Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. The Systematic Review Registration and Design
2.2. Criteria for Including Studies in a Systematic Review
- (1)
- Population of adults;
- (2)
- Confirmed diagnosis of MS;
- (3)
- Any vitamin D supplementation applied;
- (4)
- Any mental health outcome assessed (including subjective and/or objective measures).
- (1)
- Studies in animal models;
- (2)
- Confirmed concurrent diagnosis of any intellectual disabilities;
- (3)
- Confirmed concurrent diagnosis of any eating disorders;
- (4)
- Confirmed concurrent diagnosis of any other neurological disorders, changing dietary behaviors (e.g., Alzheimer’s disease, epilepsy);
- (5)
- Vitamin D applied within multiple nutrients supplementation.
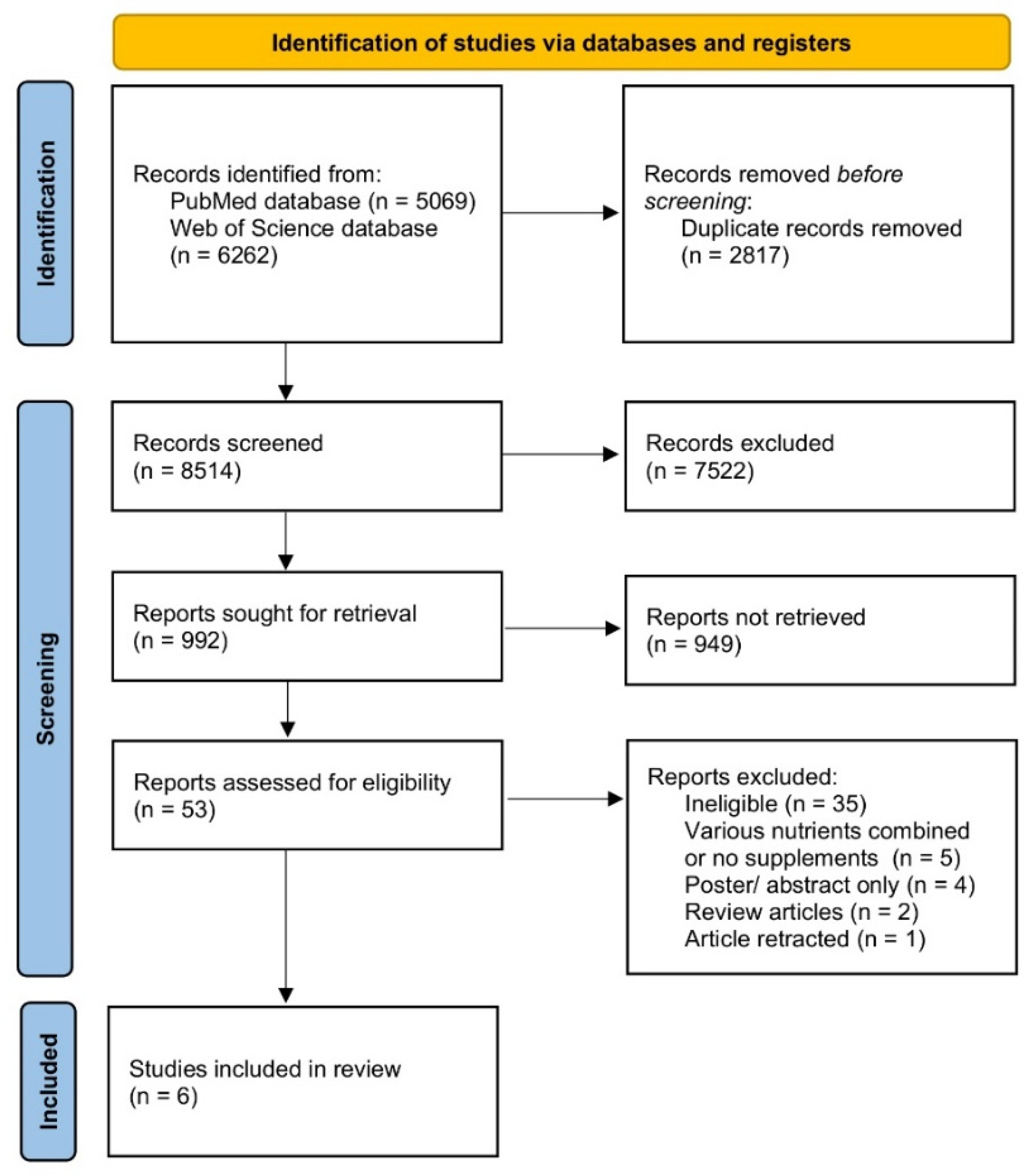
2.3. The Search and Inclusion Procedure
2.4. Data Extraction and Studies Assessment Procedure
- (1)
- The general presentation of the studies included and of the populations assessed within (authors and year; study design; country/location; population assessed; time);
- (2)
- The description of the studied group (number of participants; number of female participants; age; inclusion criteria; exclusion criteria);
- (3)
- The description of the vitamin D supplementation/intervention (vitamin D measure and dosage regimen) and of the mental health outcome (assessed outcome and psychological measure);
- (4)
- The observations and conclusions.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wootla, B.; Eriguchi, M.; Rodriguez, M. Is Multiple Sclerosis an Autoimmune Disease? Autoimmune Dis. 2012, 2012, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple Sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499849/ (accessed on 30 October 2021).
