Associations between Maternal Dietary Patterns and Infant Birth Weight in the NISAMI Cohort: A Structural Equation Modeling Analysis
Abstract
1. Introduction
2. Materials and Methods
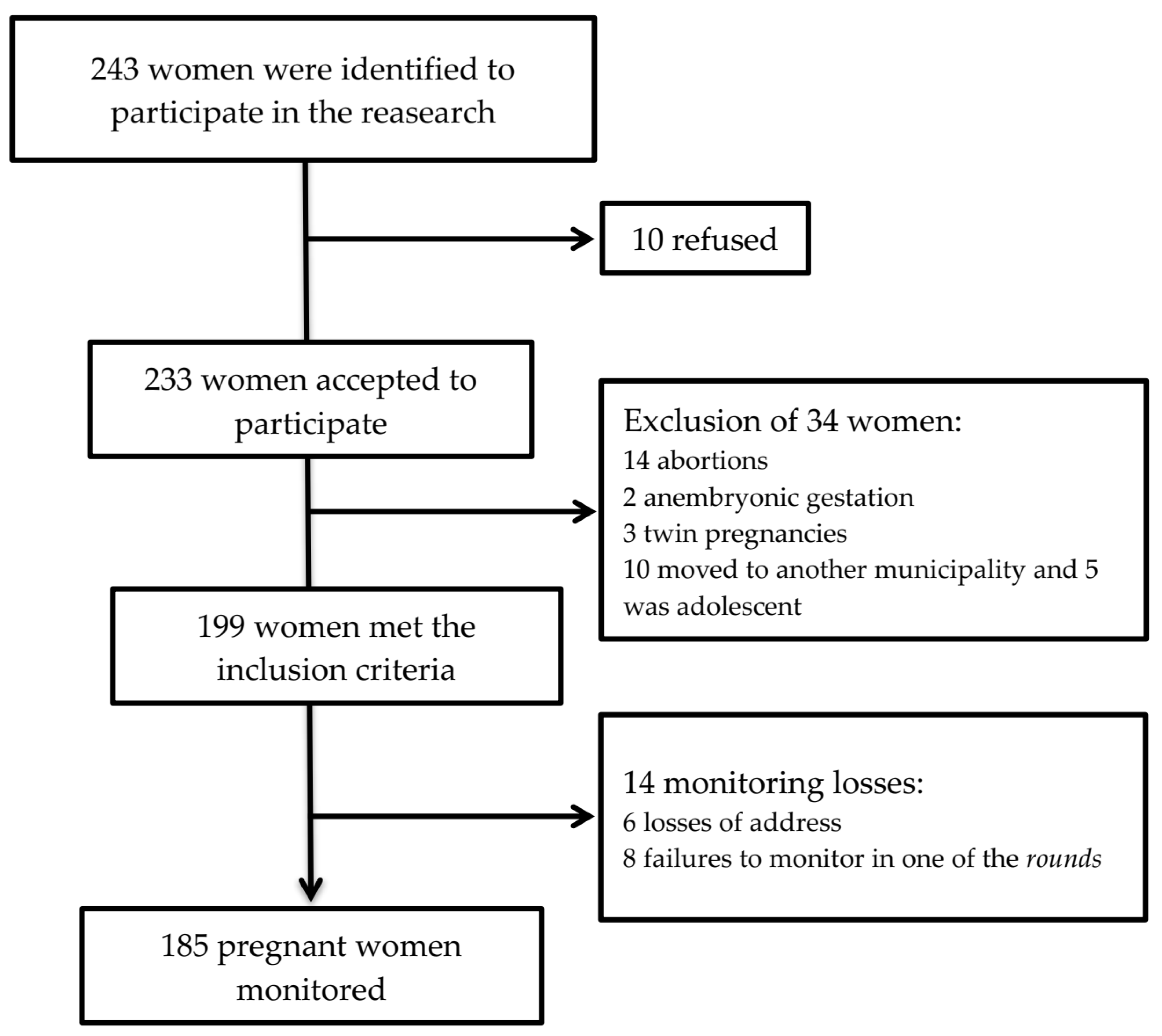
2.1. Study Design and Sample Calculation
2.2. Exclusion and Inclusion Criteria
2.3. Data Collection and Dietary Intake Assessments
2.4. Data on Study Variables
2.5. Outcomes
2.6. Data Management and Statistical Analyses
3. Results
3.1. Cohort Characteristics
3.2. Dietary Patterns during Pregnancy
3.3. Dietary Patterns during Pregnancy and Birth Weight
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.P. Developmental origins of adult health and disease. J. Epidemiol. Community Health 2004, 58, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Z. Maternal nutrition, fetal weight, body composition and disease in later life. J. Endocrinol. Investig. 2003, 26, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.A.R.; Castro, M.C.; Oliveira, C.V.R.; Malta, M.B.; Lourenco, B.H.; Cardoso, M.A. Effect of Vitamin A status during pregnancy on maternal anemia and newborn birth weight: Results from a cohort study in the Western Brazilian Amazon. Eur. J. Nutr. 2018, 59, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Carniel, E.d.F.; Zanolli, M.d.L.; Antônio, M.Â.R.d.G.M.; Morcillo, A.M. Determinants for low birth weight according to Live Born Certificates. Rev. Bras. Epidemiol. 2008, 11, 169–179. [Google Scholar] [CrossRef]
- Lima, M.C.B.d.M.; de Oliveira, G.S.; Lyra, C.; Roncalli, A.G.; Ferreira, M.A.F. The spatial inequality of low birth weight in Brazil. Cien. Saude Colet. 2013, 18, 2443–2452. [Google Scholar] [CrossRef]
- Pereira, P.P.d.S.; Da Mata, F.A.F.; Figueiredo, A.C.G.; de Andrade, K.R.C.; Pereira, M.G. Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2017, 19, 497–505. [Google Scholar] [CrossRef]
- Patra, J.; Bakker, R.; Irving, H.; Jaddoe, V.W.V.; Malini, S.; Rehm, J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG 2011, 118, 1411–1421. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and birth outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109, 729S–756S. [Google Scholar] [CrossRef]
- Knudsen, V.K.; Orozova-Bekkevold, I.M.; Mikkelsen, T.B.; Wolff, S.; Olsen, S.F. Major dietary patterns in pregnancy and fetal growth. Eur. J. Clin. Nutr. 2008, 62, 463–470. [Google Scholar] [CrossRef]
- Englund-Ogge, L.; Brantsaeter, A.L.; Juodakis, J.; Haugen, M.; Meltzer, H.M.; Jacobsson, B.; Sengpiel, V. Associations between maternal dietary patterns and infant birth weight, small and large for gestational age in the Norwegian Mother and Child Cohort Study. Eur. J. Clin. Nutr. 2018, 72, 1270–1282. [Google Scholar] [CrossRef]
