Resistant Starch Consumption Effects on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes: A Randomized Crossover Study
Abstract
:1. Introduction
2. Materials and Methods
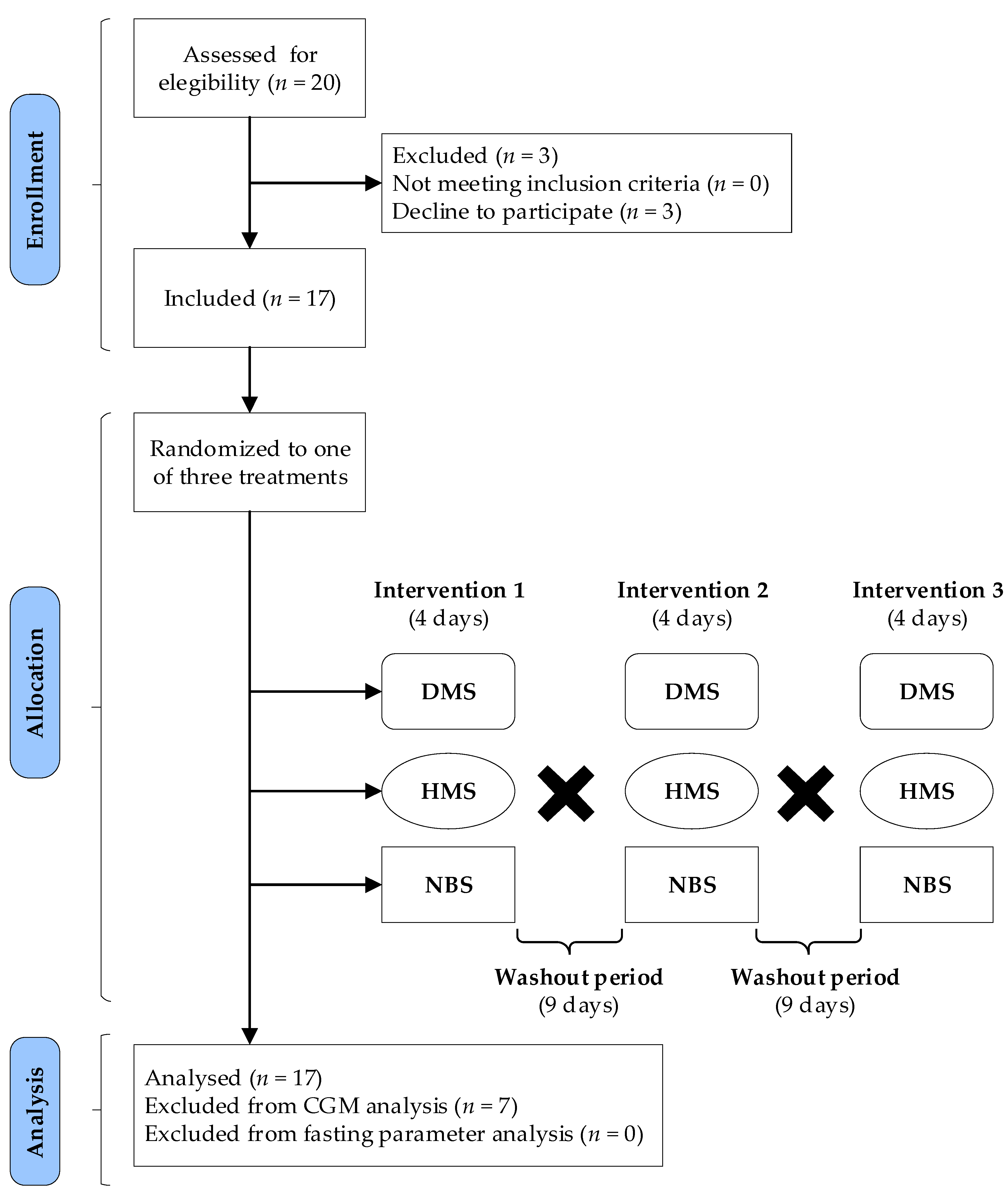
2.1. Participants
2.2. Starch Sources
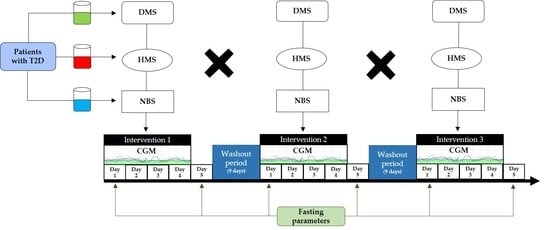
2.3. Study Design and Protocol
2.4. Treatments
2.5. Continuous Glucose Monitoring
2.6. Biochemical Determinations
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Dietary Intake
3.3. Effects of Treatments on Glycemic Control
3.4. Effects of Treatments on Glycemic Variability
3.5. Effects of Treatments on Fasting Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Ohara, M.; Fukui, T.; Ouchi, M.; Watanabe, K.; Suzuki, T.; Yamamoto, S.; Yamamoto, T.; Hayashi, T.; Oba, K.; Hirano, T. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 122, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, M.; Savorani, F.; Christensen, S.; Engelsen, S.B.; Bügel, S.; Toubro, S.; Tetens, I.; Astrup, A. Flaxseed dietary fibers suppress postprandial lipemia and appetite sensation in young men. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Khossousi, A.; Binns, C.W.; Dhaliwal, S.S.; Pal, S. The acute effects of psyllium on postprandial lipaemia and thermogenesis in overweight and obese men. Br. J. Nutr. 2008, 99, 1068–1075. [Google Scholar] [CrossRef]
- McCleary, B.V.; McLoughlin, C.; Charmier, L.M.J.; McGeough, P. Measurement of available carbohydrates, digestible, and resistant starch in food ingredients and products. Cereal. Chem. 2020, 97, 114–137. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.H.T.; Louie, J.C.Y. The relationship between resistant starch and glycemic control: A review on current evidence and possible mechanisms. Stärke 2017, 69, 1600205. [Google Scholar] [CrossRef]
- Lairon, D.; Play, B.; Jourdheuil-Rahmani, D. Digestible and indigestible carbohydrates: Interactions with postprandial lipid metabolism. J. Nutr. Biochem. 2007, 18, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Nugent, A.P. Health effects of resistant starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Francisco-Luria, M.U.; Córdova-Uscanga, R.; Rodríguez-Hernández, A.; Méndez, J.D.; Díaz-Zagoya, J.C. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int. J. Environ. Res. Public. Health 2010, 7, 1953–1962. [Google Scholar] [CrossRef] [Green Version]
- Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Gómez-Vázquez, A.; Rodríguez-Hernández, A.; Méndez, J.D.; Juárez-Rojop, I.E.; Aguilar-Mariscal, H.; Ramón-Frías, T.; Díaz-Zagoya, J.C. Potential beneficial effects of native banana starch on glycemia and insulin resistance in obese nondiabetic women. Interciencia 2012, 37, 470–476. [Google Scholar]
- Ble-Castillo, J.L.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; García-Vázquez, C.; Servin-Cruz, M.Z.; Rodríguez-Hernández, A.; Araiza-Saldaña, C.I.; Nolasco-Coleman, A.M.; Díaz-Zagoya, J.C. Acute consumption of resistant starch reduces food intake but has no effect on appetite ratings in healthy subjects. Nutrients 2017, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Domínguez, G.; Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Ble-Castillo, D.J.; García-Vázquez, C.; Olvera-Hernández, V.; Pérez-Pimienta, B.; Diaz-Zagoya, J.C.; et al. Effects of acute ingestion of native banana starch on glycemic response evaluated by continuous glucose monitoring in obese and lean subjects. Int. J. Environ. Res. Public. Health 2015, 12, 7491–7505. [Google Scholar] [CrossRef] [Green Version]
- Waliszewski, K.; Aparicio, M.; Bello, L.S.; Monroy, J. Changes of banana starch by chemical physical modification. Carbohydr. Polym. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Pérez-Lizaur, A.B.; Castro-Becerra, A.L. Sistema Mexicano de Alimentos Equivalentes, 3rd ed.; Fomento de Nutrición y Salud y Ogali: Mexico City, Mexico, 2008. [Google Scholar]
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Bjorck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.; Thompson, C.H.; Brinkworth, G.D. Glycemic variability: Assessing glycemia differently and the implications for dietary management of diabetes. Annu. Rev. Nutr. 2015, 35, 389–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Snelson, M.; Jong, J.; Manolas, D.; Kok, S.; Louise, A.; Stern, R.; Kellow, N.J. Metabolic effects of resistant starch type 2: A systematic literature review and meta-analysis of randomized controlled trials. Nutrients 2019, 11, 1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacNeil, S.; Rebry, R.M.; Tetlow, I.J.; Emes, M.J.; McKeown, B.; Graham, T.E. Resistant starch intake at breakfast affects postprandial responses in type 2 diabetics and enhances the glucose-dependent insulinotropic polypeptide—Insulin relationship following a second meal. Appl. Physiol. Nutr. Metab. 2013, 38, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Luhovyy, B.L.; Mollard, R.C.; Yurchenko, S.; Nunez, M.F.; Berengut, S.; Liu, T.T.; Smith, C.E.; Pelkman, C.L.; Anderson, G.H. The effects of whole grain high-amylose maize flour as a source of resistant starch on blood glucose, satiety, and food intake in young men. J. Food Sci. 2014, 79, H2550–H2556. [Google Scholar] [CrossRef] [PubMed]
