Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review
Abstract
1. Introduction
1.1. Mechanisms of IR
1.2. Adipocytokines and IR
1.3. Lipotoxicity and IR
2. Assessment of IR in Patients with Diabetes Mellitus
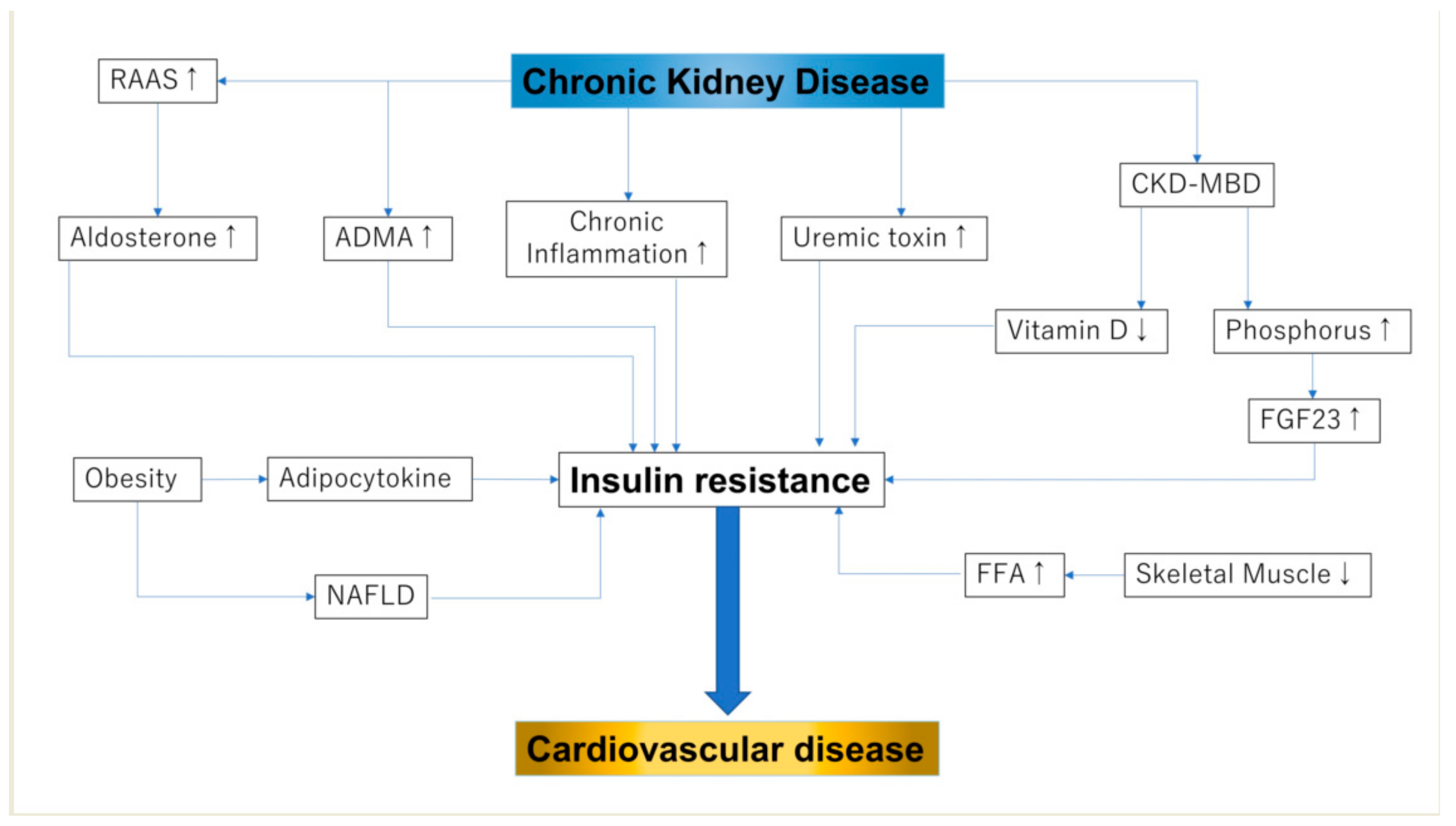
3. IR in Patients with CKD
4. CKD-MBD and IR
4.1. Vitamin D and IR
4.2. Phosphorus, FGF-23, and IR
5. Treatment of IR
Is CKD-MBD the Potential Option for Treating IR?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| chronic kidney disease | (CKD) |
| cardiovascular disease | (CVD) |
| insulin resistance | (IR) |
| CKD-mineral bone disorder | (MBD) |
| fibroblast growth factor-23 | (FGF-23) |
| tumor necrosis factor-alpha | (TNF-α), |
| free fatty acids | (FFAs) |
| insulin receptor substrate-1 | (IRS-1) |
| diacylglycerol | (DAG) |
| non-alcoholic fatty liver disease | (NAFLD) |
| forkhead transcription factor | (FOXO) |
| interleukin | (IL)-1 |
| homeostasis model assessment of insulin resistance | (HOMA-IR) |
| estimated glomerular filtration rate | (eGFR) |
| body mass index | (BMI) |
| asymmetric dimethylarginine | (ADMA) |
| blood urea nitrogen | (BUN) |
| reactive oxygen species | (ROS) |
| dimethylaminohydrolase | (DDAH) |
| parathyroid hormone | (PTH) |
| vitamin D receptor | (VDR) |
| 25 hydroxyvitamin D | (25OHD) |
| peroxisome proliferator activated receptor | (PPAR) |
| intima-media complex thickening | (mean IMT) |
| sodium-glucose transport protein 2 | (SGLT2) |
| activate protein kinase C | (PKC) |
| c-Jun N-terminal kinase | (JNK) |
References
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Lloyd, S.M.; De Craen, A.J.; Jukema, J.W.; Westendorp, R.G.; Buckley, B.M.; Kearney, P.; Briggs, A.; Stott, D.J.; et al. Contrasting associations of insulin resistance with diabetes, cardiovascular disease and all-cause mortality in the elderly: PROSPER long-term follow-up. Diabetologia 2014, 57, 2513–2520. [Google Scholar] [CrossRef]
- de Boer, I.H.; Katz, R.; Chonchol, M.B.; Fried, L.F.; Ix, J.H.; Kestenbaum, B.; Mukamal, K.J.; Peralta, C.A.; Siscovick, D.S. Insulin Resistance, Cystatin C, and Mortality Among Older Adults. Diabetes Care 2012, 35, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Carrero, J.J. Insulin resistance in chronic kidney disease. Nephrology 2017, 22, 31–34. [Google Scholar] [CrossRef]
- Hanks, L.J.; Casazza, K.; Judd, S.E.; Jenny, N.S.; Gutiérrez, O.M. Associations of Fibroblast Growth Factor-23 with Markers of Inflammation, Insulin Resistance and Obesity in Adults. PLoS ONE 2015, 10, e0122885. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Spelleken, M.; Rohrig, K.; Hauner, H.; Eckel, J. Tumor necrosis factor-alpha acutely inhibits insulin signaling in human adipocytes: Implication of the p80 tumor necrosis factor receptor. Diabetes 1998, 47, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.B.; Storlien, L.H.; Chisholm, D.J.; Kraegen, E.W. Effects of nonesterified fatty acid availability on tissue-specific glucose utilization in rats in vivo. J. Clin. Investig. 1988, 82, 293–299. [Google Scholar] [CrossRef]
