Body Composition, Anemia, and Kidney Function among Guatemalan Sugarcane Workers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic and Anthropometric Measures
3.2. Markers of Underlying Health and Anemia
3.3. Risk Factors for Lower eGFR, Elevated HbA1c and Anemia
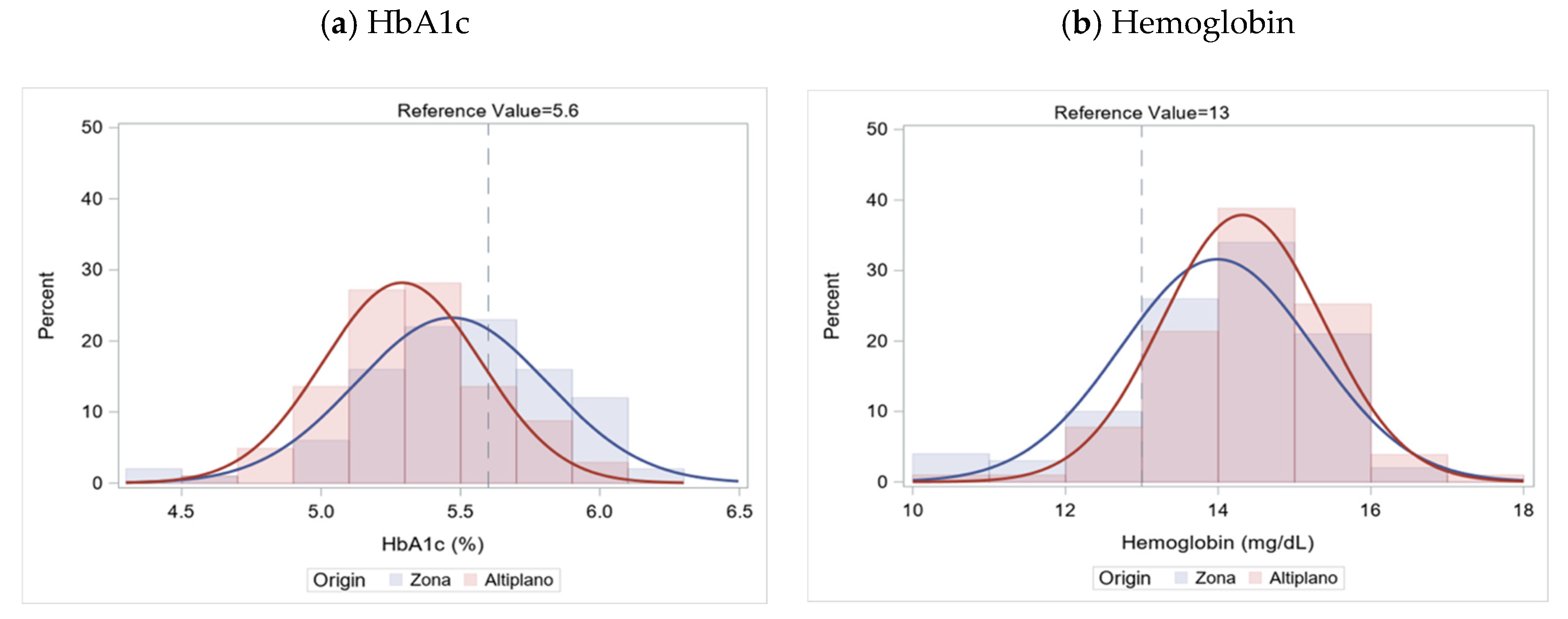
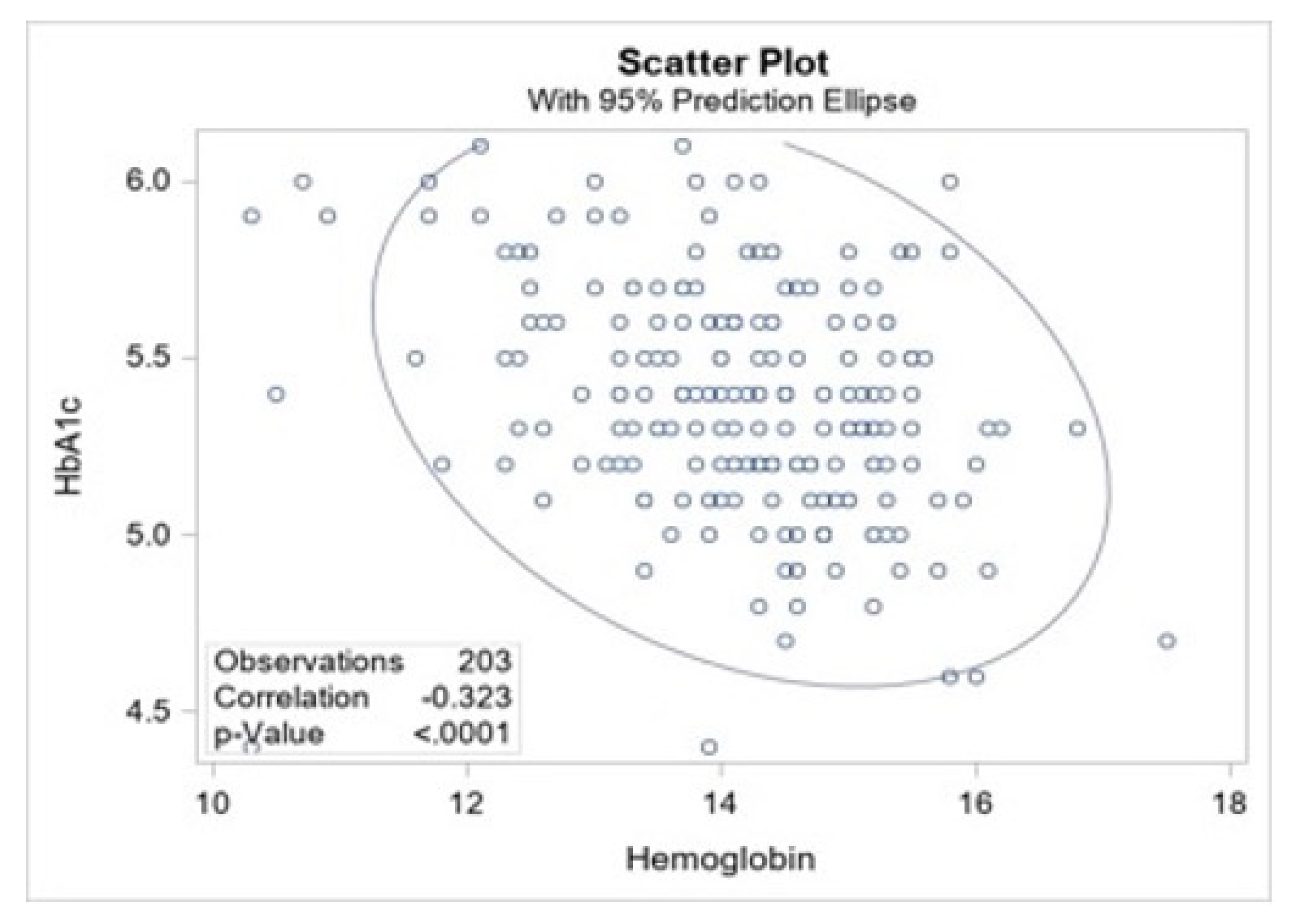
3.4. Correlation of HbA1c and Hemoglobin
3.5. Anemia Type and Kidney Function
4. Discussion
4.1. Nutritional Markers, Underlying Health and Kidney Function
4.2. Anemia
4.3. Differences by Worker Origin
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Agency for International Development Guatemala: Nutrition Profile|U.S. Agency for International Development. Available online: https://www.usaid.gov/global-health/health-areas/nutrition/countries/guatemala-nutrition-profile (accessed on 2 February 2021).