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Rolak, L.A. Multiple Sclerosis: It’s Not The Disease You Thought It Was. Clin. Med. Res. 2003, 1, 57–60. [Google Scholar] [CrossRef]
- Hong, Y.; Tang, H.R.; Ma, M.; Chen, N.; Xie, X.; He, L. Multiple sclerosis and stroke: A systematic review and meta-analysis. BMC Neurol. 2019, 19, 139. [Google Scholar] [CrossRef]
- Scalfari, A.; Knappertz, V.; Cutter, G.; Goodin, D.S.; Ashton, R.; Ebers, G.C. Mortality in patients with multiple sclerosis. Neurology 2013, 81, 184–192. [Google Scholar] [CrossRef]
- Neuhaus, M.; Calabrese, P.; Annoni, J.-M. Decision-Making in Multiple Sclerosis Patients: A Systematic Review. Mult. Scler. Int. 2018, 2018, 7835952. [Google Scholar] [CrossRef] [PubMed]
- Solaro, C.; Gamberini, G.; Masuccio, F.G. Depression in Multiple Sclerosis: Epidemiology, Aetiology, Diagnosis and Treatment. CNS Drugs 2018, 32, 117–133. [Google Scholar] [CrossRef]
- Amtmann, D.; Bamer, A.M.; Kim, J.; Chung, H.; Salem, R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil. Health J. 2018, 11, 99–107. [Google Scholar] [CrossRef]
- Gil-González, I.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M. Ángeles Quality of life in adults with multiple sclerosis: A systematic review. BMJ Open 2020, 10, e041249. [Google Scholar] [CrossRef] [PubMed]
- Fredrikson, S.; Cheng, Q.; Jiang, G.-X.; Wasserman, D. Elevated Suicide Risk among Patients with Multiple Sclerosis in Sweden. Neuroepidemiology 2003, 22, 146–152. [Google Scholar] [CrossRef]
- Bronnum-Hansen, H.; Stenager, E.; Koch-Henriksen, N. Suicide among Danes with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1457–1459. [Google Scholar] [CrossRef]
- Ragonese, P.; Aridon, P.; Salemi, G.; D’Amelio, M.; Savettieri, G. Mortality in multiple sclerosis: A review. Eur. J. Neurol. 2008, 15, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sand, I.K. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-Hydroxyvitamin D Levels and Risk of Multiple Sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.-P.; Miller, D.H.; Montalban, X.; et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Piec, P.-A.; Pons, V.; Rivest, S. Triggering Innate Immune Receptors as New Therapies in Alzheimer’s Disease and Multiple Sclerosis. Cells 2021, 10, 2164. [Google Scholar] [CrossRef] [PubMed]
- Niino, M.; Miyazaki, Y. Chapter 9—Role of Vitamin D in Multiple Sclerosis Pathogenesis and Therapy. In Nutrition and Life-Style in Neurological Autoimmune Diseases; Watson, R.R., Killgore, W.D., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 71–80. [Google Scholar]
- Martínez-Lapiscina, E.H.; Mahatanan, R.; Lee, C.-H.; Charoenpong, P.; Hong, J.-P. Associations of serum 25(OH) vitamin D levels with clinical and radiological outcomes in multiple sclerosis, a systematic review and meta-analysis. J. Neurol. Sci. 2020, 411, 116668. [Google Scholar] [CrossRef]
- Doosti-Irani, A.; Tamtaji, O.R.; Mansournia, M.A.; Mobarhan, M.G.-; Ferns, G.; Kakhaki, R.D.; Shahmirzadi, A.R.; Asemi, Z. The effects of vitamin D supplementation on expanded disability status scale in people with multiple sclerosis: A critical, systematic review and metaanalysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2019, 187, 105564. [Google Scholar] [CrossRef]
- Zheng, C.; He, L.; Liu, L.; Zhu, J.; Jin, T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2018, 23, 56–61. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef]