- Martins, A.P.B.; Benicio, M.H.D. Influence of dietary intake during gestation on postpartum weight retention. Rev. Saude Publica 2011, 45, 870–877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Shin, D.; Kim, H.; Kwon, O. Association of maternal dietary patterns during pregnancy with small-for-gestational-age infants: Korean Mothers and Children’s Environmental Health (MOCEH) study. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Abubakari, A.; Jahn, A. Maternal Dietary Patterns and Practices and Birth Weight in Northern Ghana. PLoS ONE 2016, 11, e0162285. [Google Scholar] [CrossRef]
- Chia, A.-R.; Chen, L.-W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F.-F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Maher, S.E.; O’Brien, E.C.; Moore, R.L.; Byrne, D.F.; Geraghty, A.A.; Saldova, R.; Murphy, E.F.; Van Sinderen, D.; Cotter, P.D.; McAuliffe, F.M. The association between the maternal diet and the maternal and infant gut microbiome: A systematic review. Br. J. Nutr. 2020, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Demétrio, F.; Pinto, E.d.J.; Assis, A.M.O. Factors associated with early breastfeeding cessation: A birth cohort study in two municipalities in the Recôncavo region, Bahia State, Brazil. Cad. Saude Publica 2012, 28, 641–650. [Google Scholar] [CrossRef]
- Brito, S.M. Postpartum Weight Retention Maternal: A Cohort Study in a Township of Brazilian Northeast; Universidade Federal da Bahia: Salvador, Brazil, 2015. [Google Scholar]
- Coelho, N.; Cunha, D.B.; Esteves, A.P.P.; Lacerda, E.M.; Theme Filha, M.M. Dietary patterns in pregnancy and birth weight. Rev. Saude Publica 2015, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Pfrimer, K.; Tremeschin, M.; Molina, M.d.C.; Chiarello, P.G. Consumo Alimentar—Visualizando Porções; Guanabara Koogan: Rio de Janeiro, Brasil, 2007. [Google Scholar]
- Atalah, S.E.; Castillo, L.C.; Castro, S.R.; Aldea, P.A. Propuesta de un nuevo estándar de evaluación nutricional en embarazadas. Rev. Med. Chil. 1997, 125, 1429–1436. [Google Scholar]
- Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009; ISBN 9780309131131. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric standardization reference manual. Hum. Kinet. Books 1988, 177. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Carvalho, G.Q.; Dos Santos, D.B.; Oliveira, A.M. Influence of Vitamin D serum concentration, prenatal care and social determinants on birth weight: A northeastern Brazilian cohort study. Br. J. Nutr. 2019, 122, 284–292. [Google Scholar] [CrossRef]
- da Mota Santana, J.; Assis, A.M.O.; de Oliveira Alves, W.P.; dos Santos, D.B. Association between gestational weight gain and birth weight: Nisami cohort. Rev. Bras. Saude Matern. Infant. 2020, 20, 411–420. [Google Scholar] [CrossRef]