- Unger, R.H. Lipotoxicity in the Pathogenesis of Obesity-Dependent NIDDM: Genetic and Clinical Implications. Diabetes 1995, 44, 863–870. [Google Scholar] [CrossRef]
- Lee, Y.; Ravazzola, M.; Park, B.-H.; Bashmakov, Y.K.; Orci, L.; Unger, R.H. Metabolic Mechanisms of Failure of Intraportally Transplanted Pancreatic -Cells in Rats: Role of Lipotoxicity and Prevention by Leptin. Diabetes 2007, 56, 2295–2301. [Google Scholar] [CrossRef][Green Version]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Shulman, G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 Diabetes. Hepatology 2013, 59, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, M.K.; Puri, P.; Koo, B.K.; Joo, S.K.; Jang, S.Y.; Lee, D.H.; Jung, Y.J.; Kim, B.G.; Lee, K.L.; et al. Circulating lipidomic alterations in obese and non-obese subjects with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2020, 52, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.B.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef]
- Donath, M.Y.; Zeman-Meier, D.; Böni-Schnetzler, M. Inflammation in the Pathophysiology and Therapy of Cardiometabolic Disease. Endocr. Rev. 2019, 40, 1080–1091. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Narciso, V.; Nevola, R.; Rinaldi, L.; Calvaruso, V.; Staiano, L.; Di Marco, V.; et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis 2020, 296, 40–47. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Caturano, A.; Galiero, R.; Vetrano, E.; Nevola, R.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Di Marco, V.; et al. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre-diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2345–2353. [Google Scholar] [CrossRef]
- A DeFronzo, R.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. Metab. 1979, 237, E214–E223. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Kronenberg, F.; Kielstein, J.T.; Haller, H.; Morath, C.; Ritz, E.; Fliser, D. Renal Insulin Resistance Syndrome, Adiponectin and Cardiovascular Events in Patients with Kidney Disease: The Mild and Moderate Kidney Disease Study. J. Am. Soc. Nephrol. 2005, 16, 1091–1098. [Google Scholar] [CrossRef]
- Park, J.H.; Oh, S.W.; Ahn, S.Y.; Kim, S.; Na, K.Y.; Chae, N.-W.; Kim, S.; Chin, H.J. Decreased estimated glomerular filtration rate is not directly related to increased insulin resistance. Diabetes Res. Clin. Pr. 2013, 99, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Liu, X.; Li, M.; Wu, B.; Li, Y.; Liang, Y.; Shao, X.; Holthöfer, H.; Zou, H. Association of Insulin Resistance with Chronic Kidney Disease in Non-Diabetic Subjects with Normal Weight. PLoS ONE 2013, 8, e74058. [Google Scholar] [CrossRef]
