Transdiagnostic Perspective of Impulsivity and Compulsivity in Obesity: From Cognitive Profile to Self-Reported Dimensions in Clinical Samples with and without Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Psychometric Measures
2.3. Neuropsychological Measures
2.4. Procedure
2.5. Statistical Analysis
3. Results
3.1. Descriptive for the Sample
3.2. Comparison of Impulsivity and Compulsivity Measures
3.3. Comparison of Psychological State
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- De Gonzalez, A.B.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar]
- Ferrulli, A. Obesity: Classification and Diagnosis. In Thyroid, Obesity and Metabolism; Springer: Cham, Switzerland, 2021; pp. 73–93. [Google Scholar]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Heymsfield, S.; Wadden, T. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 1490–1492. [Google Scholar] [CrossRef]
- Wenzel, J.M.; Cheer, J.F. Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling. Neuropsychopharmacology 2018, 43, 103–115. [Google Scholar]
- Amin, T.; Mercer, J.G. Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake. Curr. Obes. Rep. 2016, 5, 106–112. [Google Scholar]
- Small, D.M.; DiFeliceantonio, A.G. Neuroscience: Processed foods and food reward. Science 2019, 363, 346–347. [Google Scholar]
- Cocores, J.A.; Gold, M.S. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med. Hypotheses 2009, 73, 892–899. [Google Scholar] [CrossRef]
- Steward, T.; Miranda-Olivos, R.; Soriano-Mas, C.; Fernández-Aranda, F. Neuroendocrinological mechanisms underlying impulsive and compulsive behaviors in obesity: A narrative review of fMRI studies. Rev. Endocr. Metab. Disord. 2019, 20, 263–272. [Google Scholar]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping neural endophenotypes in addiction and obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar]
- Verdejo-García, A.; Lozano, Ó.; Moya, M.; Alcázar, M.Á.; Pérez-García, M. Psychometric properties of a spanish version of the UPPS-P impulsive behavior scale: Reliability, validity and association with trait and cognitive impulsivity. J. Pers. Assess. 2010, 92, 70–77. [Google Scholar] [CrossRef]
- Whiteside, S.P.; Lynam, D.R. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Pers. Individ. Dif. 2001, 30, 669–689. [Google Scholar] [CrossRef]
- Hamilton, K.R.; Littlefield, A.K.; Anastasio, N.C.; Cunningham, K.A.; Fink, L.H.L.; Wing, V.C.; Mathias, C.W.; Lane, S.D.; Schütz, C.G.; Swann, A.C.; et al. Rapid-response impulsivity: Definitions, measurement issues, and clinical implications. Pers. Disord. Theory Res. Treat. 2015, 6, 168–181. [Google Scholar] [CrossRef]
