarco, E.M.; Demarchi, I.G.; Lin, K. Efficacy and tolerability of the ketogenic diet and its variations for preventing migraine in adolescents and adults: A systematic review. Nutr. Rev. 2021. [Google Scholar] [CrossRef]
- Haslam, R.L.; Bezzina, A.; Herbert, J.; Spratt, N.; Rollo, M.E.; Collins, C.E. Can Ketogenic Diet Therapy Improve Migraine Frequency, Severity and Duration? Healthcare 2021, 9, 1105. [Google Scholar] [CrossRef]
- Kovács, Z.; Brunner, B.; Ari, C. Beneficial Effects of Exogenous Ketogenic Supplements on Aging Processes and Age-Related Neurodegenerative Diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef]
- Papetti, L.; Moavero, R.; Ferilli, M.A.N.; Sforza, G.; Tarantino, S.; Ursitti, F.; Ruscitto, C.; Vigevano, F.; Valeriani, M. Truths and Myths in Pediatric Migraine and Nutrition. Nutrients 2021, 13, 2714. [Google Scholar] [CrossRef]
- Qu, C.; Keijer, J.; Adjobo-Hermans, M.J.W.; van de Wal, M.; Schirris, T.; van Karnebeek, C.; Pan, Y.; Koopman, W.J.H. The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. Int. J. Biochem. Cell Biol. 2021, 138, 106050. [Google Scholar] [CrossRef] [PubMed]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Plantone, D.; Pardini, M.; Rinaldi, G. Riboflavin in Neurological Diseases: A Narrative Review. Clin. Drug Investig. 2021, 41, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J.; Jacquy, J.; Lenaerts, M. Effectiveness of high-dose riboflavin in migraine prophylaxis A randomized controlled trial. Neurology 1998, 50, 466–470. [Google Scholar] [CrossRef]
- Yee, A.J. Effectiveness of high-dose riboflavin in migraine prophylaxis. Neurology 1999, 52, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Pierelli, F.; Coppola, G.; Grieco, G.S.; Rengo, C.; Ciccolella, M.; Magis, D.; Bolla, M.; Casali, C.; Santorelli, F.M.; et al. Mitochondrial DNA haplogroups influence the therapeutic response to riboflavin in migraineurs. Neurology 2009, 72, 1588–1594. [Google Scholar] [CrossRef]
- Cutrer, F.M.; Charles, A. The neurogenic basis of migraine. Headache 2008, 48, 1411–1414. [Google Scholar] [CrossRef]
- Marashly, E.T.; Bohlega, S.A. Riboflavin Has Neuroprotective Potential: Focus on Parkinson’s Disease and Migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Nisco, A.; Barile, M. Alteration of Flavin Cofactor Homeostasis in Human Neuromuscular Pathologies. Methods Mol. Biol. (Clifton N.J.) 2021, 2280, 275–295. [Google Scholar] [CrossRef]
- Udhayabanu, T.; Manole, A.; Rajeshwari, M.; Varalakshmi, P.; Houlden, H.; Ashokkumar, B. Riboflavin Responsive Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Clin. Med. 2017, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Giancaspero, T.A.; Colella, M.; Brizio, C.; Difonzo, G.; Fiorino, G.M.; Leone, P.; Brandsch, R.; Bonomi, F.; Iametti, S.; Barile, M. Remaining challenges in cellular flavin cofactor homeostasis and flavoprotein biogenesis. Front. Chem. 2015, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Lee, G.S. Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity. Sci. Rep. 2020, 10, 19091. [Google Scholar] [CrossRef] [PubMed]
- Wiley, K.D.; Gupta, M. Vitamin B1 Thiamine Deficiency. In StatPearls; StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Braaf, M.M. Migraine headache treated successfully by head-traction manipulation and thiamin chloride. N. Y. State J. Med. 1949, 49, 1812–1816. [Google Scholar]
- Braaf, M.M. Migraine headache: An analysis of 124 cases treated by head-traction manipulation and thiamin chloride. N. Y. State J. Med. 1951, 51, 528–533. [Google Scholar]
- Subramanian, V.S.; Nabokina, S.M.; Lin-Moshier, Y.; Marchant, J.S.; Said, H.M. Mitochondrial uptake of thiamin pyrophosphate: Physiological and cell biological aspects. PLoS ONE 2013, 8, e73503. [Google Scholar] [CrossRef] [Green Version]
- Henssen, D.; Kluin, S.J.P.; Kleerebezem, J.; Van Cappellen van Walsum, A.M.; Mulleners, W.M.; Vissers, K. White matter changes in the trigeminal spinal tract in chronic migraineurs: An ex vivo study combining ultra-high field diffusion tensor imaging and polarized light imaging microscopy. Pain 2021. Publish ahead. [Google Scholar] [CrossRef]