- Chew, A.; Moran, A.; Barnoya, J. Food swamps surrounding schools in three areas of guatemala. Prev. Chronic Dis. 2020, 17, E75. [Google Scholar] [CrossRef] [PubMed]
- Mazariegos, M.; Kroker-Lobos, M.F.; Ramírez-Zea, M. Socio-economic and ethnic disparities of malnutrition in all its forms in Guatemala. Public Health Nutr. 2020, 23, s68–s76. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Baek, S.H.; Ahn, S.Y.; Chin, H.J.; Na, K.Y.; Chae, D.-W.; Kim, S. Anemia Is a Risk Factor for Acute Kidney Injury and Long-Term Mortality in Critically Ill Patients. Tohoku J. Exp. Med. 2015, 237, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossert, J.; Froissart, M. Role of anemia in progression of chronic kidney disease. Semin. Nephrol. 2006, 26, 283–289. [Google Scholar] [CrossRef]
- Hu, S.L.; Said, F.R.; Epstein, D.; Lokeshwari, M. The impact of anemia on renal recovery and survival in acute kidney injury. Clin. Nephrol. 2013, 79, 221–228. [Google Scholar] [CrossRef]
- Iseki, K.; Ikemiya, Y.; Iseki, C.; Takishita, S. Haematocrit and the risk of developing end-stage renal disease. Nephrol. Dial. Transplant. 2003, 18, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, R.S.B.; Vangala, C.; Mandayam, S.; Chavarria, D.; García-Trabanino, R.; Garcia, F.; Garcia, L.L.; Murray, K.O. Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int. 2018, 94, 1205–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Pan American Health Organization. Epidemic of Chronic Kidney Disease in Agricultural Communities in Central America. In Case Definitions, Methodological Basis and Approaches for Public Health Surveillance; PAHO: Washington, DC, USA, 2017. [Google Scholar]
- Butler-Dawson, J.; Krisher, L.; Asensio, C.; Cruz, A.; Tenney, L.; Weitzenkamp, D.; Dally, M.; Asturias, E.J.; Newman, L.S. Risk factors for declines in kidney function in sugarcane workers in guatemala. J. Occup. Environ. Med. 2018, 60, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Dally, M.; Butler-Dawson, J.; Johnson, R.J.; Krisher, L.; Jaramillo, D.; Newman, K.L.; Newman, L.S. Creatinine Fluctuations Forecast Cross-Harvest Kidney Function Decline Among Sugarcane Workers in Guatemala. Kidney Int. Rep. 2020, 5, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Dally, M.; Butler-Dawson, J.; Cruz, A.; Krisher, L.; Johnson, R.J.; Asensio, C.; Pilloni, W.D.; Asturias, E.J.; Newman, L.S. Longitudinal trends in renal function among first time sugarcane harvesters in Guatemala. PLoS ONE 2020, 15, e0229413. [Google Scholar] [CrossRef] [Green Version]
- Butler-Dawson, J.; Krisher, L.; Yoder, H.; Dally, M.; Sorensen, C.; Johnson, R.J.; Asensio, C.; Cruz, A.; Johnson, E.C.; Carlton, E.J.; et al. Evaluation of heat stress and cumulative incidence of acute kidney injury in sugarcane workers in Guatemala. Int. Arch. Occup. Environ. Health 2019, 92, 977–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, C.J.; Butler-Dawson, J.; Dally, M.; Krisher, L.; Griffin, B.R.; Johnson, R.J.; Lemery, J.; Asensio, C.; Tenney, L.; Newman, L.S. Risk Factors and Mechanisms Underlying Cross-Shift Decline in Kidney Function in Guatemalan Sugarcane Workers. J. Occup. Environ. Med. 2019, 61, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.B.N.T.; Hettiarachchi, T.W.; Sudeshika, T.; Badurdeen, Z.; Abeysundara, H.; Ranasinghe, S.; Rathnayake, M.P.; Nanayakkara, N. Snap shot view on anaemia in chronic kidney disease of uncertain aetiology. Nephrology 2019, 24, 1033–1040. [Google Scholar] [CrossRef]
- Fischer, R.S.B.; Mandayam, S.; Chavarria, D.; Vangala, C.; Nolan, M.S.; Garcia, L.L.; Palma, L.; Garcia, F.; García-Trabanino, R.; Murray, K.O. Clinical evidence of acute mesoamerican nephropathy. Am. J. Trop. Med. Hyg. 2017, 97, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.; Aragón, A.; González, M.; Weiss, I.; Glaser, J.; Rivard, C.J.; Roncal-Jiménez, C.; Correa-Rotter, R.; Johnson, R.J. Heat stress, hydration and uric acid: A cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 2016, 6, e011034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Trabanino, R.; Jarquín, E.; Wesseling, C.; Johnson, R.J.; González-Quiroz, M.; Weiss, I.; Glaser, J.; José Vindell, J.; Stockfelt, L.; Roncal, C.; et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ. Res. 2015, 142, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Hansson, E.; Glaser, J.; Jakobsson, K.; Weiss, I.; Wesseling, C.; Lucas, R.A.I.; Wei, J.L.K.; Ekström, U.; Wijkström, J.; Bodin, T.; et al. Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers. Nutrients 2020, 12, 1639. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Dillon, J.-C. Control of iron deficiency in developing countries. Sante 2002, 12, 22–30. [Google Scholar]