- Hamilton, K.R.; Mitchell, M.R.; Wing, V.C.; Balodis, I.M.; Bickel, W.K.; Fillmore, M.; Lane, S.D.; Lejuez, C.W.; Littlefield, A.K.; Luijten, M.; et al. Choice impulsivity: Definitions, measurement issues, and clinical implications. Pers. Disord. Theory Res. Treat. 2015, 6, 182–198. [Google Scholar] [CrossRef]
- de Water, E.; Mies, G.W.; Figner, B.; Yoncheva, Y.; van den Bos, W.; Castellanos, F.X.; Cillessen, A.H.N.; Scheres, A. Neural mechanisms of individual differences in temporal discounting of monetary and primary rewards in adolescents. Neuroimage 2017, 153, 198–210. [Google Scholar] [CrossRef]
- Robbins, T.; Curran, H.; De Wit, H. Special issue on impulsivity and compulsivity. Psychopharmacology 2012, 219, 251–252. [Google Scholar]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 2017, 42, 1375–1389. [Google Scholar]
- Armbruster, D.J.N.; Ueltzhöffer, K.; Basten, U.; Fiebach, C.J. Prefrontal cortical mechanisms underlying individual differences in cognitive flexibility and stability. J. Cogn. Neurosci. 2012, 24, 2385–2399. [Google Scholar] [CrossRef]
- Dajani, D.R.; Uddin, L.Q. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci. 2015, 38, 571–578. [Google Scholar]
- Widiger, T.A.; Samuel, D.B. Diagnostic categories or dimensions? A question for the Diagnostic and Statistical Manual of Mental Disorders—Fifth edition. J. Abnorm. Psychol. 2005, 114, 494–504. [Google Scholar] [CrossRef]
- Bornstein, R.F.; Natoli, A.P. Clinical utility of categorical and dimensional perspectives on personality pathology: A meta-analytic review. Pers. Disord. Theory Res. Treat. 2019, 10, 479–490. [Google Scholar] [CrossRef]
- Bottesi, G.; Ghisi, M.; Ouimet, A.J.; Tira, M.D.; Sanavio, E. Compulsivity and impulsivity in pathological gambling: Does a dimensional-transdiagnostic approach add clinical utility to DSM-5 classification? J. Gambl. Stud. 2015, 31, 825–847. [Google Scholar] [CrossRef]
- Winstanley, C.A.; Eagle, D.M.; Robbins, T.W. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin. Psychol. Rev. 2006, 26, 379–395. [Google Scholar] [CrossRef]
- Peters, J.R.; Upton, B.T.; Baer, R.A. Brief Report: Relationships Between Facets of Impulsivity and Borderline Personality Features. J. Pers. Disord. 2013, 27, 547–552. [Google Scholar] [CrossRef]
- Lavender, J.M.; Goodman, E.L.; Culbert, K.M.; Wonderlich, S.A.; Crosby, R.D.; Engel, S.G.; Mitchell, J.E.; Le Grange, D.; Crow, S.J.; Peterson, C.B. Facets of Impulsivity and Compulsivity in Women with Anorexia Nervosa. Eur. Eat. Disord. Rev. 2017, 25, 309–313. [Google Scholar] [CrossRef]
- Fineberg, N.A.; Apergis-Schoute, A.M.; Vaghi, M.M.; Banca, P.; Gillan, C.M.; Voon, V.; Chamberlain, S.R.; Cinosi, E.; Reid, J.; Shahper, S.; et al. Mapping Compulsivity in the DSM-5 Obsessive Compulsive and Related Disorders: Cognitive Domains, Neural Circuitry, and Treatment. Int. J. Neuropsychopharmacol. 2018, 21, 42–58. [Google Scholar] [CrossRef]