- Bodinham, C.L.; Al-Mana, N.M.; Smith, L.; Robertson, M.D. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br. J. Nutr. 2013, 110, 1429–1433. [Google Scholar] [CrossRef] [Green Version]
- Breyton, A.-E.; Goux, A.; Lambert-Porcheron, S.; Meynier, A.; Sothier, M.; VanDenBerghe, L.; Brack, O.; Disse, E.; Laville, M.; Vinoy, S.; et al. Starch digestibility modulation significantly improves glycemic variability in type 2 diabetic subjects: A pilot study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 237–246. [Google Scholar] [CrossRef]
- Guerci, B.; Monnier, L.; Serusclat, P.; Petit, C.; Valensi, P.; Huet, D.; Raccah, D.; Colette, C.; Quéré, S.; Dejager, S. Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: Results from the randomized optima study. Diabetes Metab. 2012, 38, 359–366. [Google Scholar] [CrossRef]
- Kodani, N.; Saisho, Y.; Tanaka, K.; Kawai, T.; Itoh, H. Effects of mitiglinide, a short-acting insulin secretagogue, on daily glycemic variability and oxidative stress markers in japanese patients with type 2 diabetes mellitus. Clin. Drug Investig. 2013, 33, 563–570. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012, 70, S10–S13. [Google Scholar] [CrossRef]
- Munster, I.P.v.; Tangerman, A.; Nagengast, F.M. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig. Dis. Sci. 1994, 39, 834–842. [Google Scholar] [CrossRef]
- Tomlin, J.; Read, N.W. The effect of resistant starch on colon function in humans. Br. J. Nutr. 2007, 64, 589–595. [Google Scholar] [CrossRef]
- Pedersen, C.; Gallagher, E.; Horton, F.; Ellis, R.J.; Ijaz, U.Z.; Wu, H.; Jaiyeola, E.; Diribe, O.; Duparc, T.; Cani, P.D.; et al. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.M.; Beyl, R.A.; Marlatt, K.L.; Martin, C.K.; Aryana, K.J.; Marco, M.L.; Martin, R.J.; Keenan, M.J.; Ravussin, E. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; et al. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with cvd risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, X. Type 2 diabetes mellitus-related environmental factors and the gut microbiota: Emerging evidence and challenges. Clinics 2020, 75, e1277. [Google Scholar] [CrossRef]