- Yip, R.; Ramakrishnan, U. Experiences and challenges in developing countries. J. Nutr. 2002, 132, 827S–830S. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; Vitamin and Mineral Nutrition Information System: Geneva, Switzerland, 2011. [Google Scholar]
- Instituto Nacional de Estadistica Guatemala (INE). República de Guatemala: Encuesta Nacional de Condiciones de Vida 2014 [Republic of Guatemala: National Survey of Living Conditions 2014]; INE Guatemala: Guatemala City, Guatemala, 2015. [Google Scholar]
- Krisher, L. A Total Worker Health® Approach to Assessing Kidney Health in Sugarcane Workers in Guatemala: An Opportunity for Nutrition Intervention; National Institute of Environmental Health Sciences (NIEHS): San José, Costa Rica, 2019. Available online: https://www.niehs.nih.gov/news/events/pastmtg/assets/docs_c_e/ckd_meetingbook_508.pdf (accessed on 10 August 2021).
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; American College of Sports Medicine, Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2021. [Google Scholar]
- Hellman, R. When are HBA1C values misleading? AACE Clin. Case Rep. 2016, 2, e377–e379. [Google Scholar] [CrossRef] [Green Version]
- NGSP: Factors that Interfere with HbA1c Test Results. Available online: http://www.ngsp.org/factors.asp (accessed on 15 June 2021).
- Crowe, J.; Wesseling, C.; Solano, B.R.; Umaña, M.P.; Ramírez, A.R.; Kjellstrom, T.; Morales, D.; Nilsson, M. Heat exposure in sugarcane harvesters in Costa Rica. Am. J. Ind. Med. 2013, 56, 1157–1164. [Google Scholar] [CrossRef]
- Paula Santos, U.; Zanetta, D.M.T.; Terra-Filho, M.; Burdmann, E.A. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015, 87, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Bodin, T.; García-Trabanino, R.; Weiss, I.; Jarquín, E.; Glaser, J.; Jakobsson, K.; Lucas, R.A.I.; Wesseling, C.; Hogstedt, C.; Wegman, D.H. WE Program Working Group Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: Phase 1. Occup. Environ. Med. 2016, 73, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Lucas, R.A.I.; Epstein, Y.; Kjellstrom, T. Excessive occupational heat exposure: A significant ergonomic challenge and health risk for current and future workers. Extrem. Physiol. Med. 2014, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Krisher, L.; Butler-Dawson, J.; Yoder, H.; Pilloni, D.; Dally, M.; Johnson, E.C.; Jaramillo, D.; Cruz, A.; Asensio, C.; Newman, L.S. Electrolyte beverage intake to promote hydration and maintain kidney function in guatemalan sugarcane workers laboring in hot conditions. J. Occup. Environ. Med. 2020, 62, e696–e703. [Google Scholar] [CrossRef]
- Jackson, A.S.; Pollock, M.L. Practical assessment of body composition. Phys. Sportsmed. 1985, 13, 76–90. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Wimbley, T.D.; Graham, D.Y. Diagnosis and management of iron deficiency anemia in the 21st century. Therap. Adv. Gastroenterol. 2011, 4, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S.; Cowie, C.C.; Li, C.; Handelsman, Y.; Bloomgarden, Z.T. Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J. Diabetes 2011, 3, 67–73. [Google Scholar] [CrossRef]
- Coban, E.; Ozdogan, M.; Timuragaoglu, A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004, 112, 126–128. [Google Scholar] [CrossRef]
- English, E.; Idris, I.; Smith, G.; Dhatariya, K.; Kilpatrick, E.S.; John, W.G. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: A systematic review. Diabetologia 2015, 58, 1409–1421. [Google Scholar] [CrossRef] [Green Version]
- Sundgot-Borgen, J.; Meyer, N.L.; Lohman, T.G.; Ackland, T.R.; Maughan, R.J.; Stewart, A.D.; Müller, W. How to minimise the health risks to athletes who compete in weight-sensitive sports review and position statement on behalf of the Ad Hoc Research Working Group on Body Composition, Health and Performance, under the auspices of the IOC Medical Commission. Br. J. Sports Med. 2013, 47, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine joint position statement. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- Bentley, M.E.; Griffiths, P.L. The burden of anemia among women in India. Eur. J. Clin. Nutr. 2003, 57, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, C.J.; Krisher, L.; Butler-Dawson, J.; Dally, M.; Dexter, L.; Asensio, C.; Cruz, A.; Newman, L.S. Workplace Screening Identifies Clinically Significant and Potentially Reversible Kidney Injury in Heat-Exposed Sugarcane Workers. Int. J. Environ. Res. Public Health 2020, 17, 8552. [Google Scholar] [CrossRef] [PubMed]
- NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?cycle=2017-2020 (accessed on 15 October 2021).