- Mobbs, O.; Crépin, C.; Thiéry, C.; Golay, A.; Van der Linden, M. Obesity and the four facets of impulsivity. Patient Educ. Couns. 2010, 79, 372–377. [Google Scholar] [CrossRef]
- Murphy, C.M.; Stojek, M.K.; MacKillop, J. Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite 2014, 73, 45–50. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar]
- VanderBroek-Stice, L.; Stojek, M.K.; Beach, S.R.H.; VanDellen, M.R.; MacKillop, J. Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite 2017, 112, 59–68. [Google Scholar] [CrossRef]
- Fagundo, A.B.; de la Torre, R.; Jiménez-Murcia, S.; Agüera, Z.; Granero, R.; Tárrega, S.; Botella, C.; Baños, R.; Fernández-Real, J.M.; Rodríguez, R.; et al. Executive functions profile in extreme eating/weight conditions: From Anorexia Nervosa to Obesity. PLoS ONE 2012, 7, e43382. [Google Scholar] [CrossRef]
- Wu, M.; Brockmeyer, T.; Hartmann, M.; Skunde, M.; Herzog, W.; Friederich, H.C. Set-shifting ability across the spectrum of eating disorders and in overweight and obesity: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 3365–3385. [Google Scholar]
- Fineberg, N.A.; Chamberlain, S.R.; Goudriaan, A.E.; Stein, D.J.; Vanderschuren, L.J.M.J.; Gillan, C.M.; Shekar, S.; Gorwood, P.A.P.M.; Voon, V.; Morein-Zamir, S.; et al. New developments in human neurocognition: Clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014, 19, 69–89. [Google Scholar]
- Munguía, L.; Lucas, I.; Jiménez-Murcia, S.; Mora-Maltas, B.; Granero, R.; Miranda-Olivos, R.; Sánchez, I.; Testa, G.; Lozano-Madrid, M.; Turton, R.; et al. Executive functions in binge spectrum eating disorders with comorbid compulsive buying. Eur. Eat. Disord. Rev. 2021, 29, 854–867. [Google Scholar] [CrossRef]
- Petry, N.M.; Barry, D.; Pietrzak, R.H.; Wagner, J.A. Overweight and obesity are associated with psychiatric disorders: Results from the national epidemiologic survey on alcohol and related conditions. Psychosom. Med. 2008, 70, 288–297. [Google Scholar] [CrossRef]
- Pavan, C.; Azzi, M.; Lancerotto, L.; Marini, M.; Busetto, L.; Bassetto, F.; Vindigni, V. Overweight/obese patients referring to plastic surgery: Temperament and personality traits. Obes. Surg. 2013, 23, 437–445. [Google Scholar] [CrossRef]
- Fassino, S.; Leombruni, P.; Pierò, A.; Abbate Daga, G.; Amianto, F.; Rovera, G.; Rovera, G.G. Temperament and character in obese women with and without binge eating disorder. Compr. Psychiatry 2002, 43, 431–437. [Google Scholar] [CrossRef]
