Levocarnitine Supplementation Suppresses Lenvatinib-Related Sarcopenia in Hepatocellular Carcinoma Patients: Results of a Propensity Score Analysis
Abstract
:1. Introduction
2. Materials and Methods
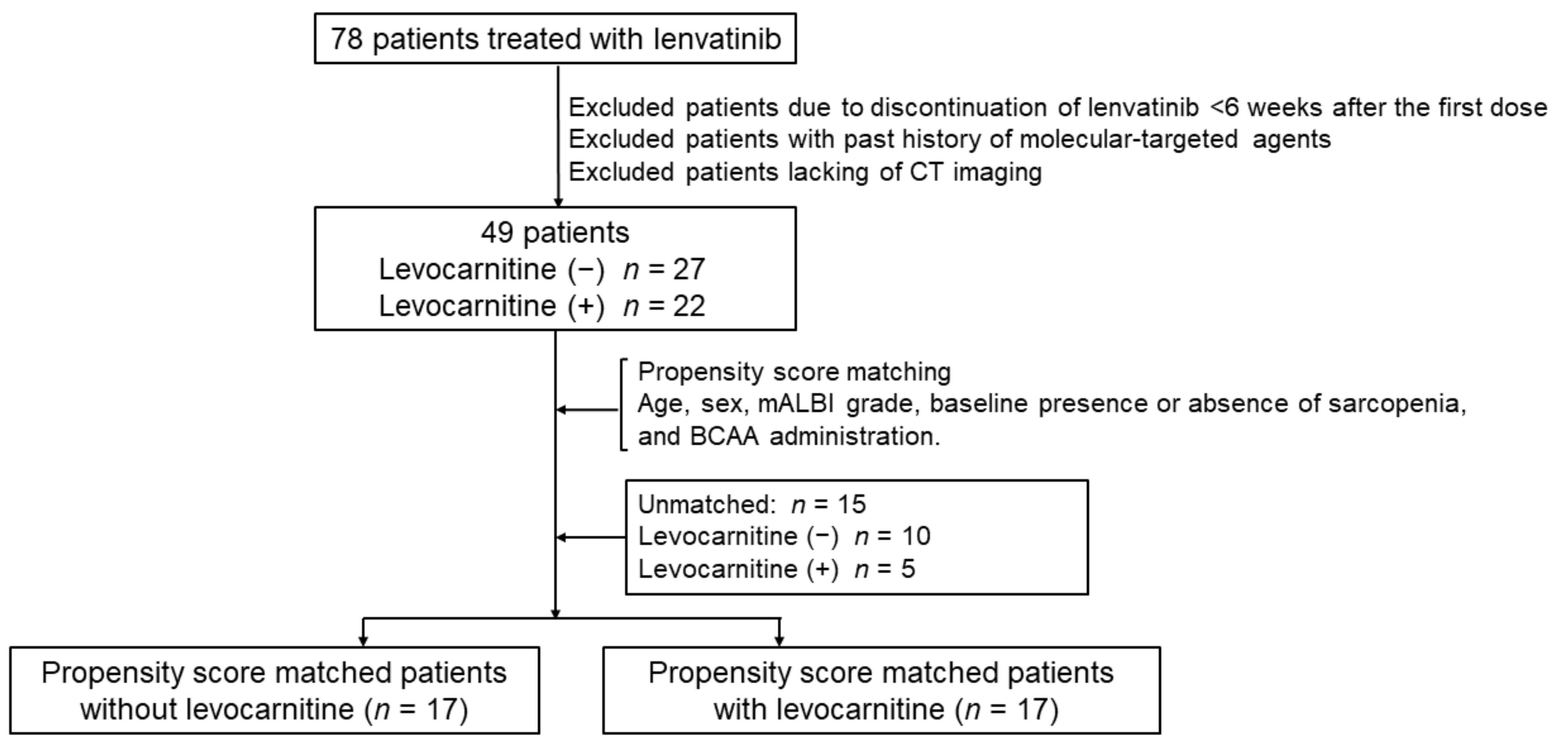
2.1. Patients
2.2. Imaging Analysis of Skeletal Muscle Mass
2.3. Measurements of Serum Ammonia and Carnitine Content
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
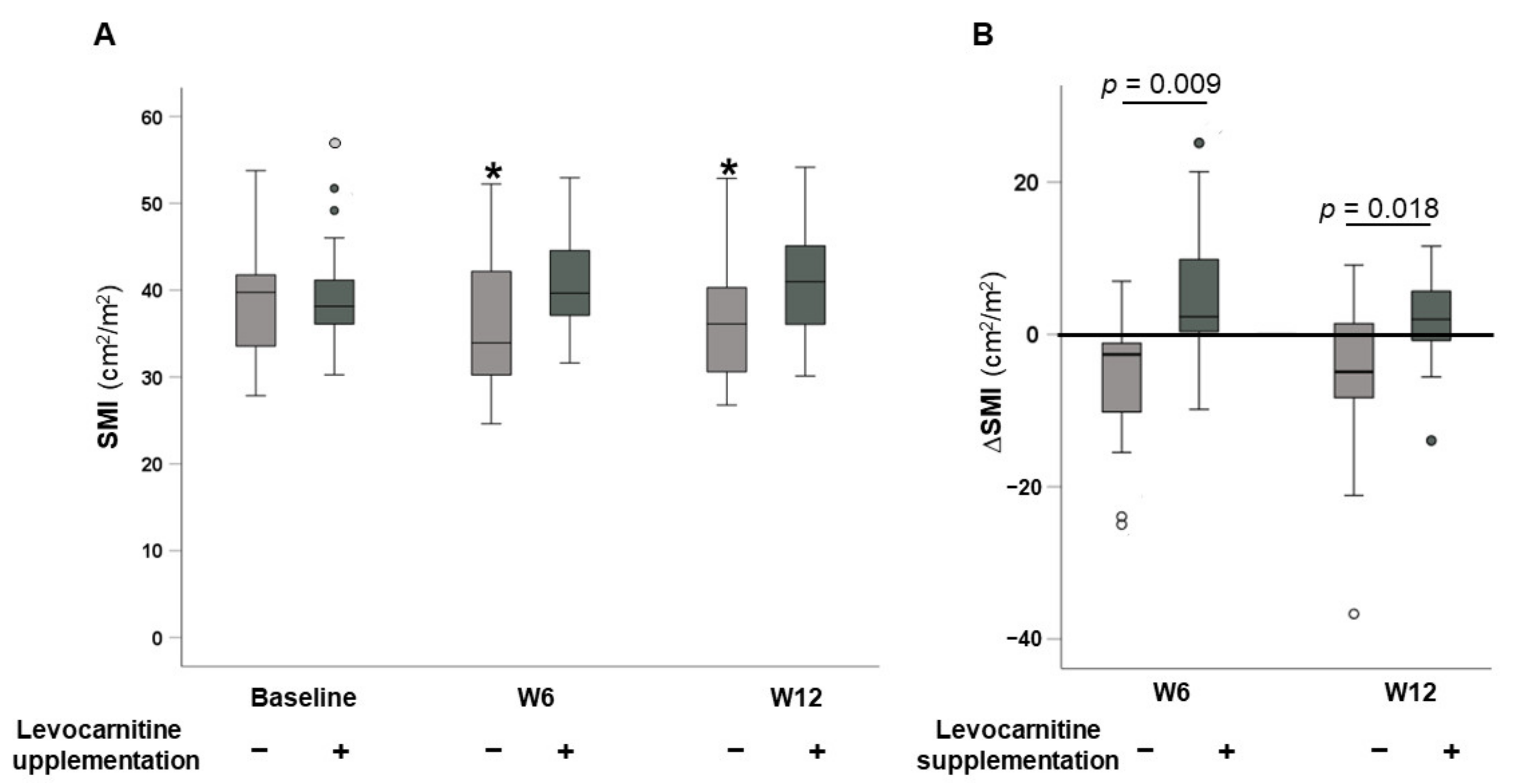
3.2. Changes in SMI
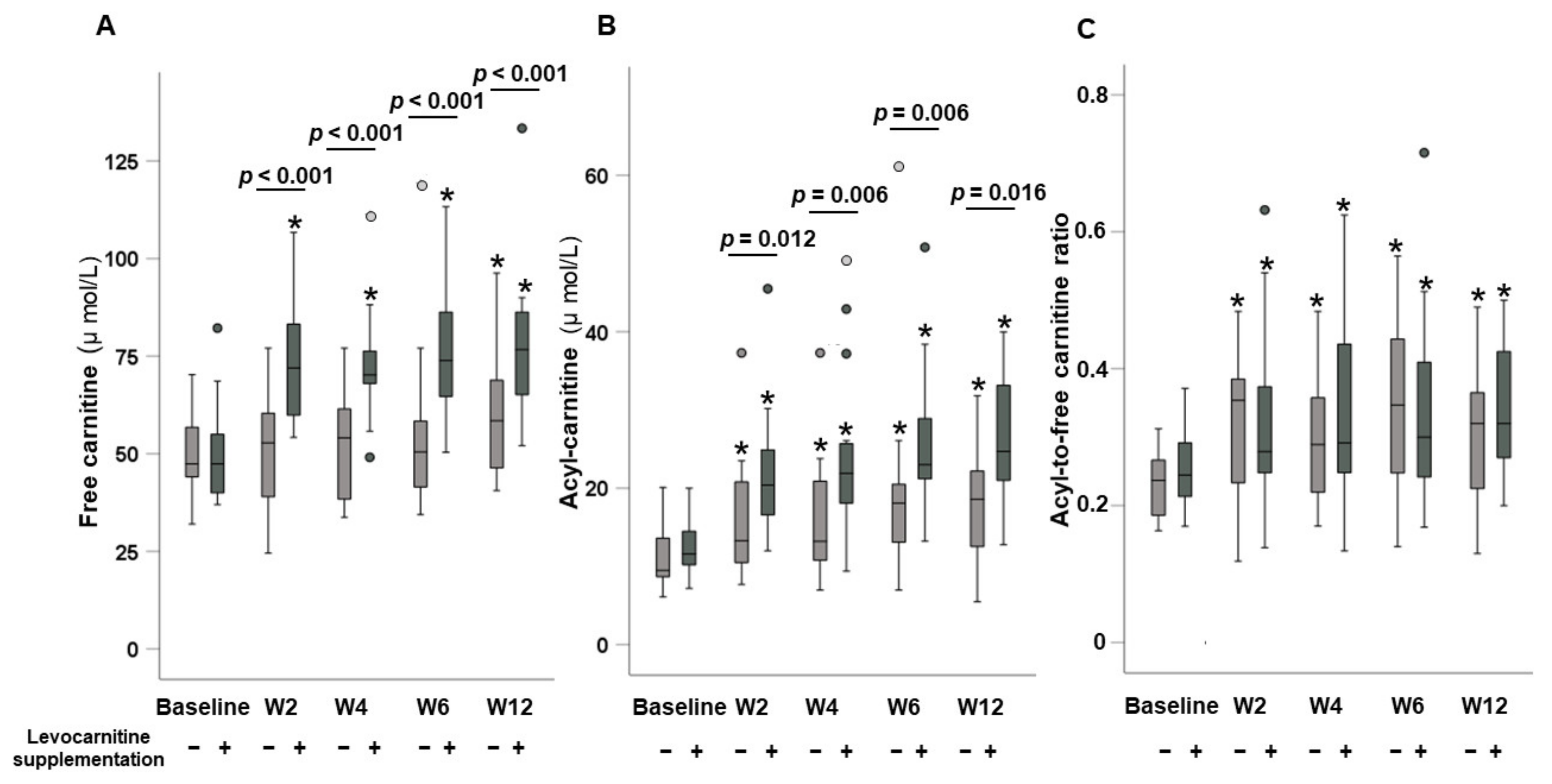
3.3. Dynamic Changes in Carnitine Concentrations
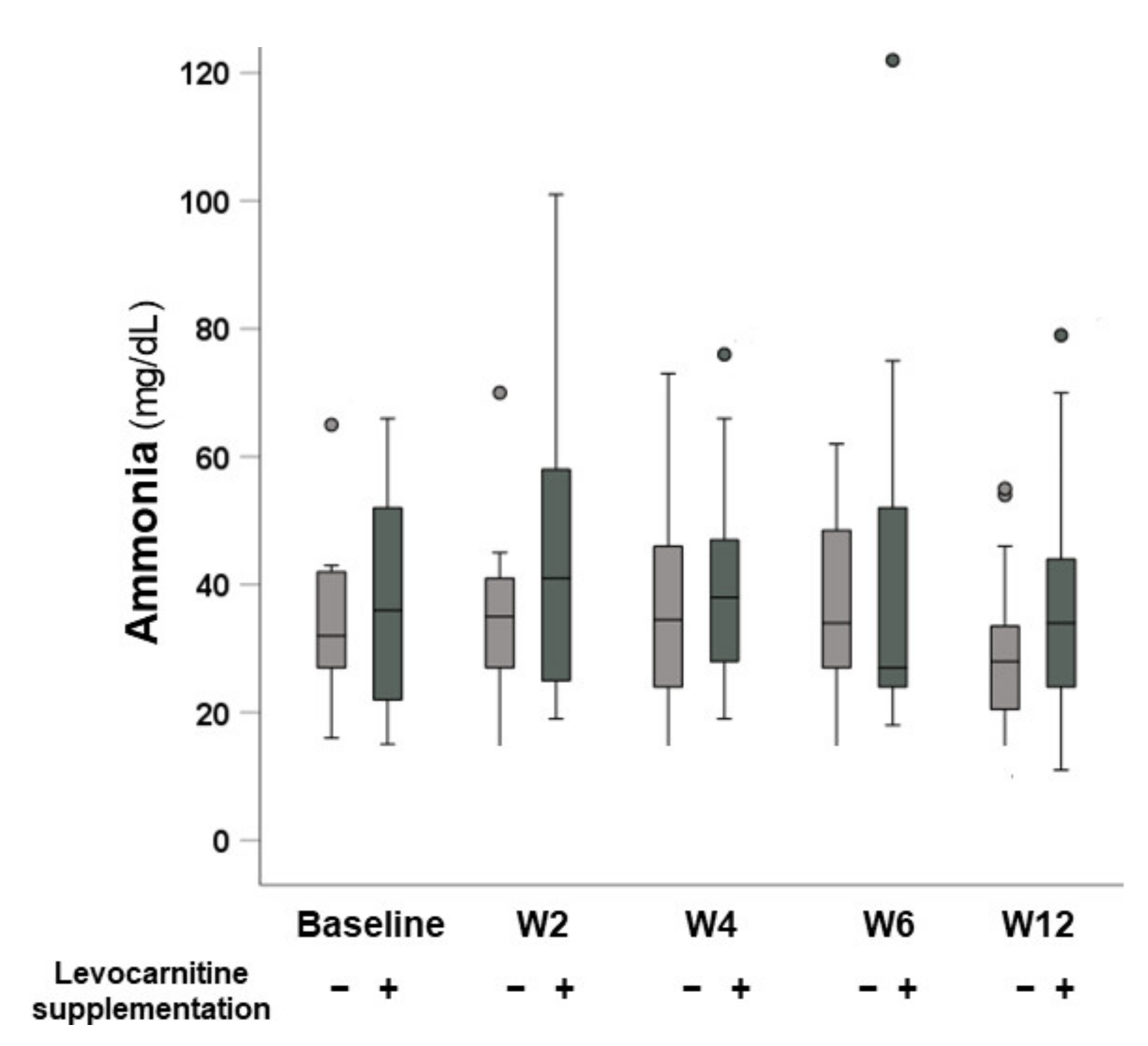
3.4. Changes in Serum Ammonia Levels
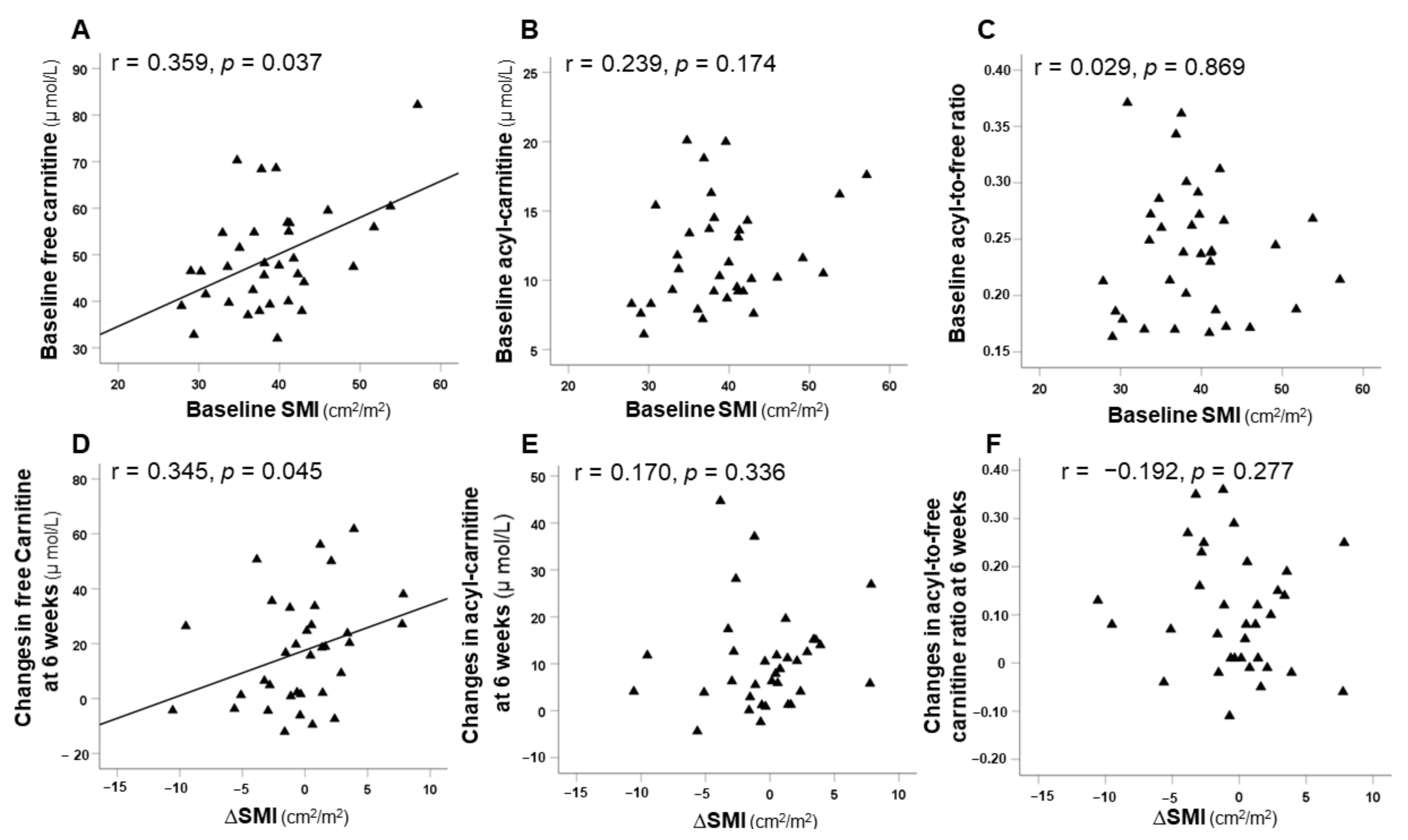
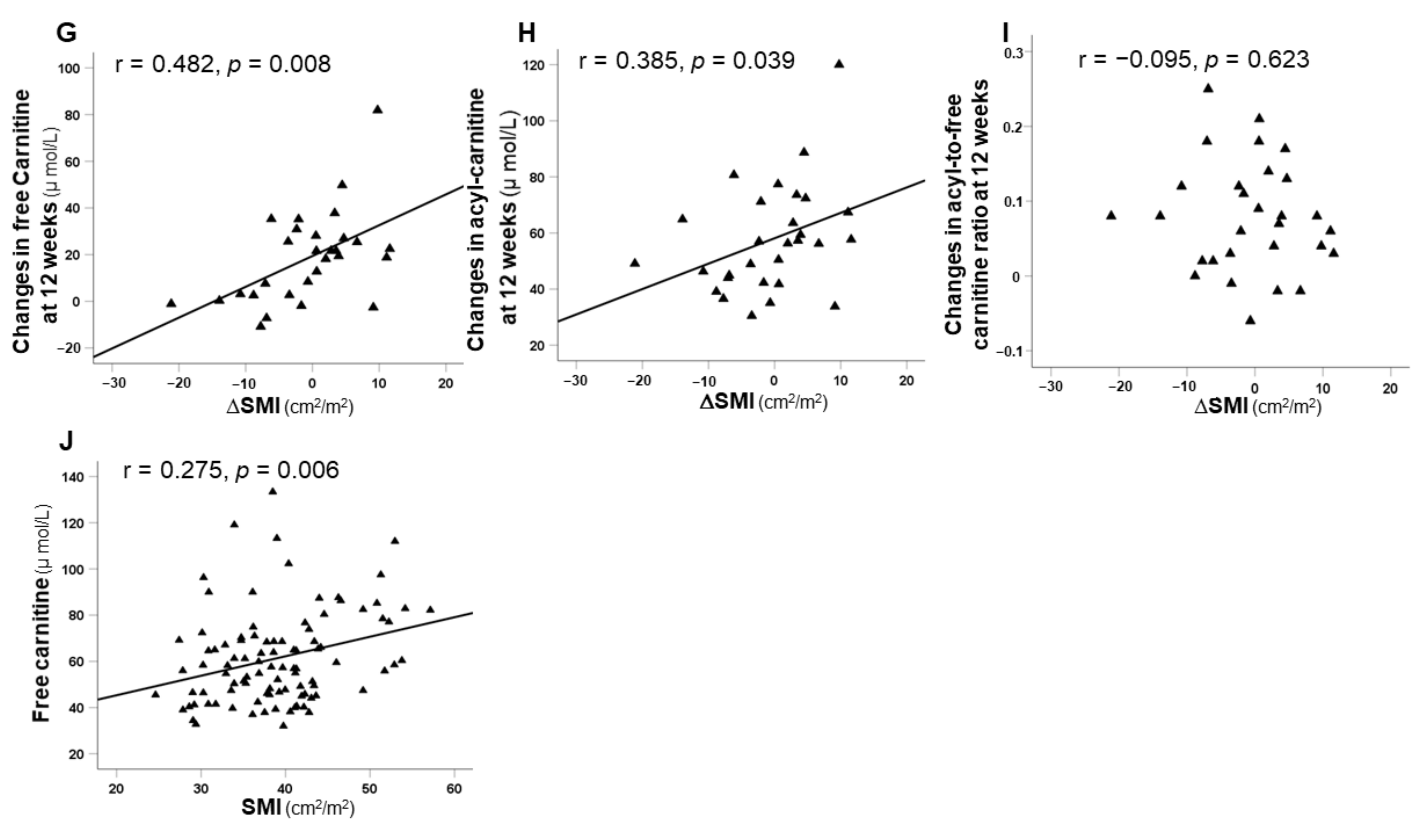