- Antonio, C.; Massimo, T.; Gianpaolo, Z.; Immacolata, P.M.; Erika, T. Oral High-Dose Thiamine Improves the Symptoms of Chronic Cluster Headache. Case Rep. Neurol. Med. 2018, 2018, 3901619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, S.; Kumar Singh, A.; Rathore, C. Chronic Migraine Responding to Intravenous Thiamine: A Report of Two Cases. Headache 2016, 56, 1204–1209. [Google Scholar] [CrossRef]
- Faraji, H.; Paknahad, Z.; Chitsaz, A. Dietary Intake of Thiamine in Migraine Patients and Healthy Subjects: A Case-Control Study. Clin. Nutr. Res. 2018, 7, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Parker, W.D., Jr.; Haas, R.; Stumpf, D.A.; Parks, J.; Eguren, L.A.; Jackson, C. Brain mitochondrial metabolism in experimental thiamine deficiency. Neurology 1984, 34, 1477–1481. [Google Scholar] [CrossRef]
- Alcázar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Et Biophys. Acta 2016, 1857, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Haas, R.H.; Passov, D.; Beal, M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann. Neurol. 1997, 42, 261–264. [Google Scholar] [CrossRef]
- Pryde, K.R.; Hirst, J. Superoxide Is Produced by the Reduced Flavin in Mitochondrial Complex I: A single, unified mechanism that applies during both forward and reverse electron transfer*. J. Biol. Chem. 2011, 286, 18056–18065. [Google Scholar] [CrossRef] [Green Version]
- Littarru, G.P.; Tiano, L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T.; Sengupta, P.; Dutta, S.; Calogero, A.E. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin. Exp. Reprod. Med. 2021, 48, 150–155. [Google Scholar] [CrossRef]
- Luo, K.; Yu, J.H.; Quan, Y.; Shin, Y.J.; Lee, K.E.; Kim, H.L.; Ko, E.J.; Chung, B.H.; Lim, S.W.; Yang, C.W. Therapeutic potential of coenzyme Q(10) in mitochondrial dysfunction during tacrolimus-induced beta cell injury. Sci. Rep. 2019, 9, 7995. [Google Scholar] [CrossRef]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Effect of coenzyme Q10 supplementation on clinical features of migraine: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Neurosci. 2020, 23, 868–875. [Google Scholar] [CrossRef]
- Sazali, S.; Badrin, S.; Norhayati, M.N.; Idris, N.S. Coenzyme Q10 supplementation for prophylaxis in adult patients with migraine-a meta-analysis. BMJ Open 2021, 11, e039358. [Google Scholar] [CrossRef]
- Hajihashemi, P.; Askari, G.; Khorvash, F.; Reza Maracy, M.; Nourian, M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia Int. J. Headache 2019, 39, 648–654. [Google Scholar] [CrossRef]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Foroushani, A.R.; Ranji-Burachaloo, S.; Djalali, M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Nutr. Neurosci. 2021, 24, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Rozen, T.D.; Oshinsky, M.L.; Gebeline, C.A.; Bradley, K.C.; Young, W.B.; Shechter, A.L.; Silberstein, S.D. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia Int. J. Headache 2002, 22, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Li, Y.; Lu, S.; Huang, W.; Di, W. Efficacy of CoQ10 as supplementation for migraine: A meta-analysis. Acta Neurol. Scand. 2019, 139, 284–293. [Google Scholar] [CrossRef]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. In StatPearls; StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shin, H.J.; Na, H.S.; Do, S.H. Magnesium and Pain. Nutrients 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Crivellaro, G.; Tottene, A.; Vitale, M.; Melone, M.; Casari, G.; Conti, F.; Santello, M.; Pietrobon, D. Specific activation of GluN1-N2B NMDA receptors underlies facilitation of cortical spreading depression in a genetic mouse model of migraine with reduced astrocytic glutamate clearance. Neurobiol. Dis. 2021, 156, 105419. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ajona, D.; Villar-Martínez, M.D.; Goadsby, P.J. Targets for migraine treatment: Beyond calcitonin gene-related peptide. Curr. Opin. Neurol. 2021, 34, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lingam, I.; Robertson, N.J. Magnesium as a Neuroprotective Agent: A Review of Its Use in the Fetus, Term Infant with Neonatal Encephalopathy, and the Adult Stroke Patient. Dev. Neurosci. 2018, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Pickering, G.; Giacomoni, E.; Cazzaniga, A.; Pellegrino, P. Headaches and Magnesium: Mechanisms, Bioavailability, Therapeutic Efficacy and Potential Advantage of Magnesium Pidolate. Nutrients 2020, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Myrdal, U.; Leppert, J.; Edvinsson, L.; Ekman, R.; Hedner, T.; Nilsson, H.; Ringqvist, I. Magnesium sulphate infusion decreases circulating calcitonin gene-related peptide (CGRP) in women with primary Raynaud’s phenomenon. Clin. Physiol. 1994, 14, 539–546. [Google Scholar] [CrossRef]