- Trirogoff, M.L.; Shintani, A.; Himmelfarb, J.; Ikizler, T.A. Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3–4 chronic kidney disease patients. Am. J. Clin. Nutr. 2007, 86, 1642–1648. [Google Scholar] [CrossRef]
- Gohda, T.; Gotoh, H.; Tanimoto, M.; Sato, M.; Io, H.; Kaneko, K.; Hamada, C.; Tomino, Y. Relationship between Abdominal Fat Accumulation and Insulin Resistance in Hemodialysis Patients. Hypertens. Res. 2008, 31, 83–88. [Google Scholar] [CrossRef]
- Ma, A.; Liu, F.; Wang, C.; Liang, K.; Yan, F.; Hou, X.; Liu, J.; Chen, L. Both insulin resistance and metabolic syndrome accelerate the progression of chronic kidney disease among Chinese adults: Results from a 3-year follow-up study. Int. Urol. Nephrol. 2018, 50, 2239–2244. [Google Scholar] [CrossRef]
- Jang, C.M.; Hyun, Y.Y.; Lee, K.B.; Kim, H. Insulin resistance is associated with the development of albuminuria in Korean subjects without diabetes. Endocrine 2014, 48, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, A.; Ning, G.; Zhang, L.; Mu, Y. Insulin resistance is associated with urinary albumin-creatinine ratio in normal weight individuals with hypertension and diabetes: The REACTION study. J. Diabetes 2019, 12, 406–416. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Ikee, R.; Hamasaki, Y.; Oka, M.; Maesato, K.; Mano, T.; Moriya, H.; Ohtake, T.; Kobayashi, S. Glucose Metabolism, Insulin Resistance, and Renal Pathology in Non-Diabetic Chronic Kidney Disease. Nephron Clin. Pr. 2008, 108, c163–c168. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet–induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef]
- Koppe, L.; Pelletier, C.C.; Alix, P.M.; Kalbacher, E.; Fouque, D.; Soulage, C.O.; Guebre-Egziabher, F. Insulin resistance in chronic kidney disease: New lessons from experimental models. Nephrol. Dial. Transplant. 2013, 29, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.S.; Zhang, L.; Mitch, W.E. Molecular mechanisms of insulin resistance in chronic kidney disease. Kidney Int. 2015, 88, 1233–1239. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, E.; Hosseinzadeh, F.M.; Hosseinzadeh-Attar, M.J. Adipokines in critical illness: A review of the evidence and knowledge gaps. Biomed. Pharmacother. 2018, 108, 1739–1750. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Kimoto, M.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; Tokuyama, H.; et al. The hydrolase DDAH2 enhances pancreatic insulin secretion by transcriptional regulation of secretagogin through a Sirt1-dependent mechanism in mice. FASEB J. 2013, 27, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, R.; Zhao, C.; Wang, Y.; Wang, J.; Liu, S.; Cao, J.; Wang, H.; Wang, T.; Guo, Y.; et al. Dimethylarginine Dimethylaminohydrolase 1 Protects Against High-Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Antioxid. Redox Signal. 2017, 26, 598–609. [Google Scholar] [CrossRef]