- Fernando, B.N.T.W.; Sudeshika, T.S.H.; Hettiarachchi, T.W.; Badurdeen, Z.; Abeysekara, T.D.J.; Abeysundara, H.T.K.; Jayasinghe, S.; Ranasighe, S.; Nanayakkara, N. Evaluation of biochemical profile of Chronic Kidney Disease of uncertain etiology in Sri Lanka. PLoS ONE 2020, 15, e0232522. [Google Scholar] [CrossRef] [PubMed]
- Raines, N.; González, M.; Wyatt, C.; Kurzrok, M.; Pool, C.; Lemma, T.; Weiss, I.; Marín, C.; Prado, V.; Marcas, E.; et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev. 2014, 16, 16–22. [Google Scholar]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.A.; Kaufman, S.; Reynolds, S.W.; McCook, B.T.; Kan, G.; Christiaens, I.; Symonds, M.E.; Olson, D.M. Differential effects of maternal nutrient restriction through pregnancy on kidney development and later blood pressure control in the resulting offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R197–R205. [Google Scholar] [CrossRef] [Green Version]
- Wood-Bradley, R.J.; Barrand, S.; Giot, A.; Armitage, J.A. Understanding the role of maternal diet on kidney development; an opportunity to improve cardiovascular and renal health for future generations. Nutrients 2015, 7, 1881–1905. [Google Scholar] [CrossRef] [Green Version]
- American Heart Association Understanding Blood Pressure Readings|American Heart Association. Available online: https://www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings (accessed on 12 August 2021).
- Hansson, E.; Glaser, J.; Weiss, I.; Ekström, U.; Apelqvist, J.; Hogstedt, C.; Peraza, S.; Lucas, R.; Jakobsson, K.; Wesseling, C.; et al. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup. Environ. Med. 2019, 76, 818–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [Green Version]
- Tefferi, A. Anemia in adults: A contemporary approach to diagnosis. Mayo Clin. Proc. 2003, 78, 1274–1280. [Google Scholar] [CrossRef] [Green Version]
- Shaskey, D.J.; Green, G.A. Sports haematology. Sports Med. 2000, 29, 27–38. [Google Scholar] [CrossRef]
- Sawka, M.N.; Convertino, V.A.; Eichner, E.R.; Schnieder, S.M.; Young, A.J. Blood volume: Importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med. Sci. Sports Exerc. 2000, 32, 332–348. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef]
- Mujica-Coopman, M.F.; Brito, A.; López de Romaña, D.; Ríos-Castillo, I.; Coris, H.; Olivares, M. Prevalence of anemia in latin america and the caribbean. Food Nutr. Bull. 2015, 36, S119–S128. [Google Scholar] [CrossRef] [Green Version]