- Sun-Edelstein, C.; Mauskop, A. Role of magnesium in the pathogenesis and treatment of migraine. Expert Rev. Neurother. 2009, 9, 369–379. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Nyári, A.; Vécsei, L. CGRP and CGRP-receptor as targets of migraine therapy: Brain Prize-2021. CNS Neurol. Disord. Drug Targets 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Bhattacharjee, M.; Islam, M.S.; Banerjee, S.; Hossain, S.; Hossain, M.I.; Haidar, M.R. Relation between Serum Magnesium Level and Migraine. Mymensingh Med. J. 2021, 30, 301–306. [Google Scholar]
- Vatzaki, E.; Straus, S.; Dogne, J.M.; Garcia Burgos, J.; Girard, T.; Martelletti, P. Latest clinical recommendations on valproate use for migraine prophylaxis in women of childbearing age: Overview from European Medicines Agency and European Headache Federation. J. Headache Pain 2018, 19, 68. [Google Scholar] [CrossRef]
- Khani, S.; Hejazi, S.A.; Yaghoubi, M.; Sharifipour, E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: A randomized controlled double-blind trial. J. Headache Pain 2021, 22, 21. [Google Scholar] [CrossRef]
- Witkowski, M.; Hubert, J.; Mazur, A. Methods of assessment of magnesium status in humans: A systematic review. Magnes. Res. 2011, 24, 163–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trauninger, A.; Pfund, Z.; Koszegi, T.; Czopf, J. Oral magnesium load test in patients with migraine. Headache 2002, 42, 114–119. [Google Scholar] [CrossRef]
- Jain, A.C.; Sethi, N.C.; Balbar, P.K. A clinical electroencephalographic and trace element study with special reference to zinc, copper and magnesium in serum and cerebrospinal fluid (CSF) in cases of migraine. J. Neurol. 1985, 232, S161. [Google Scholar]
- Ramadan, N.M.; Halvorson, H.; Vande-Linde, A.; Levine, S.R.; Helpern, J.A.; Welch, K.M. Low brain magnesium in migraine. Headache 1989, 29, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Samaie, A.; Asghari, N.; Ghorbani, R.; Arda, J. Blood Magnesium levels in migraineurs within and between the headache attacks: A case control study. Pan Afr. Med. J. 2012, 11, 46. [Google Scholar]
- Barbiroli, B.; Iotti, S.; Cortelli, P.; Martinelli, P.; Lodi, R.; Carelli, V.; Montagna, P. Low brain intracellular free magnesium in mitochondrial cytopathies. J Cereb. Blood Flow Metab. 1999, 19, 528–532. [Google Scholar] [CrossRef]
- Vikelis, M.; Dermitzakis, E.V.; Vlachos, G.S.; Soldatos, P.; Spingos, K.C.; Litsardopoulos, P.; Kararizou, E.; Argyriou, A.A. Open Label Prospective Experience of Supplementation with a Fixed Combination of Magnesium, Vitamin B2, Feverfew, Andrographis Paniculata and Coenzyme Q10 for Episodic Migraine Prophylaxis. J. Clin. Med. 2020, 10, 67. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Song, T.J.; Kim, B.S.; Chu, M.K. Therapeutic role of melatonin in migraine prophylaxis: Is there a link between sleep and migraine? Prog. Brain Res. 2020, 255, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.B.W.; Davis-Martin, R.E.; Houle, T.T.; Turner, D.P.; Smitherman, T.A. Perceived triggers of primary headache disorders: A meta-analysis. Cephalalgia Int. J. Headache 2018, 38, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, R.J.; Stacpoole, P.W. Sleep disorders associated with primary mitochondrial diseases. J. Clin. Sleep Med. 2014, 10, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in Mitochondria: Mitigating Clear and Present Dangers. Physiology (Bethesda) 2020, 35, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Alstadhaug, K.B.; Odeh, F.; Salvesen, R.; Bekkelund, S.I. Prophylaxis of migraine with melatonin: A randomized controlled trial. Neurology 2010, 75, 1527–1532. [Google Scholar] [CrossRef]