- Menon, V.; Vellekkatt, F. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2018, 65, 74–80. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Edmondson, D.; Wasson, L.T.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K. Vitamin D Supplementation for Depressive Symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Spedding, S. Vitamin D and Depression: A Systematic Review and Meta-Analysis Comparing Studies with and without Biological Flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, Y.; Huang, W. The effect of vitamin D supplement on negative emotions: A systematic review and meta-analysis. Depress. Anxiety 2020, 37, 549–564. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. The Influence of Vitamin D Intake and Status on Mental Health in Children: A Systematic Review. Nutrients 2021, 13, 952. [Google Scholar] [CrossRef] [PubMed]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Influence of Vitamin D Supplementation on Mental Health in Diabetic Patients: A Systematic Review. Nutrients 2021, 13, 3678. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Inflammatory Bowel Diseases and Irritable Bowel Syndrome Patients: A Systematic Review. Nutrients 2021, 13, 3662. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and me-ta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connel, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 July 2021).
- Cui, C.-J.; Wang, G.-J.; Yang, S.; Huang, S.-K.; Qiao, R.; Cui, W. Tissue Factor-bearing MPs and the risk of venous thrombosis in cancer patients: A meta-analysis. Sci. Rep. 2018, 8, 1675. [Google Scholar] [CrossRef]
- Ashtari, F.; Toghianifar, N.; Esfahani, S.H.Z.; Mansourian, M. High dose Vitamin D intake and quality of life in relapsing-remitting multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Neurol. Res. 2016, 38, 888–892. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.-H.; Bol, Y.; Damoiseaux, J.; Smolders, J.; Hupperts, R. Vitamin D 3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. J. Neurol. Sci. 2017, 378, 30–35. [Google Scholar] [CrossRef]
- Kotb, M.A.; Kamal, A.M.; Aldossary, N.M.; Bedewi, M.A. Effect of vitamin D replacement on depression in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2019, 29, 111–117. [Google Scholar] [CrossRef]
- Beckmann, Y.; Türe, S.; Duman, S.U. Vitamin D deficiency and its association with fatigue and quality of life in multiple sclerosis patients. EPMA J. 2020, 11, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.L.; Simpson, S.J.; Jelinek, G.A.; Neate, S.L.; De Livera, A.M.; Brown, C.R.; O’Kearney, E.; Marck, C.H.; Weiland, T.J. Longitudinal Associations of Modifiable Lifestyle Factors With Positive Depression-Screen Over 2.5-Years in an International Cohort of People Living With Multiple Sclerosis. Front. Psychiatry 2018, 9, 526. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; Jelinek, P.; Weiland, T.; Nag, N.; Neate, S.; Jelinek, G. Self-reported use of vitamin D supplements is associated with higher physical quality of life scores in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102760. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHOQOL: Measuring Quality of Life. Available online: https://www.who.int/tools/whoqol (accessed on 30 October 2021).