- Ananda Jayalal, T.B.; Jayaruwan Bandara, T.W.M.A.; Mahawithanage, S.T.C.; Wansapala, M.A.J.; Galappaththi, S.P.L. A quantitative analysis of chronic exposure of selected heavy metals in a model diet in a CKD hotspot in Sri Lanka. BMC Nephrol. 2019, 20, 208. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.-J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant. Soil. 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Kroker-Lobos, M.F.; Alvarez, C.S.; Rivera-Andrade, A.; Smith, J.W.; Egner, P.; Torres, O.; Lazo, M.; Freedman, N.D.; Guallar, E.; Graubard, B.I.; et al. Association between aflatoxin-albumin adduct levels and tortilla consumption in Guatemalan adults. Toxicol. Rep. 2019, 6, 465–471. [Google Scholar] [CrossRef]
- Beutler, E.; Waalen, J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef] [Green Version]
- Billett, H.H. Hemoglobin and Hematocrit. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
| Variable | All Participants (N = 203) | Altiplano (n = 103) | Zona (n = 100) | p-Value |
|---|---|---|---|---|
| Mean (SD) or N (%) | ||||
| Age (years) | 28 (7) | 28 (7) | 28 (8) | 0.67 |
| Height (cm) | 160.2 (5.5) | 159.5 (4.5) | 160.8 (6.3) | 0.09 |
| Weight (kg) | 55.7 (7.5) | 55.5 (6.5) | 55.9 (8.4) | 0.72 |
| Body mass index (kg/m2) | 21.7 (2.5) | 21.8 (2.1) | 21.6 (2.7) | 0.52 |
| --Underweight (BMI ≤ 18.5), n(%) | 10 (5%) | 6 (6%) | 4 (4%) | |
| --Overweight (BMI ≥ 25), n(%) | 14 (7%) | 7 (7%) | 7 (7%) | 0.90 |
| Body density (kg/L) | 1.1 (0.0) | 1.1 (0.0) | 1.1 (0.0) | 0.99 |
| Body fat % | 8.5 (4.0) | 8.5 (3.5) | 8.5 (4.5) | 0.96 |
| --Essential body fat % (2–5%), n(%) | 23 (11%) | 9 (9%) | 14 (14%) | 0.23 |
| Fat mass (kg) | 5.0 (3.8) | 4.9 (2.6) | 5.0 (3.8) | 0.71 |
| Fat free mass * (kg) | 50.8 (5.2) | 50.7 (4.8) | 50.9 (5.7) | 0.78 |
| Biomarker | All Participants (N = 203) | Altiplano (N = 103) | Zona (N = 100) | p-Value | Reference Values |
|---|---|---|---|---|---|
| Mean (SD) or N (%) | |||||
| Serum Creatinine | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.33 | 0.6–1.2 mg/dL |
| eGFR | 114.2 (17.8) | 113.2 (16.6) | 115.2 (19.0) | 0.44 | ≥90 mL/min/1.73 m2 |
| --60–89 eGFR (n (%)) | 22 (11%) | 11 (11%) | 11 (11%) | 0.93 | 60–89 mL/min/1.73 m2 |
| --30–59 eGFR (n (%)) | 3 (1%) | 1 (1%) | 2 (2%) | 30–59 mL/min/1.73 m2 | |
| Serum Albumin | 4.8 (0.2) | 4.8 (0.2) | 4.8 (0.2) | 0.44 | 3.4–5.4 g/dL |
| BUN | 13.5 (3.7) | 12.3 (3.5) | 14.6 (3.5) | <0.01 * | 7–20 mg/dL |
| --Elevated BUN (n (%)) | 12 (6%) | 5 (5%) | 7 (7%) | 0.52 | >20 mg/dL |
| HbA1c | 5.4 (0.3) | 5.3 (0.3) | 5.5 (0.3) | <0.01 * | 3.6–5.6% |
| --Elevated HbA1c (n (%)) | 42 (21%) | 12 (12%) | 30 (30%) | <0.01 * | 5.7–6.4% |
| Systolic BP, mean (SD) | 114.3 (12.3) | 116.2 (11.6) | 112.3 (12.8) | 0.03 | <120 mmHg |
| --High Systolic (n (%)) | 88 (44%) | 52 (51%) | 36 (37%) | 0.05 | ≥120 mmHg |
| Diastolic BP, mean (SD) | 70.6 (7.7) | 71.5 (7.9) | 69.6 (7.5) | 0.09 | <80 mmHg |
| --High diastolic (n (%)) | 39 (19.4%) | 24 (23.3%) | 15 (15.3%) | 0.15 | ≥80 mmHg |
| Blood pressure (n (%)) | |||||