3.5. Correlation between SMI and Carnitine Concentrations
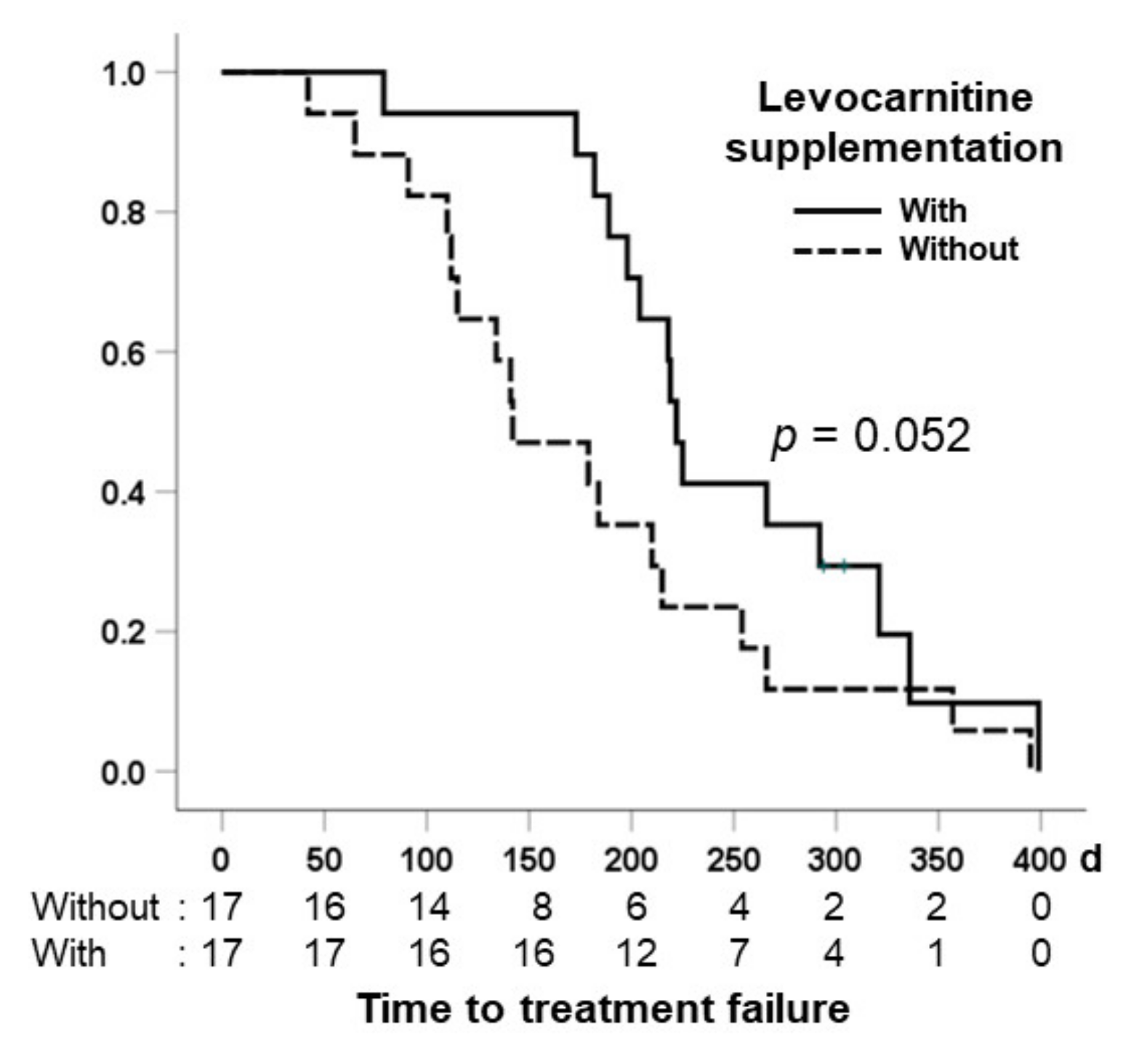
3.6. Comparison of Time to Treatment Failure in the Two Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zschäbitz, S.; Grüllich, C. Lenvantinib: A Tyrosine Kinase Inhibitor of VEGFR 1-3, FGFR 1-4, PDGFRα, KIT and RET. Recent Results Cancer Res. 2018, 211, 187–198. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol. Res. 2020, 50, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya KHino KNishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Ponziani, F.R.; Pompili, M.; Bria, E.; Tortora, G.; Gasbarrini, A.; et al. Skeletal Muscle Loss during Multikinase Inhibitors Therapy: Molecular Pathways, Clinical Implications, and Nutritional Challenges. Nutrients 2020, 12, 3101. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid Depletion of Subcutaneous Adipose Tissue during Sorafenib Treatment Predicts Poor Survival in Patients with Hepatocellular Carcinoma. Cancers 2020, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Kawano, R.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; et al. No Muscle Depletion with High Visceral Fat as a Novel Beneficial Biomarker of Sorafenib for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Uojima, H.; Chuma, M.; Tanaka, Y.; Hidaka, H.; Nakazawa, T.; Iwabuchi, S.; Kobayashi, S.; Hattori, N.; Ogushi, K.; Morimoto, M.; et al. Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer 2020, 9, 193–206. [Google Scholar] [CrossRef]
- Uchikawa, S.; Kawaoka, T.; Namba, M.; Kodama, K.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Tsuge, M.; Hiramatsu, A.; et al. Skeletal Muscle Loss during Tyrosine Kinase Inhibitor Treatment for Advanced Hepatocellular Carcinoma Patients. Liver Cancer 2020, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Levolger, S.; van Vugt, J.L.; de Bruin, R.W.; IJzermans, J.N. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br. J. Surg. 2015, 102, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.A. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 2004, 1033, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Imai, K.; Suetugu, A.; Takai, K.; Shimizu, M. Usefulness of Carnitine Supplementation for the Complications of Liver Cirrhosis. Nutrients 2020, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Ando, H.; Ishizuka, K.; Kitagawa, R.; Okubo, S.; Saito, H.; Kokubu, S.; Miyazaki, A.; Ikejima, K.; Shiina, S.; et al. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0229772. [Google Scholar] [CrossRef] [Green Version]
- Popuri, K.; Cobzas, D.; Esfandiari, N.; Baracos, V.; Jägersand, M. Body Composition Assessment in Axial CT Images Using FEM-Based Automatic Segmentation of Skeletal Muscle. IEEE Trans. Med. Imaging 2016, 35, 512–520. [Google Scholar] [CrossRef]
- Takahashi, M.; Ueda, S.; Misaki, H.; Sugiyama, N.; Matsumoto, K.; Matsuo, N.; Murao, S. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin. Chem. 1994, 40, 817–821. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 2002, 13, 2276–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. Nutr. Metab. 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicka, A.K.; Hartmane, D.; Lipinska, P.; Wojtowicz, E.; Lysiak-Szydlowska, W.; Olek, R.A. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients 2018, 10, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanuma, M.; Nagai, H.; Igarashi, Y. Sorafenib Might Induce Sarcopenia in Patients With Hepatocellular Carcinoma by Inhibiting Carnitine Absorption. Anticancer Res. 2020, 40, 4173–4182. [Google Scholar] [CrossRef] [PubMed]
- Japan Pediatric Society. Available online: http://www.jpeds.or.jp/modules/guidelines/index.php?content_id=2 (accessed on 4 December 2021).