- Hosoya, K.; Minakuchi, H.; Wakino, S.; Fujimura, K.; Hasegawa, K.; Komatsu, M.; Yoshifuji, A.; Futatsugi, K.; Shinozuka, K.; Washida, N.; et al. Insulin resistance in chronic kidney disease is ameliorated by spironolactone in rats and humans. Kidney Int. 2015, 87, 749–760. [Google Scholar] [CrossRef]
- Adler, G.K.; Murray, G.R.; Turcu, A.F.; Nian, H.; Yu, C.; Solorzano, C.C.; Manning, R.; Peng, D.; Luther, J.M. Primary Aldosteronism Decreases Insulin Secretion and Increases Insulin Clearance in Humans. Hypertension 2020, 75, 1251–1259. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R. The Use of Fibroblast Growth Factor 23 Testing in Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 1283–1303. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Gal-Moscovici, A.; Sprague, S.M. Use of vitamin D in chronic kidney disease patients. Kidney Int. 2010, 78, 146–151. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B. Vitamin D and diabetes. J. Steroid Biochem. Mol. Biol. 2010, 121, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A. Obesity, Metabolic Syndrome, and Type 2 Diabetes: Inflammatory Basis of Glucose Metabolic Disorders. Nutr. Rev. 2008, 65, S152–S156. [Google Scholar] [CrossRef]
- Oh, J.; Riek, A.E.; Darwech, I.; Funai, K.; Shao, J.; Chin, K.; Sierra, O.L.; Carmeliet, G.; Ostlund, R.E., Jr.; Bernal-Mizrachi, C. Deletion of Macrophage Vitamin D Receptor Promotes Insulin Resistance and Monocyte Cholesterol Transport to Accelerate Atherosclerosis in Mice. Cell Rep. 2015, 10, 1872–1886. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, Y.; Wang, W.; Scott, J.; Kim, K.H.; Sun, Z.; Guo, Q.; Lu, Y.; Gonzales, N.M.; Wu, H.; et al. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology 2019, 71, 1559–1574. [Google Scholar] [CrossRef]
- Leung, P.S. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef]
- Vaidya, A.; Williams, J. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism 2012, 61, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline Serum 25-Hydroxy Vitamin D Is Predictive of Future Glycemic Status and Insulin Resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef]
- A Alvarez, J.; Ashraf, A.P.; Hunter, G.R.; A Gower, B. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am. J. Clin. Nutr. 2010, 92, 1344–1349. [Google Scholar] [CrossRef]
- Weiler, H.A.; Lowe, J.; Krahn, J.; Leslie, W.D. Osteocalcin and vitamin D status are inversely associated with homeostatic model assessment of insulin resistance in Canadian Aboriginal and white women: The First Nations Bone Health Study. J. Nutr. Biochem. 2013, 24, 412–418. [Google Scholar] [CrossRef]
- Heaney, R.P.; French, C.B.; Nguyen, S.; Ferreira, M.; Baggerly, L.L.; Brunel, L.; Veugelers, P. A Novel Approach Localizes the Association of Vitamin D Status with Insulin Resistance to One Region of the 25-Hydroxyvitamin D Continuum12. Adv. Nutr. 2013, 4, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Kao, W.L.; Crainiceanu, C.; Sozio, S.M.; Oberai, P.C.; Shafi, T.; Coresh, J.; Powe, N.R.; Plantinga, L.C.; Jaar, B.G.; et al. Biomarkers of Vascular Calcification and Mortality in Patients with ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef]