- Kremenchutzky, M.; Walt, L. Perceptions of health status in multiple sclerosis patients and their doctors. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2013, 40, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Fiol, M.P.; Gaitán, M.I.; Correale, J. Quality of Life Assessment in Multiple Sclerosis: Different Perception between Patients and Neurologists. Front. Neurol. 2018, 8, 729. [Google Scholar] [CrossRef]
- Bassi, M.; Falautano, M.; Cilia, S.; Goretti, B.; Grobberio, M.; Pattini, M.; Pietrolongo, E.; Viterbo, R.G.; Amato, M.P.; Benin, M.; et al. The coexistence of well- and ill-being in persons with multiple sclerosis, their caregivers and health professionals. J. Neurol. Sci. 2014, 337, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Stangel, M.; Penner, I.-K.; Kallmann, B.A.; Lukas, C.; Kieseier, B.C. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: The multiple sclerosis decision model. Ther. Adv. Neurol. Disord. 2015, 8, 3–13. [Google Scholar] [CrossRef]
- Dehnavi, S.R.; Heidarian, F.; Shaygannejad, V.; Ashtari, F. Psychological well-being in people with multiple sclerosis in an Iranian population. J. Res. Med. Sci. 2015, 20, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Jalilian, M.; Abdi, A.; Khazaie, H.; Sarbarzeh, P.A. Surveying sleep quality and fatigue in multiple sclerosis patients at a multiple sclerosis center in Kermanshah, Iran, in 2017. Neurobiol. Sleep Circadian Rhythm. 2020, 8, 100050. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.B.; Williams, J.V.A.; Lavorato, D.H.; Berzins, S.; Metz, L.M.; Bulloch, A.G.M. Health Status, Stress and Life Satisfaction in a Community Population with MS. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2012, 39, 206–212. [Google Scholar] [CrossRef][Green Version]
- Mikula, P.; Nagyova, I.; Vitkova, M.; Szilasiova, J. Management of multiple sclerosis: The role of coping self-efficacy and self-esteem. Psychol. Health Med. 2018, 23, 964–969. [Google Scholar] [CrossRef]
- Hart, S.L.; Vella, L.; Mohr, D.C. Relationships among depressive symptoms, benefit-finding, optimism, and positive affect in multiple sclerosis patients after psychotherapy for depression. Health Psychol. 2008, 27, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Briones-Buixassa, L.; Milà, R.; Aragonès, J.M.; Bufill, E.; Olaya, B.; Arrufat, F.X. Stress and multiple sclerosis: A systematic review considering potential moderating and mediating factors and methods of assessing stress. Health Psychol. Open 2015, 2, 2055102915612271. [Google Scholar] [CrossRef] [PubMed]
- Lorefice, L.; Fenu, G.; Trincas, G.; Moro, M.F.; Frau, J.; Coghe, G.C.; Cocco, E.; Marrosu, M.G.; Carta, M.G. Progressive multiple sclerosis and mood disorders. Neurol. Sci. 2015, 36, 1625–1631. [Google Scholar] [CrossRef]
- Hartoonian, N.; Terrill, A.L.; Beier, M.L.; Turner, A.P.; Day, M.A.; Alschuler, K.N. Predictors of anxiety in multiple sclerosis. Rehabil. Psychol. 2015, 60, 91–98. [Google Scholar] [CrossRef]
- Sparaco, M.; Lavorgna, L.; Bonavita, S. Psychiatric disorders in multiple sclerosis. J. Neurol. 2021, 268, 45–60. [Google Scholar] [CrossRef]
- Kalb, R.; Feinstein, A.; Rohrig, A.; Sankary, L.; Willis, A. Depression and Suicidality in Multiple Sclerosis: Red Flags, Management Strategies, and Ethical Considerations. Curr. Neurol. Neurosci. Rep. 2019, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138–145. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Bitarafan, S.; Harirchian, M.H.; Nafissi, S.; Sahraian, M.A.; Togha, M.; Siassi, F.; Saedisomeolia, A.; Alipour, E.; Moham-madpour, N.; Chamary, M.; et al. Dietary intake of nutrients and its correlation with fatigue in multiple sclerosis patients. Iran J. Neurol. 2014, 13, 28–32. [Google Scholar]
- Bettencourt, A.; Boleixa, D.; Reguengo, H.; Samões, R.; Santos, E.; Oliveira, J.C.; Silva, B.; Costa, P.P.; da Silva, A.M. Serum 25-hydroxyvitamin D levels in multiple sclerosis patients from the north of Portugal. J. Steroid Biochem. Mol. Biol. 2018, 180, 137–141. [Google Scholar] [CrossRef]
- Shaygannejad, V.; Golabchi, K.; Haghighi, S.; Dehghan, H.; Moshayedi, A. A Comparative Study of 25 (OH) Vitamin D Se-rum Levels in Patients with Multiple Sclerosis and Control Group in Isfahan, Iran. Int. J. Prev. Med. 2010, 1, 195–201. [Google Scholar] [PubMed]