- Uemura, F.; Okada, Y.; Torimoto, K.; Tanaka, Y. Enlarged glycemic variability in sulfonylurea-treated well-controlled type 2 diabetics identified using continuous glucose monitoring. Sci. Rep. 2021, 11, 4875. [Google Scholar] [CrossRef]
- Parada, J.; Santos, J.L. Interactions between starch, lipids, and proteins in foods: Microstructure control for glycemic response modulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 2362–2369. [Google Scholar] [CrossRef]
- Robertson, M.D.; Wright, J.W.; Loizon, E.; Debard, C.; Vidal, H.; Shojaee-Moradie, F.; Russell-Jones, D.; Umpleby, A.M. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J. Clin. Endocr. Metab. 2012, 97, 3326–3332. [Google Scholar] [CrossRef] [Green Version]
- Robertson, M.D.; Currie, J.M.; Morgan, L.M.; Jewell, D.P.; Frayn, K.N. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia 2003, 46, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Al-Mana, N.M.; Robertson, M.D. Acute effect of resistant starch on food intake, appetite and satiety in overweight/obese males. Nutrients 2018, 10, 1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Total |
|---|---|
| Subjects (n) | 10 |
| Sex, M/F [n (%)] | 5/5 (50.0/50.0) |
| Age (years) | 48.5 ± 9.12 |
| Weight (kg) | 77.32 ± 11.58 |
| BMI (kg/m2) | 29.05 ± 3.84 |
| HbA1c (%) | 9.58 ± 2.13 |
| Fasting glycemia (mg/dL) | 203 ± 77.22 |
| Insulin (μUI/mL) | 7.4 ± 4.74 |
| Triglycerides (mg/dL) | 148 (103.3, 266.8) |
| Cholesterol (mg/dL) | 189 ± 27.38 |
| Medication | |
| Only metformin (n) | 4 |
| Metformin + Glibenclamide (n) | 6 |
| Ingredients | Treatments | p | ||
|---|---|---|---|---|
| DMS | HMS | NBS | ||
| Energy (kcal) | 1711 ± 77.87 | 1558 ± 57.19 | 1576 ± 106 | 0.1135 |
| Carbohydrates (g) | 198.9 ± 9.15 | 215.7 ± 14.34 | 194.7 ± 13.89 | 0.2691 |
| Proteins (g) | 63.28 ± 2.65 | 69.53 ± 6.23 | 64.18 ± 3.24 | 0.2030 |
| Fat (g) | 54.35 ± 1.96 | 64.65 ± 11.17 | 59.58 ± 7.38 | 0.4405 |
| Fiber (g) | 13.8 ± 0.98 | 14.58 ± 1.41 | 12.65 ± 1.78 | 0.1358 |
| GC Indexes | Period (Days) | Treatments | ||
|---|---|---|---|---|
| DMS | HMS | NBS | ||
| 24 h MBG | 2 | 164.2 (151.3, 177.9) | 180.2 (166.7, 193.8) a | 178.7 (166.4, 187.6) b |
| 3 | 163.9 (154.7, 167.2) | 183.6 (175.8, 190.1) ac | 173.3 (170.6, 177.1) bc | |
| 4 | 151.3 (144.5, 162.1) | 189.2 (173.5, 200.8) ac | 172.8 (164.1, 179.2) bc | |
| Maximum glucose | 2 | 241.4 ± 90.08 | 249.8 ± 85.25 | 243.7 ± 68.60 |
| 3 | 230.8 ± 83.48 | 244.3 ± 86.98 | 228 ± 71.13 | |
| 4 | 213.2 ± 83.81 | 260.4 ± 80.31 | 233 ± 82.19 | |
| Minimum glucose | 2 | 93.50 (84.50, 119.3) | 108 (88.25, 211) | 110 (94.75, 181.3) |