- Kendrick, J.; Cheung, A.K.; Kaufman, J.S.; Greene, T.; Roberts, W.L.; Smits, G.; Chonchol, M.; The HOST Investigators. FGF-23 Associates with Death, Cardiovascular Events, and Initiation of Chronic Dialysis. J. Am. Soc. Nephrol. 2011, 22, 1913–1922. [Google Scholar] [CrossRef]
- Mirza, M.A.; Alsiö, J.; Hammarstedt, A.; Erben, R.G.; Michaëlsson, K.; Tivesten, Å.; Marsell, R.; Orwoll, E.; Karlsson, M.K.; Ljunggren, Ö.; et al. Circulating Fibroblast Growth Factor-23 Is Associated With Fat Mass and Dyslipidemia in Two Independent Cohorts of Elderly Individuals. Arter. Thromb. Vasc. Biol. 2011, 31, 219–227. [Google Scholar] [CrossRef]
- Chen, P.C.; Chang, Y.D.; Lee, M.C.; Hsu, B.G. High Serum Fibroblast Growth Factor 23 Level Is Associated with Metabolic Syndrome in Kidney Transplantation Patients. Transplant Proc. 2020, 520, 3168–3172. [Google Scholar] [CrossRef] [PubMed]
- Holecki, M.; Chudek, J.; Owczarek, A.; Olszanecka-Glinianowicz, M.; Bożentowicz-Wikarek, M.; Dulawa, J.; Mossakowska, M.; Zdrojewski, T.; Skalska, A.; Więcek, A. Inflammation but not obesity or insulin resistance is associated with increased plasma fibroblast growth factor 23 concentration in the elderly. Clin. Endocrinol. 2015, 82, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.; El Nokeety, M.M.; Heikal, A.A.; Abdulazim, D.O.; Naguib, M.M.; El Din, U.A.A.S. Fibroblast growth factor-23 is a strong predictor of insulin resistance among chronic kidney disease patients. Ren. Fail. 2018, 40, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Brown, E.J.; Wright, C.M.; Bhat, S.; Banerjee, R.R.; Dai, C.Y.; Enders, G.H.; Silberg, D.G.; Wen, X.; Wu, G.D.; et al. A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA 2001, 98, 502–506. [Google Scholar] [CrossRef]
- Nakashima, A.; Yokoyama, K.; Kawanami, D.; Ohkido, I.; Urashima, M.; Utsunomiya, K.; Yokoo, T. Association between resistin and fibroblast growth factor 23 in patients with type 2 diabetes mellitus. Sci. Rep. 2018, 8, 13999. [Google Scholar] [CrossRef] [PubMed]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farré, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.L.; Faulconbridge, L.F.; Webb, V.L.; Wadden, T.A. Behavioral and pharmacologic therapies for obesity. Nat. Rev. Endocrinol. 2010, 6, 578–588. [Google Scholar] [CrossRef]
- Pitta, E.; Liaras, K. Thiazoles and Thiazolidinones as Antioxidants. Curr. Med. Chem. 2013, 20, 4460–4480. [Google Scholar] [CrossRef]