- Haines, S.T.; Park, S. Vitamin D Supplementation: What’s Known, What to Do, and What’s Needed. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 354–382. [Google Scholar] [CrossRef]
- Balvers, M.; Brouwer-Brolsma, E.M.; Endenburg, S.; de Groot, L.; Kok, F.J.; Gunnewiek, J.K. Recommended intakes of vitamin D to optimise health, associated circulating 25-hydroxyvitamin D concentrations, and dosing regimens to treat deficiency: Workshop report and overview of current literature. J. Nutr. Sci. 2015, 4, e23. [Google Scholar] [CrossRef] [PubMed]
| PICOS Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Multiple sclerosis adult patients | Children and adolescents with multiple sclerosis, individuals with any intellectual disabilities, any eating disorders, or any other neurological disorders |
| Intervention/exposure | Any vitamin D supplementation applied | Vitamin D applied within multiple nutrients supplementation |
| Comparison | Influence on a mental health outcomes assessed while compared with baseline/placebo/various doses and regimens | Lack of comparison |
| Outcome | Any mental health outcome assessed | Cognitive function assessed |
| Study design | Articles published in peer-reviewed journals, in English | Articles not published in English, reviews, meta-analyses, expert opinions, letters to editor, comments, studies in animal models, methodological articles, case reports, conference reports |
| Ref. | Authors, Year | Study Design | Country/Detailed Location | Study Group | Time |
|---|---|---|---|---|---|
| [35] | Ashtari et al., 2016 | Randomized, double-blind, placebo-controlled clinical trial | Iran | Patients with relapsing remitting multiple sclerosis | 22 December 2013–June 2014 * |
| [36] | Rolf et al., 2017 | Randomized placebo-controlled pilot study within SOLARIUM sub-study of the SOLAR trial | The Netherlands | Patients with relapsing remitting multiple sclerosis recruited in four hospitals in The Netherlands from SOLARIUM sub-study of the SOLAR trial | February 2011–April 2015 * |
| [37] | Kotb et al., 2019 | Prospective cross-sectional observational study | Saudi Arabia/Alkharj | Patients with relapsing remitting multiple sclerosis from Prince Sattam Bin-Abdulaziz University Hospital | 2013–2018 |
| [38] | Beckmann et al., 2020 | Prospective study | Not specified | Patients with multiple sclerosis from multiple sclerosis outpatient clinic | 2016–2017 |
| [39] | Taylor et al., 2018 | Longitudinal prospective study within Health Outcomes and Lifestyle In a Sample of people with Multiple Sclerosis (HOLISM) Study | Participants living in 57 countries, while 88% resided in the United States, Australia, United Kingdom, New Zealand, and Canada | Adults with multiple sclerosis from HOLISM Study | 2012–2015 * |
| [40] | Simpson-Yap et al., 2021 | Longitudinal prospective study | International cohort | Adults with multiple sclerosis from HOLISM Study | 2012–2015 * |
| Ref. | Number of Participants (Number of Females) | Age (Mean Years with SD) | Inclusion Criteria/Exclusion Criteria |
|---|---|---|---|
| [35] | 94 (80) | 31.4 ± 7.6 for vitamin D supplementation group 34.6 ± 10.1 for placebo group | Inclusion: aged 18–55 years; definite diagnosis of relapsing remitting multiple sclerosis according to McDonald criteria; EDSS score <4; no relapse 30 days before inclusion; negative β-HCG test for women in child-bearing age Exclusion: pregnancy; lactation; any disease other than MS; 25(OH)D serum level >85 ng/mL; past history of renal or hepatic disease; relapse during the study; received corticosteroids in the previous 30 days; calcium >11 mg/dL; aspartate transaminase or alanine transaminase >3 times normal values; alkaline phosphatase >2.5 times normal values |
| [36] | 40 (26) | 38.5 ± 7.8 for vitamin D supplementation group 37.6 ± 9.6 for placebo group | Inclusion: aged 18–55 years; diagnosed with relapsing remitting multiple sclerosis according to the original or 2005 revised McDonald criteria confirmed by MRI; treated with interferon-β1α; first clinical event in the previous 5 years; active disease in the previous 18 months, but not in the 30 days prior to inclusion Exclusion: use of oral or systemic glucocorticoids or ACTH within 30 days prior to inclusion; a history or presence of severe depression; a history of suicide attempt or current suicidal ideation; current or past drug or alcohol abuse; missing data |