- Ebrahimi-Monfared, M.; Sharafkhah, M.; Abdolrazaghnejad, A.; Mohammadbeigi, A.; Faraji, F. Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial. Restor Neurol. Neurosci. 2017, 35, 385–393. [Google Scholar] [CrossRef]
- Fallah, R.; Fazelishoroki, F.; Sekhavat, L. A Randomized Clinical Trial Comparing the Efficacy of Melatonin and Amitriptyline in Migraine Prophylaxis of Children. Iran J. Child Neurol. 2018, 12, 47–54. [Google Scholar]
- Gonçalves, A.L.; Martini Ferreira, A.; Ribeiro, R.T.; Zukerman, E.; Cipolla-Neto, J.; Peres, M.F. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Bougea, A.; Spantideas, N.; Lyras, V.; Avramidis, T.; Thomaidis, T. Melatonin 4 mg as prophylactic therapy for primary headaches: A pilot study. Funct. Neurol. 2016, 31, 33–37. [Google Scholar] [CrossRef]
- Peres, M.F.; Zukerman, E.; da Cunha Tanuri, F.; Moreira, F.R.; Cipolla-Neto, J. Melatonin, 3 mg, is effective for migraine prevention. Neurology 2004, 63, 757. [Google Scholar] [CrossRef]
- Plasencia-García, B.O.; Romero-Guillena, S.L.; Quirós-López, A.; Ruiz-Doblado, S. Agomelatine and migraine management: A successfully treated case series. Adv. Psychopharmacol. 2015, 5, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Long, R.; Zhu, Y.; Zhou, S. Therapeutic role of melatonin in migraine prophylaxis: A systematic review. Medicine 2019, 98, e14099. [Google Scholar] [CrossRef]
- Kirkland, J.B.; Meyer-Ficca, M.L. Niacin. Adv. Food Nutr. Res. 2018, 83, 83–149. [Google Scholar] [CrossRef]
- Pirinen, E.; Auranen, M.; Khan, N.A.; Brilhante, V.; Urho, N.; Pessia, A.; Hakkarainen, A.; Kuula, J.; Heinonen, U.; Schmidt, M.S.; et al. Niacin Cures Systemic NAD(+) Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020, 31, 1078–1090.e1075. [Google Scholar] [CrossRef]
- Velling, D.A.; Dodick, D.W.; Muir, J.J. Sustained-release niacin for prevention of migraine headache. Mayo Clin. Proc. 2003, 78, 770–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giniatullin, R. Serotonin in migraine: The puzzling role of ionotropic 5-HT(3) receptor in the context of established therapeutic effect of metabotropic 5-HT(1) subtypes. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T.; Shibata, K. Nutritional aspect of tryptophan metabolism. Int. J. Tryptophan Res. 2013, 6, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Gedye, A. Hypothesized treatment for migraines using low doses of tryptophan, niacin, calcium, caffeine, and acetylsalicylic acid. Med. Hypotheses 2001, 56, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Prousky, J.; Seely, D. The treatment of migraines and tension-type headaches with intravenous and oral niacin (nicotinic acid): Systematic review of the literature. Nutr. J. 2005, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Mason, B.N.; Russo, A.F. Vascular Contributions to Migraine: Time to Revisit? Front. Cell Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Scaglia, F. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 2015, 116, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tarighat Esfanjani, A.; Mahdavi, R.; Ebrahimi Mameghani, M.; Talebi, M.; Nikniaz, Z.; Safaiyan, A. The effects of magnesium, L-carnitine, and concurrent magnesium-L-carnitine supplementation in migraine prophylaxis. Biol. Trace Elem. Res. 2012, 150, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.; Brenner, E.; Linde, M.; Gravdahl, G.B.; Tronvik, E.A.; Engstrøm, M.; Sonnewald, U.; Helde, G.; Stovner, L.J.; Sand, T. Acetyl-l-carnitine versus placebo for migraine prophylaxis: A randomized, triple-blind, crossover study. Cephalalgia Int. J. Headache 2015, 35, 987–995. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Charleston, L.t.; Khalil, S.; Young, W.B. Carnitine Responsive Migraine Headache Syndrome: Case Report and Review of the Literature. Curr. Pain Headache Rep. 2021, 25, 26. [Google Scholar] [CrossRef]