- Manjal, S.K.; Kaur, R.; Bhatia, R.; Kumar, K.; Singh, V.; Shankar, R.; Kaur, R.; Rawal, R.K. Synthetic and medicinal perspective of thiazolidinones: A review. Bioorganic Chem. 2017, 75, 406–423. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Mazzone, T.; Meyer, P.M.; Feinstein, S.B.; Davidson, M.H.; Kondos, G.T.; D’Agostino, R.B.; Perez, A.; Provost, J.-C.; Haffner, S.M. Effect of Pioglitazone Compared with Glimepiride on Carotid Intima-Media Thickness in Type 2 Diabetes. JAMA 2006, 296, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2018, 70, 711–724. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.P.; Jenkins, E.C.; Estes, S.K.; Castaneda, A.V.; Ueta, K.; Farmer, T.D.; Puglisi, A.E.; Swift, L.L.; Printz, R.L.; Shiota, M. Correcting Postprandial Hyperglycemia in Zucker Diabetic Fatty Rats with an SGLT2 Inhibitor Restores Glucose Effectiveness in the Liver and Reduces Insulin Resistance in Skeletal Muscle. Diabetes 2017, 66, 1172–1184. [Google Scholar] [CrossRef]
- Spoto, B.; Pizzini, P.; Cutrupi, S.; Tripepi, G.; Curatola, G.; Mallamaci, F.; Zoccali, C. Vitamin D receptor activation by paricalcitol and insulin resistance in CKD. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, L.; Asadi, S.; Al-Mousavi, Z.; Niknam, R. A randomized controlled clinical trial comparing calcitriol versus cholecalciferol supplementation to reduce insulin resistance in patients with non-alcoholic fatty liver disease. Clin. Nutr. 2020, 40, 2999–3005. [Google Scholar] [CrossRef]
- Trummer, C.; Theiler-Schwetz, V.; Kollmann, M.; Wölfler, M.; Münzker, J.; Pilz, S.; Pieber, T.R.; Heijboer, A.C.; Obermayer-Pietsch, B.; Lerchbaum, E. Effects of vitamin D supplementation on metabolic and endocrine parameters in healthy premenopausal women: A randomized controlled trial. Clin. Nutr. 2019, 39, 718–726. [Google Scholar] [CrossRef]
- de Boer, I.H.; Sachs, M.; Hoofnagle, A.N.; Utzschneider, K.M.; Kahn, S.E.; Kestenbaum, B.; Himmelfarb, J. Paricalcitol does not improve glucose metabolism in patients with stage 3–4 chronic kidney disease. Kidney Int. 2013, 83, 323–330. [Google Scholar] [CrossRef]
- Wallace, H.J.; Holmes, L.; Ennis, C.N.; Cardwell, C.R.; Woodside, J.V.; Young, I.; Bell, P.M.; Hunter, S.J.; McKinley, M.C. Effect of vitamin D3 supplementation on insulin resistance and β-cell function in prediabetes: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2019, 110, 1138–1147. [Google Scholar] [CrossRef]
- Wood, A.D.; Secombes, K.R.; Thies, F.; Aucott, L.; Black, A.J.; Mavroeidi, A.; Simpson, W.G.; Fraser, W.D.; Reid, D.M.; Macdonald, H.M. Vitamin D3Supplementation Has No Effect on Conventional Cardiovascular Risk Factors: A Parallel-Group, Double-Blind, Placebo-Controlled RCT. J. Clin. Endocrinol. Metab. 2012, 97, 3557–3568. [Google Scholar] [CrossRef]