- López-Pantoja, J.L.; Cabranes, J.A.; Sanchez-Quintero, S.; Velao, M.; Sanz, M.; Torres-Pardo, B.; Ancín, I.; Cabrerizo, L.; Rubio, M.A.; Lopez-lbor, J.J.; et al. Personality profiles between obese and control subjects assessed with five standardized personality scales. Actas Esp. Psiquiatr. 2012, 40, 266–274. [Google Scholar]
- Sarisoy, G.; Atmaca, A.; Ecemiş, G.; Gümüş, K.; Pazvantoǧlu, O. Personality characteristics and body image in obese individuals. Asia-Pac. Psychiatry 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Villarejo, C.; Jiménez-Murcia, S.; Álvarez-Moya, E.; Granero, R.; Penelo, E.; Treasure, J.; Vilarrasa, N.; Gil-Montserrat De Bernabé, M.; Casanueva, F.F.; Tinahones, F.J.; et al. Loss of control over eating: A description of the eating disorder/obesity spectrum in women. Eur. Eat. Disord. Rev. 2014, 22, 25–31. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar]
- Schoffelmeer, A.N.M.; Drukarch, B.; De Vries, T.J.; Hogenboom, F.; Schetters, D.; Pattij, T. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J. Neurosci. 2011, 31, 1284–1291. [Google Scholar] [CrossRef]
- Könner, A.C.; Hess, S.; Tovar, S.; Mesaros, A.; Sánchez-Lasheras, C.; Evers, N.; Verhagen, L.A.W.; Brönneke, H.S.; Kleinridders, A.; Hampel, B.; et al. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 2011, 13, 720–728. [Google Scholar] [CrossRef]
- Sevak, R.J.; Koek, W.; Galli, A.; France, C.P. Insulin replacement restores the behavioral effects of quinpirole and raclopride in streptozotocin-treated rats. J. Pharmacol. Exp. Ther. 2007, 320, 1216–1223. [Google Scholar] [CrossRef]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA. 2015, 112, 3463–3468. [Google Scholar] [CrossRef]
- Speed, N.; Saunders, C.; Davis, A.R.; Owens, W.A.; Matthies, H.J.G.; Saadat, S.; Kennedy, J.P.; Vaughan, R.A.; Neve, R.L.; Lindsley, C.W.; et al. Impaired striatal akt signaling disrupts dopamine homeostasis and increases feeding. PLoS ONE 2011, 6, e25169. [Google Scholar] [CrossRef]
- Williams, J.M.; Owens, W.A.; Turner, G.H.; Saunders, C.; Dipace, C.; Blakely, R.D.; France, C.P.; Gore, J.C.; Daws, L.C.; Avison, M.J.; et al. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007, 5, e274. [Google Scholar] [CrossRef]
- Mallorquí-Bagué, N.; Lozano-Madrid, M.; Toledo, E.; Corella, D.; Salas-Salvadó, J.; Cuenca-Royo, A.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; et al. Type 2 diabetes and cognitive impairment in an older population with overweight or obesity and metabolic syndrome: Baseline cross-sectional analysis of the PREDIMED-plus study. Sci. Rep. 2018, 8, 16128. [Google Scholar] [CrossRef]
- Mallorquí-Bagué, N.; Lozano-Madrid, M.; Vintró-Alcaraz, C.; Forcano, L.; Díaz-López, A.; Galera, A.; Fernández-Carrión, R.; Granero, R.; Jiménez-Murcia, S.; Corella, D.; et al. Effects of a psychosocial intervention at one-year follow-up in a PREDIMED-plus sample with obesity and metabolic syndrome. Sci. Rep. 2021, 11, 9144. [Google Scholar] [CrossRef]