| [37] | 35 (19) | 27.0 ± 4.0 | Inclusion: aged ≥18 years; relapsing remitting multiple sclerosis according to McDonald criteria; no exacerbations; no gadolinium enhancing lesions on MRI; no corticosteroid therapy within four weeks prior to recruitment; regular treatment with interferon-β Exclusion: treatment other than interferon; high-dose of vitamin D (daily intake of at least 25 µg) before inclusion to the study; immunomodulatory therapy changed within the past 3 months; history of systemic glucocorticoid therapy or relapse within 30 days; severe depression; pregnancy; serum creatinine >1.5 mg/dL; hypersensitivity to vitamin D preparations; history of hyperparathyroidism, tuberculosis, sarcoidosis, or nephrolithiasis |
| [38] | 149 (107) | 37.52 ± 9.82 | Inclusion: diagnosed with multiple sclerosis according to 2010 revised McDonald criteria Exclusion: disorders related to vitamin D deficiency (e.g., parathyroid pathologies); other acute or chronic disease at time of blood sampling determined by routine tests; receiving vitamins (vitamin D or multivitamin compounds) as supplements in the 6 months preceding data collection; relapse in the last 30 days; pregnancy; breastfeeding; other neurological or immune-mediated disease; skin diseases; medication use with a medical recommendation to avoid exposure to the sun; applying hydrochlorothiazide, barbiturates, phenytoin or digitalis |
| [39] | 1401 (1150) | 48.4 ± 10.5 | Inclusion: aged ≥18 years, diagnosed with multiple sclerosis (MS) by a medical doctor Exclusion: missing data * |
| [40] | 1401 (1152) | 48.4 ± 10.5 | Inclusion: aged ≥18 years, diagnosed with multiple sclerosis (MS) by a medical doctor Exclusion: missing data * |
| Ref. | Vitamin D Measure | Assessed Vitamin D Supplementation | Mental Health Outcome | Psychological Measure |
|---|---|---|---|---|
| [35] | 25(OH)D blood level | 1250 µg/5 days for 3 months vs. placebo | Quality of life | Multiple sclerosis quality of life (MSQOL-54)—Persian version |
| [36] | 25(OH)D blood level | 175 µg/day for 4 weeks followed by 350 µg/day for 44 weeks vs. placebo | (1) Depressive symptoms (2) Fatigue | (1) Depression subscale of the Hospital Anxiety and Depression Scale (HADS) (2) Dutch version of the Fatigue Severity Scale (FSS) |
| [37] | 25(OH)D blood level | 250 µg/day for 12 months | Depressive symptoms | Beck’s depression inventory (BDI) |
| [38] | 25(OH)D blood level | In patients with serum 25(OH)D <30 ng/mL–1250 µg/week for 8 weeks (to reach a minimum serum 25(OH)D level of 30 ng/mL) followed by 37.5–50 µg/day In patients with serum 25(OH)D of 20–30 ng/mL–37.5–50 µg/days | (1) Quality of life (2) Fatigue | (1) Multiple sclerosis-related quality of life inventory (MSQOLI) (2) Fatigue Severity Scale (FSS) |
| [39] | Taking vitamin D supplement | Declared vitamin D supplementation taken vs. not taken in the follow up of 2.5 years | Depression | Patient Health Questionnaire-2 (PHQ-2) Patient, Health Questionniare-9 (PHQ-9) |
| [40] | Taking vitamin D supplement | Declared vitamin D supplementation taken vs. not taken in the follow up of 2.5 years | Quality of life | Multiple sclerosis quality of life (MSQOL-54) |
| Ref. | Observations | Conclusions |
|---|---|---|
| [35] | After 3 months, the vitamin D group had a significant difference in mental health component of quality of life with placebo group, 62.41 ± 13.99 vs. 60.99 ± 17.99 (p = 0.041). Change in health component of quality of life was 75.74 ± 25.73 and 70.59 ± 26.45 in vitamin D and placebo group, respectively (p = 0.036). | Mental quality of life improved significantly after taking high dose vitamin D for 3 months in vitamin D group relative to placebo. Also a positive change in health status was reported by patients receiving high dose vitamin D relative to placebo group. |