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–181627. [Google Scholar] [CrossRef] [Green Version]
- Hey, P.; Gow, P.; Testro, A.G.; Apostolov, R.; Chapman, B.; Sinclair, M. Nutraceuticals for the treatment of sarcopenia in chronic liver disease. Clin. Nutr. ESPEN 2021, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Picca, A.; Marzetti, E.; Calvani, R.; Conta, G.; Del Chierico, F.; Capuani, G.; Faccia, M.; Fianchi, F.; Funaro, B.; et al. GuLiver study group. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 2021, 41, 1320–1334. [Google Scholar] [CrossRef]
- Hiramatsu, A.; Aikata, H.; Uchikawa, S.; Ohya, K.; Kodama, K.; Nishida, Y.; Daijo, K.; Osawa, M.; Teraoka, Y.; Honda, F.; et al. Levocarnitine use is associated with improvement in sarcopenia in patients with liver cirrhosis. Hepatol. Commun. 2019, 3, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, K.; Ishikawa, T.; Hoshii, A.; Hokari, T.; Suzuki, M.; Noguchi, H.; Hirosawa, H.; Koyama, F.; Kobayashi, M.; Hirosawa, S.; et al. Effect of levocarnitine administration in patients with chronic liver disease. Exp. Ther. Med. 2020, 20, 94. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kiguchi, D.; Ninomiya, T.; Hirooka, M.; Abe, M.; Matsuura, B.; Hiasa, Y.; Michitaka, K. Can L-carnitine supplementation and exercise improve muscle complications in patients with liver cirrhosis who receive branched-chain amino acid supplementation? Eur. J. Gastroenterol. Hepatol. 2019, 31, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Ohya, K.; Kawaoka, T.; Namba, M.; Uchikawa, S.; Kodama, K.; Morio, K.; Nakahara, T.; Murakami, E.; Hiramatsu, A.; Tsuge, M.; et al. Early changes in ammonia levels and liver function in patients with advanced hepatocellular carcinoma treated by lenvatinib therapy. Sci. Rep. 2019, 20, 12101. [Google Scholar] [CrossRef] [PubMed]
- Narita, R.; Kotoh, K.; Yoneda, A.; Motomura, M.; Harada, M. Factors Raising Serum Ammonia Level During Lenvatinib Treatment of Patients With Hepatocellular Carcinoma. Anticancer Res. 2020, 40, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Giordano, M.; Pennisi, G.; Bella, R.; Rampello, L.; Malaguarnera, M.; Li Volti, G.; Galvano, F. Oral acetyl-L carnitine therapy reduces fatigue in overt hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2011, 93, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajima, Y.; Takahashi, H.; Akiyama, T.; Murayama, K.; Iwane, S.; Kuwashiro, T.; Tanaka, K.; Kawazoe, S.; Ono, N.; Eguchi, T.; et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J. Gastroenterol. 2018, 53, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, A.K.; Renzi, G.; Olek, R.A. The bright and the dark sides of L-carnitine supplementation: A systematic review. J. Int. Soc. Sports Nutr. 2020, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Díez-Ricote, L.; Ruiz-Valderrey, P.; Micó, V.; Blanco-Rojo, R.; Tomé-Carneiro, J.; Dávalos, A.; Ordovás, J.M.; Daimiel, L. Trimethylamine n-Oxide (TMAO) Modulates the Expression of Cardiovascular Disease-Related microRNAs and Their Targets. Int. J. Mol. Sci. 2021, 22, 11145. [Google Scholar] [CrossRef] [PubMed]