| 3 | 105.5 (85.76, 147.5) | 106.5 (85.50, 218.5) | 96 (89.75, 143.8) | |
| 4 | 91 (73.75, 129) | 106 (81, 217) | 93.50 (83, 199.8) | |
| HBGI | 2 | 3.55 (0.97, 15.61) | 3.62 (1.20, 29.35) | 3.71 (2.25, 18.81) |
| 3 | 3.03 (1.51, 14.07) | 4.82 (1.09, 27.99) | 3.19 (1.54, 16.28) | |
| 4 | 3.17 (0.49, 9.79) | 5.37 (2.20, 24.16) a | 2.51 (1.25, 18.69) | |
| LBGI | 2 | 0.404 (0.002, 0.818) | 0.028 (0.0, 0.606) | 0.005 (0.0, 0.465) |
| 3 | 0.061 (0.0, 0.687) | 0.061 (0.0, 0.915) | 0.392 (0.0, 0.641) | |
| 4 | 0.352 (0.0, 1.20) | 0.059 (0.0, 0.836) | 0.453 (0.0, 0.775) | |
| LAGE | 2 | 128.1 ± 61.42 | 115.5 ± 44.94 | 115 ± 45.22 |
| 3 | 114.2 ± 53.42 | 111.7 ± 50.66 | 109.6 ± 32.20 | |
| 4 | 109.1 ± 56.11 | 129.5 ± 52.56 | 99.80 ± 31.45 | |
| TAR (%) | 2 | 14.24 (0.0, 59.20) | 12.50 (0.00, 100.4) | 11.63 (4.42, 99.57) |
| 3 | 6.94 (0.00, 62.07) | 17.71 (0.00, 100.4) | 9.37 (2.60, 94.10) | |
| 4 | 7.98 (0.00, 61.02) | 25.52 (1.82, 100.4) | 48.09 (3.12, 100.4) | |
| TBR (%) | 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | |
| 4 | 0 | 0 (0.00, 0.35) | 0 | |
| TIR (%) | 2 | 81.95 (21.79, 100.4) | 88.03 (0.00, 100.4) | 88.72 (0.00, 96.96) |
| 3 | 84.03 (38.20, 100.4) | 81.77 (0.00, 100.4) | 90.97 (6.24, 97.75) | |
| 4 | 92.36 (39.33, 100.4) | 74.83 (0.00, 95.40) | 0 (0.00, 100.3) | |
| GV Indexes | Period (Days) | Treatments | ||
|---|---|---|---|---|
| DMS | HMS | NBS | ||
| SD | 2 | 1.80 ± 1.04 | 1.54 ± 0.75 | 1.57 ± 0.75 |
| 3 | 1.57 ± 0.82 | 1.49 ± 0.69 | 1.35 ± 0.46 | |
| 4 | 1.32 (0.68, 1.91) | 1.68 (1.23, 2.55) | 1.18 (0.79, 1.41) | |
| CV | 2 | 18.75 ± 8.46 | 16.01 ± 6.99 | 16.27 ± 6.37 |
| 3 | 17 ± 6.76 | 15.12 ± 6.44 | 14.23 ± 3.14 | |
| 4 | 15.88 ± 5.95 | 18.14 ± 7.74 | 13.30 ± 3.84 | |
| CONGA | 2 | 8.56 ± 3.49 | 9.16 ± 3.97 | 9.13 ± 3.31 |
| 3 | 8.30 ± 3.07 | 9.34 ± 3.90 | 8.88 ± 3.47 | |
| 4 | 6.65 (5.88, 9.98) | 7.44 (6.47, 13.58) a | 6.84 (5.94, 12.37) | |
| GRADE | 2 | 7.29 ± 7.57 | 9.46 ± 9.41 | 9.67 ± 8.50 |
| 3 | 7.34 ± 7.19 | 10.19 ± 9.27 | 9.03 ± 8.27 | |
| 4 | 6.65 ± 7.08 | 10.80 ± 8.85 a | 8.78 ± 8.55 | |
| MAG | 2 | 1.18 ± 0.45 | 1.33 ± 0.48 | 1.27 ± 0.32 |
| 3 | 1.16 ± 0.48 | 1.33 ± 0.41 | 1.22 ± 0.32 | |
| 4 | 1.11 ± 0.51 | 1.41 ± 0.49 | 1.19 ± 0.33 | |
| J-Index | 2 | 44.61 ± 35.78 | 49.36 ± 39.65 | 46.73 ± 30.27 |
| 3 | 40.45 ± 29.51 | 49.79 ± 37.72 | 43.42 ± 31.47 | |
| 4 | 27.90 (16.53, 45.60) | 35.76 (24.36, 82.68) a | 25.32 (20.24, 66.64) | |
| MAGE | 2 | 4.84 ± 2.53 | 4.05 ± 1.54 | 4.23 ± 1.90 |