- Qiao Qiu, W.; Lyn Price, L.; Hibberd, P.; Buell, J.; Collins, L.; Leins, D.; Mkaya Mwamburi, D.; Rosenberg, I.; Smaldone, L.; Scott, T.M.; et al. Executive dysfunction in homebound older people with diabetes mellitus. J. Am. Geriatr. Soc. 2006, 54, 496–501. [Google Scholar] [CrossRef]
- Yeung, S.E.; Fischer, A.L.; Dixon, R.A. Exploring Effects of Type 2 Diabetes on Cognitive Functioning in Older Adults. Neuropsychology 2009, 23, 1–9. [Google Scholar]
- Sun, D.M.; Ma, Y.; Sun, Z.B.; Xie, L.; Huang, J.Z.; Chen, W.S.; Duan, S.X.; Lin, Z.R.; Guo, R.W.; Le, H.B.; et al. Decision-making in primary onset middle-age type 2 diabetes mellitus: A BOLD-fMRI study. Sci. Rep. 2017, 7, 10246. [Google Scholar] [CrossRef]
- Cloninger, C.R. The Temperament and Character Inventory-Revised; Center for Psychobiology of Personality, Washington University: St. Louis, MO, USA, 1999. [Google Scholar]
- Gutiérrez-Zotes, J.A.; Bayón, C.; Montserrat, C.; Valero, J.; Labad, A.; Cloninger, C.R.; Fernández-Aranda, F. Inventario del Temperamento y el Carácter-Revisado (TCI-R). Baremación y datos normativos en una muestra de población general. Actas Esp. Psiquiatr. 2004, 32, 8–15. [Google Scholar]
- Derogatis, L.R. SCL-90-R: Symptom Checklist-90-R: Administration, Scoring and Procedures Manuall—II for the Revised Version; Clinical Psychometric Research: Towson, MD, USA, 1994. [Google Scholar]
- Derogatis, L.R. SCL-90-R Cuestionario de 90 Síntomas Revisado Manual; González de Rivera y Revuelta, J.L., de las Cuevas, C., Rodríguez-Abuin, M., Rodríguez Pulido, F., Eds.; TEA Ediciones: Madrid, Spain, 2002. [Google Scholar]
- Harris, M.E. Wisconsin Card Sorting Test: Computer Version; Psychological Assessment Resources: Lutz, FL, USA, 1990; Volume 4. [Google Scholar]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Del Barrio, V. Diagnostic and Statistical Manual of Mental Disorders. In The Curated Reference Collection in Neuroscience and Biobehavioral Psychology; American Psychiatric Association: Washington, DC, USA, 2016; ISBN 9780128093245. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. Available online: https://www.stata.com/ (accessed on 4 December 2021).
- Finner, H.; Roters, M. On the False Discovery Rate and Expected Type I Errors. Biom. J. 2001, 43, 985–1005. [Google Scholar]
- Albrecht, D.S.; Kareken, D.A.; Christian, B.T.; Dzemidzic, M.; Yoder, K.K. Cortical dopamine release during a behavioral response inhibition task. Synapse 2014, 68, 266–274. [Google Scholar] [CrossRef][Green Version]