- Tokuchi, Y.; Suda, G.; Kimura, M.; Maehara, O.; Kitagataya, T.; Kubo, A.; Yoshida, S.; Fu, Q.; Yang, Z.; Hosoda, S.; et al. Possible correlation between increased serum free carnitine levels and increased skeletal muscle mass following HCV eradication by direct acting antivirals. Sci. Rep. 2021, 11, 16616. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 34) | Without Levocarnitine Supplementation (n = 17) | With Levocarnitine Supplementation n = 17 | p Value | |
|---|---|---|---|---|
| Age, years | 77.5 (52–90) | 77 (67–90) | 79 (52–87) | 0.959 |
| Sex, male/female | 28/6 | 15/2 | 13/4 | 0.656 |
| HBV/HCV/NBNC | 4/10/20 | 2/5/10 | 2/5/10 | 1.000 |
| ECOG PS, 0/1 | 29/5 | 14/3 | 15/2 | 1.000 |
| Naïve, yes/no | 30/2 | 15/1 | 15/1 | 1.000 |
| Body weight, kg | 59.1 (38.6–81.4) | 59.0 (38.6–81.4) | 61 (41.2–69.1) | 0.959 |
| BMI, kg/m2 | 22.82 (15.88–27.77) | 21.82 (17.25–27.77) | 23.31 (15.58–27.62) | 0.221 |
| Initial dose, 12 mg/8 mg | 15/19 | 7/10 | 8/9 | 0.730 |
| BCLC staging, B/C | 23/11 | 11/6 | 12/5 | 0.714 |
| Albumin, g/dL | 3.8 (2.9–4.7) | 3.7 (3.3–4.2) | 3.9 (2.9–4.7) | 0.407 |
| Total bilirubin, mg/dL | 0.7 (0.3–1.4) | 0.6 (0.4–1.2) | 0.9 (0.4–1.4) | 0.061 |
| Platelet count, ×104/μL | 18.2 (7.6–46.7) | 20.4 (7.6–35.5) | 16.0 (7.8–46.7) | 0.865 |
| Ammonia, mg/dL | 33.5 (15–66) | 32 (16–65) | 36 (15–66) | 0.850 |
| Free carnitine, µmol/L | 47.4 (32–82.2) | 47.4 (32–70.3) | 47.4 (37–82.2) | 0.986 |
| Acyl-carnitine, µmol/L | 10.65 (6.1–20.1) | 9.5 (6.1–20.1) | 11.6 (7.2–20.0) | 0.255 |
| AC/FC ratio | 0.24 (0.16–0.37) | 0.24 (0.16–0.31) | 0.25 (0.17–0.37) | 0.221 |
| AFP, ng/mL | 32.1 (0.9–70,000) | 21.2 (0.9–35,500) | 85.5 (1.6–70,000) | 0.730 |
| DCP, mAU/mL | 215.5 (16–5,520,000) | 1730 (21–72,300) | 63 (15–10,000) | 0.008 |
| Child–Pugh score, 5/6 | 24/10 | 11/6 | 13/4 | 0.452 |
| ALBI score | −2.51 (−3.21–−1.64) | −2.56 (−3.02–−2.11) | −2.51 (−3.11–−1.64) | 0.783 |
| mALBI | 15/12/7 | 7/7/3 | 8/5/4 | 0.543 |
| BCAA supplementation, yes/no | 11/23 | 5/12 | 6/11 | 0.714 |
| SMI, cm2/m2 | 38.45 (29.01–59.79) | 39.75 (27.86–53.77) | 38.15 (30.27–57.14) | 0.796 |
| Sarcopenia, yes/no | 26/8 | 13/4 | 13/4 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okubo, H.; Ando, H.; Nakadera, E.; Ikejima, K.; Shiina, S.; Nagahara, A. Levocarnitine Supplementation Suppresses Lenvatinib-Related Sarcopenia in Hepatocellular Carcinoma Patients: Results of a Propensity Score Analysis. Nutrients 2021, 13, 4428. https://doi.org/10.3390/nu13124428
Okubo H, Ando H, Nakadera E, Ikejima K, Shiina S, Nagahara A. Levocarnitine Supplementation Suppresses Lenvatinib-Related Sarcopenia in Hepatocellular Carcinoma Patients: Results of a Propensity Score Analysis. Nutrients. 2021; 13(12):4428. https://doi.org/10.3390/nu13124428
Chicago/Turabian StyleOkubo, Hironao, Hitoshi Ando, Eisuke Nakadera, Kenichi Ikejima, Shuichiro Shiina, and Akihito Nagahara. 2021. "Levocarnitine Supplementation Suppresses Lenvatinib-Related Sarcopenia in Hepatocellular Carcinoma Patients: Results of a Propensity Score Analysis" Nutrients 13, no. 12: 4428. https://doi.org/10.3390/nu13124428
APA StyleOkubo, H., Ando, H., Nakadera, E., Ikejima, K., Shiina, S., & Nagahara, A. (2021). Levocarnitine Supplementation Suppresses Lenvatinib-Related Sarcopenia in Hepatocellular Carcinoma Patients: Results of a Propensity Score Analysis. Nutrients, 13(12), 4428. https://doi.org/10.3390/nu13124428