| 3 | 4.04 (1.59, 5.57) | 3.83 (1.99, 6.09) | 3.36 (2.57, 4.27) | |
| 4 | 3.62 ± 2.11 | 4.78 ± 1.81 | 3.41 ± 1.57 | |
| MODD | 2–4 | 1.73 ± 0.80 | 1.75 ± 0.89 | 1.57 ± 0.58 |
| Parameter | Treatments | p | ||
|---|---|---|---|---|
| DMS | HMS | NBS | ||
| Glucose (mg/dL) | −14.41 ± 32.35 | −1.00 ± 34.47 | −36.47 ± 36.86 | 0.0074 b |
| Insulin (µUI/mL) | −4.0 (−7, 0.5) | −1.0 (−6.0, 4.0) | 0.0 (−3.5, 1.5) | 0.3837 |
| Triglycerides (mg/dL) | −11.00 (−490.0, 18.00) | −32.0 (−98.5, −5.5) | −24.00 (−389.0, 13.0) | 0.9752 |
| Cholesterol (mg/dL) | −4.94 ± 32.99 | −0.94 ± 33.55 | −10.76 ± 44.81 | 0.7125 |
| GLP-1 (pM/L) | −4.496 ± 15.03 | £ | 5.379 ± 22.09 | 0.2344 |
| HOMA-IR | −1.91± 2.2 | 0.28 ± 5.77 | −1.88 ± 3.03 | 0.1167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Córdova, Y.; Ble-Castillo, J.L.; García-Vázquez, C.; Olvera-Hernández, V.; Ramos-García, M.; Navarrete-Cortes, A.; Jiménez-Domínguez, G.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Martínez-López, M.C.; et al. Resistant Starch Consumption Effects on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes: A Randomized Crossover Study. Nutrients 2021, 13, 4052. https://doi.org/10.3390/nu13114052
Arias-Córdova Y, Ble-Castillo JL, García-Vázquez C, Olvera-Hernández V, Ramos-García M, Navarrete-Cortes A, Jiménez-Domínguez G, Juárez-Rojop IE, Tovilla-Zárate CA, Martínez-López MC, et al. Resistant Starch Consumption Effects on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes: A Randomized Crossover Study. Nutrients. 2021; 13(11):4052. https://doi.org/10.3390/nu13114052
Chicago/Turabian StyleArias-Córdova, Yolanda, Jorge Luis Ble-Castillo, Carlos García-Vázquez, Viridiana Olvera-Hernández, Meztli Ramos-García, Adrián Navarrete-Cortes, Guadalupe Jiménez-Domínguez, Isela Esther Juárez-Rojop, Carlos Alfonso Tovilla-Zárate, Mirian Carolina Martínez-López, and et al. 2021. "Resistant Starch Consumption Effects on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes: A Randomized Crossover Study" Nutrients 13, no. 11: 4052. https://doi.org/10.3390/nu13114052
APA StyleArias-Córdova, Y., Ble-Castillo, J. L., García-Vázquez, C., Olvera-Hernández, V., Ramos-García, M., Navarrete-Cortes, A., Jiménez-Domínguez, G., Juárez-Rojop, I. E., Tovilla-Zárate, C. A., Martínez-López, M. C., & Méndez, J. D. (2021). Resistant Starch Consumption Effects on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes: A Randomized Crossover Study. Nutrients, 13(11), 4052. https://doi.org/10.3390/nu13114052