- Bari, A.; Robbins, T.W. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: Possible relevance to ADHD. Psychopharmacology 2013, 230, 89–111. [Google Scholar] [CrossRef]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; Smith, C.E.; et al. Dopaminergic network differences in human impulsivity. Science 2010, 329, 532. [Google Scholar] [CrossRef]
- Eckstrand, K.L.; Mummareddy, N.; Kang, H.; Cowan, R.; Zhou, M.; Zald, D.; Silver, H.J.; Niswender, K.D.; Avison, M.J. An insulin resistance associated neural correlate of impulsivity in type 2 diabetes mellitus. PLoS ONE 2017, 12, e0189113. [Google Scholar] [CrossRef]
- Eisenstein, S.A.; Gredysa, D.M.; Antenor-Dorsey, J.A.; Green, L.; Arbeláez, A.M.; Koller, J.M.; Black, K.J.; Perlmutter, J.S.; Moerlein, S.M.; Hershey, T. Insulin, central dopamine D2 receptors, and monetary reward discounting in obesity. PLoS ONE 2015, 10, e0133621. [Google Scholar] [CrossRef]
- Sullivan, S.; Cloninger, C.R.; Przybeck, T.R.; Klein, S. Personality characteristics in obesity and relationship with successful weight loss. Int. J. Obes. 2007, 31, 669–674. [Google Scholar] [CrossRef]
- Gerlach, G.; Herpertz, S.; Loeber, S. Personality traits and obesity: A systematic review. Obes. Rev. 2015, 16, 32–63. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Calugi, S.; El Ghoch, M. Are Personality Characteristics as Measured by the Temperament and Character Inventory (TCI) Associated with Obesity Treatment Outcomes? A Systematic Review. Curr. Obes. Rep. 2018, 7, 27–36. [Google Scholar]
- Dalle Grave, R.; Calugi, S.; Marchesini, G.; Beck-Peccoz, P.; Bosello, O.; Compare, A.; Cuzzolaro, M.; Grossi, E.; Mannucci, E.; Molinari, E.; et al. Personality features of obese women in relation to binge eating and night eating. Psychiatry Res. 2013, 207, 86–91. [Google Scholar] [CrossRef]
- Xiao, L.; Bechara, A.; Grenard, L.J.; Stacy, W.A.; Palmer, P.; Wei, Y.; Jia, Y.; Fu, X.; Johnson, C.A. Affective decision-making predictive of Chinese adolescent drinking behaviors. J. Int. Neuropsychol. Soc. 2009, 15, 547–557. [Google Scholar] [CrossRef]
- Zermatten, A.; Van Der Linden, M.; D′Acremont, M.; Jermann, F.; Bechara, A. Impulsivity and decision making. J. Nerv. Ment. Dis. 2005, 193, 647–650. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar]
- Edwards, C.G.; Walk, A.M.; Thompson, S.V.; Mullen, S.P.; Holscher, H.D.; Khan, N.A. Disordered eating attitudes and behavioral and neuroelectric indices of cognitive flexibility in individuals with overweight and obesity. Nutrients 2018, 10, 1902. [Google Scholar] [CrossRef]
| AN-R | OB − T2D | HC | OB + T2D | GD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 107 | N = 115 | N = 171 | N = 67 | N = 121 | |||||||
| n | % | n | % | n | % | n | % | n | % | p | |
| Sex | |||||||||||
| Women | 97 | 90.7% | 107 | 93.0% | 144 | 84.2% | 48 | 71.6% | 19 | 15.7% | <0.001 * |