| --Normal | 98 (49%) | 42 (41%) | 56 (57%) | 0.02 | <120 and < 80 mm Hg |
| --Elevated | 103 (51%) | 61 (59%) | 42 (42%) | ≥120 and/or ≥ 80 mm Hg | |
| Blood Count Measures | Cohort (n = 203) | Altiplano (n = 103) | Zona (n = 100) | p-Value | Reference Values |
|---|---|---|---|---|---|
| Mean (SD) or N (%) | |||||
| Hemoglobin | 14.2 (1.2) | 14.3 (1.1) | 14.0 (1.3) | 0.04 | 13–18 g/dL |
| --Anemia, n (%) | 27 (13%) | 10 (10%) | 17 (17%) | 0.13 | <13 g/dL |
| Hematocrit | 41.4 (3.3) | 41.8 (3.0) | 41.0 (3.6) | 0.07 | 42–50% |
| --Low Hematocrit, n (%) | 109 (54%) | 51 (50%) | 58 (53%) | 0.23 | <42% |
| MCV | 90.4 (3.5) | 90.1 (3.4) | 90.8 (3.6) | 0.11 | 82–92 fL |
| MCH | 31.0 (1.3) | 30.9 (1.3) | 31.1 (1.3) | 0.20 | 27–32 pg |
| MCHC | 34.3 (0.5) | 34.3 (0.5) | 34.3 (0.6) | 0.88 | 32–36 g/dL |
| RDW | 12.8 (0.9) | 12.6 (0.8) | 12.9 (0.9) | <0.01 * | 11.8–15.6% |
| % Lymphocytes | 31.6 (7.1) | 30.9 (7.6) | 32.4 (6.6) | 0.14 | 20–45% |
| # Lymphocytes | 2.9 (0.7) | 2.8 (0.6) | 2.9 (0.7) | 0.17 | 1.09–2.99 K/uL |
| % Monocytes | 9.4 (3.9) | 7.7 (1.3) | 11.2 (4.7) | <0.01 * | 3–8% |
| # Monocytes | 0.9 (0.4) | 0.7 (0.2) | 1.0 (0.5) | <0.01 * | 0.24–0.79 K/uL |
| % Segmented neutrophils | 59.2 (8.5) | 61.5 (8.3) | 56.9 (8.1) | <0.01 * | 40–60% |
| # Segmented neutrophils | 5.6 (1.8) | 5.9 (2.1) | 5.21 (1.3) | <0.01 * | 1.63–6.96 K/uL |
| Leukocytes | 9.3 (2.0) | 9.4 (2.2) | 9.1 (1.7) | 0.38 | 5–10 K/uL |
| Erythrocytes | 4.6 (0.4) | 4.7 (0.4) | 4.5 (0.4) | 0.01 * | 4.5–6.2 M/uL |
| Platelets | 324.7 (71.5) | 339.9 (70.5) | 309.0 (69.3) | <0.01 * | 150–500 K/uL |
| Measures | Estimate (95% CI) | p-Value |
|---|---|---|
| Elevated HbA1C (≥5.7) (ref: No) | −7.7 (−13.1, −2.3) | <0.01 * |
| Anemia (Hb < 13 g/dL) | −18.2 (−24.2, −12.2) | <0.01 * |
| Low hematocrit (<42%) (ref: No) | −7.7 (−12.0, −3.5) | <0.01 * |
| Serum albumin (g/dL) | −11.9 (−21.1, −2.7) | <0.01 * |
| BUN (mg/dL) | −2.9 (−3.3, −2.4) | <0.01 * |
| Systolic blood pressure (mmHg) | 0.0 (−0.2, 0.2) | 0.99 |
| Diastolic blood pressure (mmHg) | −0.0 (−0.3, 0.3) | 0.95 |
| Body fat % | −0.1 (−0.6, 0.5) | 0.79 |
| Fat mass (kg) | −0.2 (−0.8, 0.5) | 0.68 |
| Fat free mass (kg) | −0.2 (−0.6, 0.2) | 0.32 |
| BMI (kg/m2) | −0.2 (−1.1, 0.7) | 0.64 |
| Measures | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| eGFR (ml/min per 1.73 m2) | 0.95 (0.92, 0.97) | <0.01 * |
| Elevated HbA1c (≥5.7%) (ref: No) | 3.30 (1.35, 8.08) | <0.01 * |
| Serum albumin (g/dL) | 0.12 (0.02, 0.78) | 0.03 |
| BUN (mg/dL) | 1.27 (1.13, 1.43) | <0.01 * |
| Systolic blood pressure (mmHg) | 0.98 (0.95, 1.02) | 0.32 |
| Diastolic blood pressure (mmHg) | 0.98 (0.93, 1.04) | 0.48 |
| Body fat % | 0.84 (0.72, 0.99) | 0.03 |
| Fat mass (kg) | 0.75 (0.58, 0.96) | 0.02 |