- He, S.; Yu, S.; Zhou, Z.; Wang, C.; Wu, Y.; Li, W. Effect of vitamin D supplementation on fasting plasma glucose, insulin resistance and prevention of type 2 diabetes mellitus in non-diabetics: A systematic review and meta-analysis. Biomed. Rep. 2018, 8, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. Vitamin D Supplementation, Glycemic Control, and Insulin Resistance in Prediabetics: A Meta-Analysis. J. Endocr. Soc. 2018, 2, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Łagowska, K.; Bajerska, J.; Jamka, M. The Role of Vitamin Oral Supplementation in Insulin Resistance in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 1637. [Google Scholar] [CrossRef]
- Wojcik, M.; Dolezal-Oltarzewska, K.; Janus, D.; Drozdz, D.; Sztefko, K.; Starzyk, J.B. FGF23 contributes to insulin sensitivity in obese adolescents—Preliminary results. Clin. Endocrinol. 2011, 77, 537–540. [Google Scholar] [CrossRef]
- Block, G.A.; Spiegel, D.M.; Ehrlich, J.; Mehta, R.; Lindbergh, J.; Dreisbach, A.; Raggi, P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005, 68, 1815–1824. [Google Scholar] [CrossRef]
- Kakuta, T.; Tanaka, R.; Hyodo, T.; Suzuki, H.; Kanai, G.; Nagaoka, M.; Takahashi, H.; Hirawa, N.; Oogushi, Y.; Miyata, T.; et al. Effect of Sevelamer and Calcium-Based Phosphate Binders on Coronary Artery Calcification and Accumulation of Circulating Advanced Glycation End Products in Hemodialysis Patients. Am. J. Kidney Dis. 2011, 57, 422–431. [Google Scholar] [CrossRef]
- Toida, T.; Fukudome, K.; Fujimoto, S.; Yamada, K.; Sato, Y.; Chiyotanda, S.; Kitamura, K. Effect of lanthanum carbonate vs. calcium carbonate on serum calcium in hemodialysis patients: A crossover study. Clin. Nephrol. 2012, 78, 216–223. [Google Scholar] [CrossRef]
- Spatz, C.; Roe, K.; Lehman, E.; Verma, N. Effect of a Non-Calcium-Based Phosphate Binder on Fibroblast Growth Factor 23 in Chronic Kidney Disease. Nephron Clin. Pr. 2013, 123, 61–66. [Google Scholar] [CrossRef]
- Covic, A.; Passlick-Deetjen, J.; Kroczak, M.; Büschges-Seraphin, B.; Ghenu, A.; Ponce, P.; Marzell, B.; De Francisco, A.L. A comparison of calcium acetate/magnesium carbonate and sevelamer-hydrochloride effects on fibroblast growth factor-23 and bone markers: Post hoc evaluation from a controlled, randomized study. Nephrol. Dial. Transplant. 2013, 28, 2383–2392. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, A.; Kato, K.; Ohkido, I.; Yokoo, T. Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review. Nutrients 2021, 13, 4349. https://doi.org/10.3390/nu13124349
Nakashima A, Kato K, Ohkido I, Yokoo T. Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review. Nutrients. 2021; 13(12):4349. https://doi.org/10.3390/nu13124349
Chicago/Turabian StyleNakashima, Akio, Kazuhiko Kato, Ichiro Ohkido, and Takashi Yokoo. 2021. "Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review" Nutrients 13, no. 12: 4349. https://doi.org/10.3390/nu13124349
APA StyleNakashima, A., Kato, K., Ohkido, I., & Yokoo, T. (2021). Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review. Nutrients, 13(12), 4349. https://doi.org/10.3390/nu13124349