| Men | 10 | 9.3% | 8 | 7.0% | 27 | 15.8% | 19 | 28.4% | 102 | 84.3% | |
| Education Primary | 29 | 27.1% | 48 | 41.7% | 16 | 9.4% | 48 | 71.6% | 72 | 59.5% | <0.001 * |
| Secondary | 48 | 44.9% | 53 | 46.1% | 104 | 60.8% | 17 | 25.4% | 32 | 26.4% | |
| University | 30 | 28.0% | 14 | 12.2% | 51 | 29.8% | 2 | 3.0% | 17 | 14.0% | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | |
| Age (years) | 25.31 | 8.30 | 41.39 | 11.87 | 29.71 | 13.28 | 54.61 | 11.33 | 38.30 | 13.56 | <0.001 * |
| BMI (kg/m2) | 16.36 | 2.17 | 44.60 | 6.70 | 22.34 | 3.14 | 41.96 | 8.59 | 26.38 | 5.90 | <0.001 * |
| AN-R | OB − T2D | HC | OB + T2D | GD | Polynomial Contrasts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 107 | N = 115 | N = 171 | N = 67 | N = 121 | Trends (p-Value) | |||||||||
| Impulsivity | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | O1 | O2 | O3 | O4 |
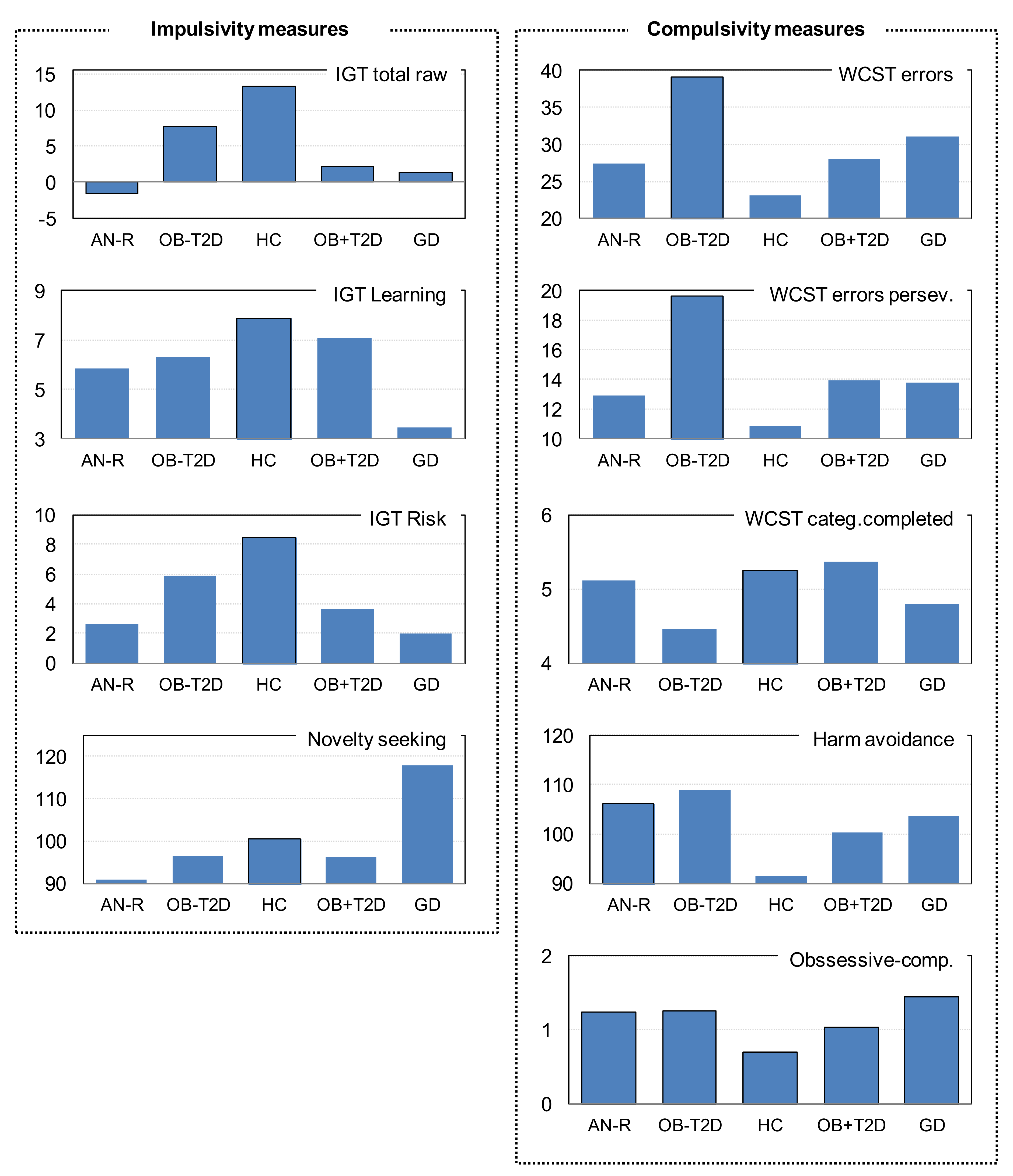
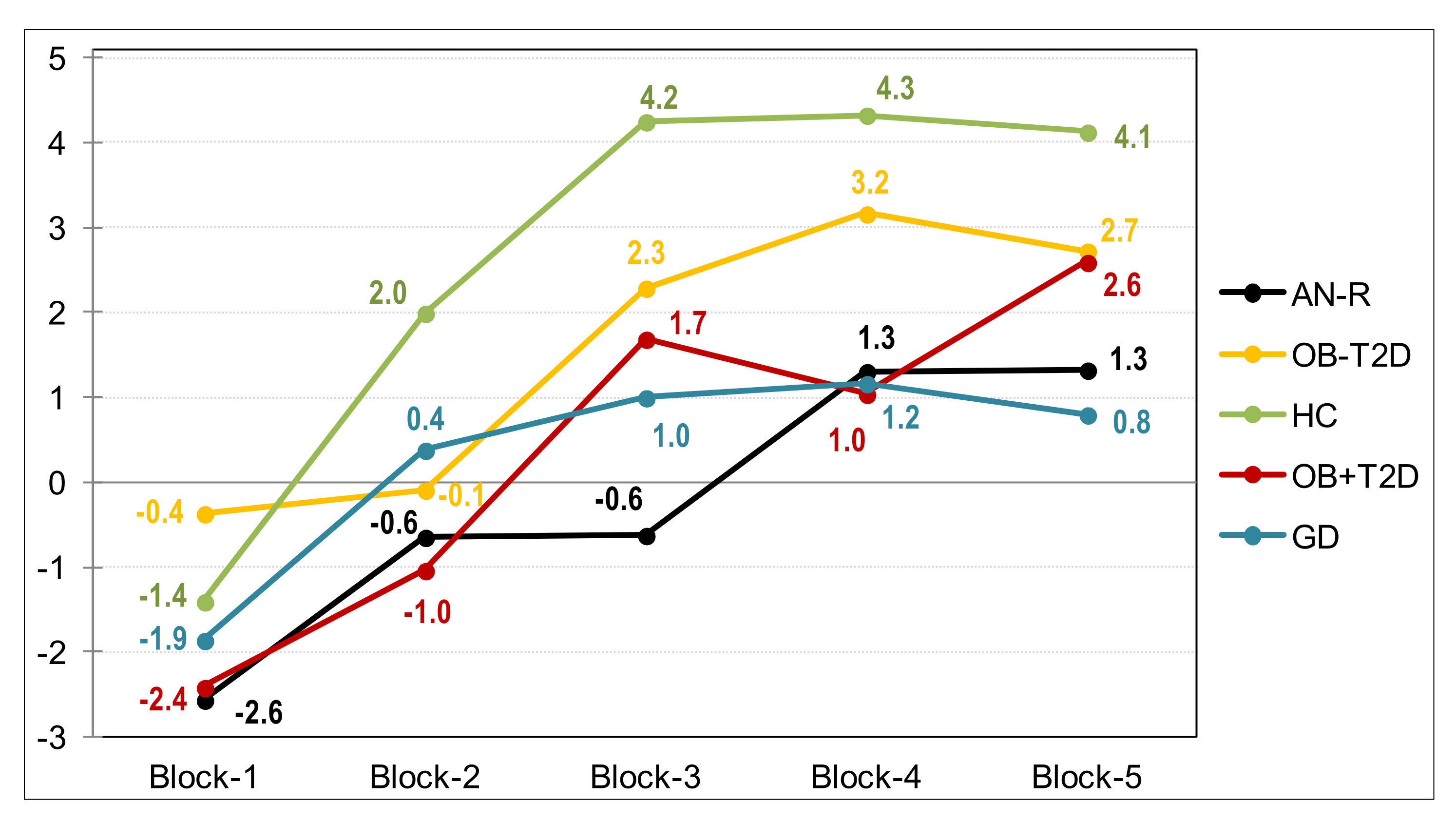
| IGT Total raw | −1.62 | 20.33 | 7.71 | 22.12 | 13.24 | 28.52 | 2.10 | 20.31 | 1.41 | 24.58 | 0.964 | 0.004 * | 0.106 | 0.308 |
| IGT Learning | 5.84 | 12.94 | 6.35 | 14.65 | 7.87 | 16.84 | 7.08 | 14.38 | 3.44 | 14.38 | 0.511 | 0.177 | 0.477 | 0.909 |
| IGT Risk index | 2.63 | 13.83 | 5.89 | 13.42 | 8.46 | 17.02 | 3.63 | 13.28 | 1.96 | 15.22 | 0.561 | 0.029 * | 0.481 | 0.478 |
| TCI-R Novelty seeking | 90.94 | 12.56 | 96.53 | 13.21 | 100.33 | 11.45 | 96.15 | 12.11 | 117.82 | 9.29 | 0.001 * | 0.001 * | 0.001 * | 0.035 * |
| Compulsivity | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | O1 | O2 | O3 | O4 |
| WCST Errors | 27.40 | 16.71 | 39.19 | 25.96 | 23.06 | 16.04 | 27.98 | 24.61 | 31.04 | 24.66 | 0.636 | 0.733 | 0.001 * | 0.027 * |
| WCST Errors perseve. | 12.94 | 7.71 | 19.67 | 14.61 | 10.84 | 7.24 | 13.96 | 13.21 | 13.80 | 10.88 | 0.348 | 0.735 | 0.001 * | 0.011 |
| WCST Categ.compl. | 5.11 | 1.20 | 4.45 | 2.22 | 5.25 | 1.21 | 5.37 | 2.12 | 4.80 | 1.97 | 0.650 | 0.549 | 0.001 * | 0.416 |
| TCI-R Harm avoid. | 106.02 | 18.63 | 108.88 | 16.87 | 91.38 | 16.21 | 100.21 | 14.07 | 103.51 | 18.77 | 0.046 * | 0.002 * | 0.015 * | 0.004 * |
| SCL-90R Obs.-comp. | 1.24 | 0.91 | 1.26 | 0.73 | 0.70 | 0.56 | 1.04 | 0.67 | 1.45 | 0.87 | 0.505 | 0.001 * | 0.016 * | 0.055 |
| Pairwise | AN-R/ | AN-R/ | AN-R/ | AN-R/ | OB − T2D/ | OB − T2D/ | OB − T2D/ | HC/ | HC/ | OB + T2D/ | ||||
| comparisons | OB − T2D | HC | OB + T2D | GD | HC | OB + T2D | GD | OB + T2D | GD | GD | η2 | |||
| Impulsivity | ||||||||||||||
| IGT Total raw | 0.142 | <0.001 * | 0.572 | 0.493 | 0.291 | 0.159 | 0.224 | 0.046 * | 0.002 * | 0.895 | 0.048 | |||
| IGT Learning | 0.897 | 0.307 | 0.760 | 0.381 | 0.638 | 0.764 | 0.365 | 0.821 | 0.056 | 0.259 | 0.008 | |||