| Fat free mass (kg) | 0.88 (0.80, 0.96) | <0.01 * |
| BMI (kg/m2) | 0.69 (0.53, 0.89) | <0.01 * |
| Measures | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| eGFR (mL/min/1.73 m2) | 0.97 (0.95, 0.99) | 0.01 * |
| Anemia (Hb < 13 g/dL) | 3.30 (1.35, 8.05) | <0.01 * |
| Low hematocrit (<42%) (ref: No) | 2.48 (1.16, 5.29) | 0.02 |
| Serum albumin | 0.37 (0.08, 1.73) | 0.21 |
| BUN | 1.14 (1.03, 1.26) | 0.01 * |
| Systolic blood pressure (mmHg) | 1.02 (0.99, 1.05) | 0.24 |
| Diastolic blood pressure (mmHg) | 1.01 (0.96, 1.05) | 0.85 |
| Body fat % | 1.02 (0.93, 1.11) | 0.71 |
| Fat mass (kg) | 1.03 (0.93, 1.13) | 0.63 |
| Fat free mass (kg) | 0.98 (0.92, 1.05) | 0.62 |
| BMI (kg/m2) | 1.05 (0.92, 1.12) | 0.49 |
| Anemia Type | Cohort (n = 27) | Altiplano (n = 10) | Zona (n = 17) |
|---|---|---|---|
| N(%) | |||
| Microcytic | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypochromic Microcytic | 1 (4%) | 0 (0%) | 1 (6%) |
| Macrocytic | 4 (15%) | 1 (10%) | 3 (18%) |
| Borderline Macrocytic | 3 (11%) | 0 (0%) | 3 (18%) |
| Normochromic Normocytic | 19 (70%) | 9 (90%) | 10 (59%) |
| Anemia Type | eGFR, Mean (SD) | Range | p-Value |
|---|---|---|---|
| Non-anemic (Hb ≥ 13 mg/dL) (n = 175) | 117 (14) | 67–137 | <0.01 * |
| Anemic (Hb < 13 mg/dL) (n = 27) | 96 (28) | 32–135 | |
| --Hypochromic Microcytic (n = 1) | 110 (-) | - | |
| --Macrocytic (n = 4) | 89 (45) | 32–135 | |
| --Borderline Macrocytic (n = 3) | 80 (19) | 60–97 | |
| --Normochromic Normocytic (n = 19) | 100 (25) | 36–134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krisher, L.; Butler-Dawson, J.; Schlosser, K.; Asensio, C.; Sinibaldi, E.; Yoder, H.; Dexter, L.; Dally, M.; Pilloni, D.; Cruz, A.; et al. Body Composition, Anemia, and Kidney Function among Guatemalan Sugarcane Workers. Nutrients 2021, 13, 3928. https://doi.org/10.3390/nu13113928
Krisher L, Butler-Dawson J, Schlosser K, Asensio C, Sinibaldi E, Yoder H, Dexter L, Dally M, Pilloni D, Cruz A, et al. Body Composition, Anemia, and Kidney Function among Guatemalan Sugarcane Workers. Nutrients. 2021; 13(11):3928. https://doi.org/10.3390/nu13113928
Chicago/Turabian StyleKrisher, Lyndsay, Jaime Butler-Dawson, Karen Schlosser, Claudia Asensio, Elisa Sinibaldi, Hillary Yoder, Lynn Dexter, Miranda Dally, Daniel Pilloni, Alex Cruz, and et al. 2021. "Body Composition, Anemia, and Kidney Function among Guatemalan Sugarcane Workers" Nutrients 13, no. 11: 3928. https://doi.org/10.3390/nu13113928
APA StyleKrisher, L., Butler-Dawson, J., Schlosser, K., Asensio, C., Sinibaldi, E., Yoder, H., Dexter, L., Dally, M., Pilloni, D., Cruz, A., Jaramillo, D., & Newman, L. S. (2021). Body Composition, Anemia, and Kidney Function among Guatemalan Sugarcane Workers. Nutrients, 13(11), 3928. https://doi.org/10.3390/nu13113928