| [36] | Pre- and post-supplementation depression scores, measured using the Hospital Anxiety Depression Scale (HADS) depression subscale (HADS-D), showed a significant decrease within the vitamin D3 group (median HADS-D 4.0 to 3.0, p = 0.02), a trend towards a decrease within the placebo group (median HADS-D 3.0 to 2.0, p = 0.06), but no significantly different reductions between groups (p = 0.78). | There was no evidence for a reduction of depressive symptoms upon vitamin D3 supplementation in relapsing remitting multiple sclerosis patients. |
| [37] | Depressive symptoms were high at baseline and improved with vitamin D replacement although, Expanded Disability Status Scale (EDSS) score was not improving. Vitamin D levels correlated negatively with depressive symptoms at baseline and follow up periods. | Lower vitamin D levels are associated with higher depressive scores, and vitamin D replacement could improve depressive symptoms in patients with relapsing remitting multiple sclerosis. |
| [38] | After vitamin D supplementation, health-related quality of life and fatigue scores improved significantly. There was a direct association between health-related quality of life with absence of fatigue and vitamin D status at the end of study. | The 90% frequency of multiple sclerosis patients with vitamin D deficiency, together with the significant association of vitamin D status with the absence of fatigue and improved physical and functional well-being, points to vitamin D supplementation as a potential therapy to enhance the patient’s quality of life. |
| [39] | Vitamin D supplementation at baseline was associated with lower frequency of positive depression-screen 2.5 years later. After adjusting for potential confounders, vitamin D supplementation was not associated with a change in risk for depression. | Vitamin D supplementation was associated with lower frequencies of depression risk, but this association was no longer significant after adjusting for potential confounders. |
| [40] | At 2.5-year follow-up, quality of life scores were higher among participants reporting taking vitamin D supplements (physical: aβ = 3.58, 95% CI = 1.35–5.80; mental: aβ = 3.08, 95% CI = 0.72–5.44), particularly average daily dose over 125 µg/d. Baseline-reported vitamin D supplementation was associated with greater increase in physical (aβ = 1.02, 95% CI = 0.22–1.81), but not mental quality of life (aβ = 0.11, 95% CI = −1.00–1.23). | Self-reported vitamin D supplement use was cross-sectionally associated with higher physical and mental quality of life, but prospectively only with increased physical quality of life. |
| Ref. | Studied Outcome | Conclusion about General Influence of Vitamin D on Mental Health a | Quality of the Study Based on the Study Design b | Quality of the Study Based on the NOS Score c | ||
|---|---|---|---|---|---|---|
| [35] | Quality of life | Supporting | Randomized against placebo | +++ | 8 | +++ |
| [36] | Depressive symptoms; fatigue | Not supporting | Randomized against placebo | +++ | 6 | ++ |
| [37] | Depressive symptoms | Supporting | Prospective with supplementation applied | ++ | 5 | ++ |
| [38] | Quality of life; fatigue | Supporting | Prospective with supplementation applied | ++ | 6 | ++ |
| [39] | Depression | Not supporting | Prospective based on self-reporting | + | 7 | +++ |
| [40] | Quality of life | Supporting | Prospective based on self-reporting | + | 6 | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review. Nutrients 2021, 13, 4207. https://doi.org/10.3390/nu13124207
Głąbska D, Kołota A, Lachowicz K, Skolmowska D, Stachoń M, Guzek D. Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review. Nutrients. 2021; 13(12):4207. https://doi.org/10.3390/nu13124207
Chicago/Turabian StyleGłąbska, Dominika, Aleksandra Kołota, Katarzyna Lachowicz, Dominika Skolmowska, Małgorzata Stachoń, and Dominika Guzek. 2021. "Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review" Nutrients 13, no. 12: 4207. https://doi.org/10.3390/nu13124207
APA StyleGłąbska, D., Kołota, A., Lachowicz, K., Skolmowska, D., Stachoń, M., & Guzek, D. (2021). Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review. Nutrients, 13(12), 4207. https://doi.org/10.3390/nu13124207