| IGT Risk index | 0.407 | 0.004 * | 0.806 | 0.807 | 0.429 | 0.361 | 0.222 | 0.164 | 0.005 * | 0.605 | 0.024 | |||
| TCI-R Novelty seeking | 0.068 | <0.001 * | 0.102 | <0.001 * | 0.133 | 0.843 | <0.001 * | 0.121 | <0.001 * | <0.001 * | 0.282 † | |||
| Compulsivity | ||||||||||||||
| WCST Errors | 0.025 * | 0.104 | 0.915 | 0.321 | <0.001 * | 0.001 * | 0.059 | 0.288 | 0.010 * | 0.479 | 0.043 | |||
| WCST Errors perseve. | 0.013 * | 0.125 | 0.715 | 0.647 | <0.001 * | 0.001 * | 0.008 * | 0.189 | 0.063 | 0.943 | 0.042 | |||
| WCST Categ.compl. | 0.126 | 0.496 | 0.555 | 0.306 | 0.023 * | 0.001 * | 0.318 | 0.761 | 0.072 | 0.107 | 0.029 | |||
| TCI-R Harm avoid. | 0.512 | <0.001 * | 0.199 | 0.408 | <0.001 * | 0.002 * | 0.132 | 0.022 * | <0.001 * | 0.358 | 0.124 † | |||
| SCL-90R Obs.-comp. | 0.937 | <0.001 * | 0.314 | 0.119 | <0.001 * | 0.073 | 0.218 | 0.046 * | <0.001 * | 0.010 * | 0.109 † | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, G.; Mora-Maltas, B.; Camacho-Barcia, L.; Granero, R.; Lucas, I.; Agüera, Z.; Jiménez-Murcia, S.; Baños, R.; Bertaina-Anglade, V.; Botella, C.; et al. Transdiagnostic Perspective of Impulsivity and Compulsivity in Obesity: From Cognitive Profile to Self-Reported Dimensions in Clinical Samples with and without Diabetes. Nutrients 2021, 13, 4426. https://doi.org/10.3390/nu13124426
Testa G, Mora-Maltas B, Camacho-Barcia L, Granero R, Lucas I, Agüera Z, Jiménez-Murcia S, Baños R, Bertaina-Anglade V, Botella C, et al. Transdiagnostic Perspective of Impulsivity and Compulsivity in Obesity: From Cognitive Profile to Self-Reported Dimensions in Clinical Samples with and without Diabetes. Nutrients. 2021; 13(12):4426. https://doi.org/10.3390/nu13124426
Chicago/Turabian StyleTesta, Giulia, Bernat Mora-Maltas, Lucía Camacho-Barcia, Roser Granero, Ignacio Lucas, Zaida Agüera, Susana Jiménez-Murcia, Rosa Baños, Valerie Bertaina-Anglade, Cristina Botella, and et al. 2021. "Transdiagnostic Perspective of Impulsivity and Compulsivity in Obesity: From Cognitive Profile to Self-Reported Dimensions in Clinical Samples with and without Diabetes" Nutrients 13, no. 12: 4426. https://doi.org/10.3390/nu13124426
APA StyleTesta, G., Mora-Maltas, B., Camacho-Barcia, L., Granero, R., Lucas, I., Agüera, Z., Jiménez-Murcia, S., Baños, R., Bertaina-Anglade, V., Botella, C., Bulló, M., Casanueva, F. F., Dalsgaard, S., Fernández-Real, J.-M., Franke, B., Frühbeck, G., Fitó, M., Gómez-Martínez, C., Pintó, X., ... Fernández-Aranda, F. (2021). Transdiagnostic Perspective of Impulsivity and Compulsivity in Obesity: From Cognitive Profile to Self-Reported Dimensions in Clinical Samples with and without Diabetes. Nutrients, 13(12), 4426. https://doi.org/10.3390/nu13124426