- Liu, J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: An overview. Neurochem. Res. 2008, 33, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.L.; Vohra, H.; Zhang, Y.; Sutton, M.; Whitworth, J.A. The effect of alpha-lipoic acid on mitochondrial superoxide and glucocorticoid-induced hypertension. Oxidative Med. Cell. Longev. 2013, 2013, 517045. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Awad, T.G.; Al-Adl, N.M. Efficacy of combined topiramate/thioctic acid therapy in migraine prophylaxis. Saudi Pharm. J. 2010, 18, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Cavestro, C.; Bedogni, G.; Molinari, F.; Mandrino, S.; Rota, E.; Frigeri, M.C. Alpha-Lipoic Acid Shows Promise to Improve Migraine in Patients with Insulin Resistance: A 6-Month Exploratory Study. J. Med. Food 2018, 21, 269–273. [Google Scholar] [CrossRef]
- Magis, D.; Ambrosini, A.; Sándor, P.; Jacquy, J.; Laloux, P.; Schoenen, J. A randomized double-blind placebo-controlled trial of thioctic acid in migraine prophylaxis. Headache 2007, 47, 52–57. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Xu, Y.; Ma, D.; Wang, M. The Transient Receptor Potential Ankyrin Type 1 Plays a Critical Role in Cortical Spreading Depression. Neuroscience 2018, 382, 23–34. [Google Scholar] [CrossRef]
- Rezaei Kelishadi, M.; Alavi Naeini, A.; Askari, G.; Khorvash, F.; Heidari, Z. The efficacy of alpha-lipoic acid in improving oxidative, inflammatory, and mood status in women with episodic migraine in a randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14455. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Huang, Z.; Deutschman, C.S.; Levy, R.J. Caffeine restores myocardial cytochrome oxidase activity and improves cardiac function during sepsis. Crit. Care Med. 2009, 37, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Buse, D.C.; Reed, M.L.; Fanning, K.M.; Bostic, R.; Dodick, D.W.; Schwedt, T.J.; Munjal, S.; Singh, P.; Lipton, R.B. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: Results of the migraine in America symptoms and treatment (MAST) study. J. Headache Pain 2020, 21, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, N.; Huang, L.; Hong, J.; Zhao, X.; Chen, Y.; Hu, J.; Cong, X.; Xie, Y.; Pu, J. Elevated homocysteine levels in patients with heart failure: A systematic review and meta-analysis. Medicine 2021, 100, e26875. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine—from disease biomarker to disease prevention. .J Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Meschi, T.; Cervellin, G.; Borghi, L. Homocysteine and migraine. A narrative review. Clin. Chim. Acta 2014, 433, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.; Sechi, E.; Fois, C.; Kumar, N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr. Rev. 2016, 74, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Fila, M.; Chojnacki, C.; Chojnacki, J.; Blasiak, J. Is an "Epigenetic Diet" for Migraines Justified? The Case of Folate and DNA Methylation. Nutrients 2019, 11, 2763. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.K.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef]
- Sadeghi, O.; Maghsoudi, Z.; Askari, G.; Khorvash, F.; Feizi, A. Association between serum levels of homocysteine with characteristics of migraine attacks in migraine with aura. J. Res. Med Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 1041–1045. [Google Scholar]
- Christen, W.G.; Cook, N.R.; Van Denburgh, M.; Zaharris, E.; Albert, C.M.; Manson, J.E. Effect of Combined Treatment With Folic Acid, Vitamin B(6), and Vitamin B(12) on Plasma Biomarkers of Inflammation and Endothelial Dysfunction in Women. J. Am. Heart Assoc. 2018, 7, e008517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusitanond, P.; Eikelboom, J.W.; Hankey, G.J.; Thom, J.; Gilmore, G.; Loh, K.; Yi, Q.; Klijn, C.J.; Langton, P.; van Bockxmeer, F.M.; et al. Homocysteine-lowering treatment with folic acid, cobalamin, and pyridoxine does not reduce blood markers of inflammation, endothelial dysfunction, or hypercoagulability in patients with previous transient ischemic attack or stroke: A randomized substudy of the VITATOPS trial. Stroke 2005, 36, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Eikelboom, J.W.; Loh, K.; Tang, M.; Pizzi, J.; Thom, J.; Yi, Q. Sustained homocysteine-lowering effect over time of folic acid-based multivitamin therapy in stroke patients despite increasing folate status in the population. Cerebrovasc. Dis. 2005, 19, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O'Brien, P.J. Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways. Chem. -Biol. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, O.; Nasiri, M.; Maghsoudi, Z.; Pahlavani, N.; Rezaie, M.; Askari, G. Effects of pyridoxine supplementation on severity, frequency and duration of migraine attacks in migraine patients with aura: A double-blind randomized clinical trial study in Iran. Iran J. Neurol. 2015, 14, 74–80. [Google Scholar]
- Askari, G.; Nasiri, M.; Mozaffari-Khosravi, H.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The effects of folic acid and pyridoxine supplementation on characteristics of migraine attacks in migraine patients with aura: A double-blind, randomized placebo-controlled, clinical trial. Nutrition 2017, 38, 74–79. [Google Scholar] [CrossRef]
- Lea, R.; Colson, N.; Quinlan, S.; Macmillan, J.; Griffiths, L. The effects of vitamin supplementation and MTHFR (C677T) genotype on homocysteine-lowering and migraine disability. Pharm. Genom. 2009, 19, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yu, Y.; He, J.; Guo, L.; Li, H.; Teng, J. Effects of MTHFR C677T and A1298C Polymorphisms on Migraine Susceptibility: A Meta-Analysis of 26 Studies. Headache 2019, 59, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Nasir, B.; Avgan, N.; Ghassabian, S.; Oliver, C.; Lea, R.; Smith, M.; Griffiths, L. The effect of 1 mg folic acid supplementation on clinical outcomes in female migraine with aura patients. J. Headache Pain 2016, 17, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togha, M.; Razeghi Jahromi, S.; Ghorbani, Z.; Martami, F.; Seifishahpar, M. Serum Vitamin B12 and Methylmalonic Acid Status in Migraineurs: A Case-Control Study. Headache 2019, 59, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Mayans, L. Headache: Migraine. FP Essent 2018, 473, 11–16. [Google Scholar] [PubMed]
- Onderwater, G.L.J.; van Oosterhout, W.P.J.; Schoonman, G.G.; Ferrari, M.D.; Terwindt, G.M. Alcoholic beverages as trigger factor and the effect on alcohol consumption behavior in patients with migraine. Eur. J. Neurol. 2019, 26, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.D.; Padmavathi, P.; Kavitha, G.; Saradamma, B.; Varadacharyulu, N. Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties. Mol. Cell Biochem. 2013, 375, 39–47. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Do, T.P.; Al-Saoudi, A.; Ashina, M. Future prophylactic treatments in migraine: Beyond anti-CGRP monoclonal antibodies and gepants. Rev. Neurol. (Paris) 2021, 177, 827–833. [Google Scholar] [CrossRef]
- Monteith, T.S.; Goadsby, P.J. Acute migraine therapy: New drugs and new approaches. Curr. Treat. Options Neurol. 2011, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT(1) receptor agonists effective anti-migraine drugs? Expert Opin. Pharm. 2021, 22, 1221–1225. [Google Scholar] [CrossRef]
- Assas, B.M. Anti-migraine agents from an immunological point of view. J. Transl. Med. 2021, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Deen, M.; Correnti, E.; Kamm, K.; Kelderman, T.; Papetti, L.; Rubio-Beltrán, E.; Vigneri, S.; Edvinsson, L.; Maassen Van Den Brink, A.; On behalf of the European Headache Federation School of Advanced Studies (EHF-SAS). Blocking CGRP in migraine patients—A review of pros and cons. J. Headache Pain 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loder, E.W.; Burch, R.C. Who should try new antibody treatments for migraine? JAMA Neurol. 2018, 75, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fila, M.; Chojnacki, C.; Chojnacki, J.; Blasiak, J. Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine. Nutrients 2021, 13, 4433. https://doi.org/10.3390/nu13124433
Fila M, Chojnacki C, Chojnacki J, Blasiak J. Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine. Nutrients. 2021; 13(12):4433. https://doi.org/10.3390/nu13124433
Chicago/Turabian StyleFila, Michal, Cezary Chojnacki, Jan Chojnacki, and Janusz Blasiak. 2021. "Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine" Nutrients 13, no. 12: 4433. https://doi.org/10.3390/nu13124433
APA StyleFila, M., Chojnacki, C., Chojnacki, J., & Blasiak, J. (2021). Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine. Nutrients, 13(12), 4433. https://doi.org/10.3390/nu13124433