- Santana, J.M.; Alves de Oliveira Queiroz, V.; Monteiro Brito, S.; Barbosa Dos Santos, D.; Marlucia Oliveira Assis, A. Food Consumption Patterns During Pregnancy: A Longitudinal Study in a Region of the North East of Brazil. Nutr. Hosp. 2015, 32, 130–138. [Google Scholar] [CrossRef]
- Kline, R. Principles and Practice of Structural Equation Modeling, 4th ed.; Guilford Publications: New York, NY, USA, 2016; ISBN 146252334X. [Google Scholar]
- Okubo, H.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Murakami, K.; Hirota, Y.; Kanzaki, H.; Kitada, M.; Horikoshi, Y.; Ishiko, O.; et al. Maternal dietary patterns in pregnancy and fetal growth in Japan: The Osaka Maternal and Child Health Study. Br. J. Nutr. 2012, 107, 1526–1533. [Google Scholar] [CrossRef]
- Sunawang; Utomo, B.; Hidayat, A.; Kusharisupeni; Subarkah. Preventing low birthweight through maternal multiple micronutrient supplementation: A cluster-randomized, controlled trial in Indramayu, West Java. Food Nutr. Bull. 2009, 30, S488–S495. [Google Scholar] [CrossRef]
- Ramon, R.; Ballester, F.; Iniguez, C.; Rebagliato, M.; Murcia, M.; Esplugues, A.; Marco, A.; Garcia de la Hera, M.; Vioque, J. Vegetable but not fruit intake during pregnancy is associated with newborn anthropometric measures. J. Nutr. 2009, 139, 561–567. [Google Scholar] [CrossRef]
- Loy, S.-L.; Marhazlina, M.; Azwany, Y.N.; Hamid Jan, J.M. Higher intake of fruits and vegetables in pregnancy is associated with birth size. Southeast Asian J. Trop. Med. Public Health 2011, 42, 1214–1223. [Google Scholar]
- Alsharairi, N.A. The Infant Gut Microbiota and Risk of Asthma: The Effect of Maternal Nutrition during Pregnancy and Lactation. Microorganisms 2020, 8, 1119. [Google Scholar] [CrossRef]
- Chia, A.-R.; de Seymour, J.V.; Colega, M.; Chen, L.-W.; Chan, Y.-H.; Aris, I.M.; Tint, M.-T.; Quah, P.L.; Godfrey, K.M.; Yap, F.; et al. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am. J. Clin. Nutr. 2016, 104, 1416–1423. [Google Scholar] [CrossRef]
- Lu, M.-S.; Chen, Q.-Z.; He, J.-R.; Wei, X.-L.; Lu, J.-H.; Li, S.-H.; Wen, X.-X.; Chan, F.-F.; Chen, N.-N.; Qiu, L.; et al. Maternal Dietary Patterns and Fetal Growth: A Large Prospective Cohort Study in China. Nutrients 2016, 8, 257. [Google Scholar] [CrossRef]
- Kaiser, L.; Allen, L.H. Position of the American Dietetic Association: Nutrition and Lifestyle for a Healthy Pregnancy Outcome. J. Am. Diet. Assoc. 2008, 108, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Martins, A.P.B.; Martins, C.A.; Garzillo, J.; Canella, D.S.; Baraldi, L.G.; Barciotte, M.; da Costa Louzada, M.L.; et al. Dietary guidelines to nourish humanity and the planet in the twenty-first century. A blueprint from Brazil. Public Health Nutr. 2015, 18, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef]
- Fazio, E.; Nomura, R.M.Y.; Dias, M.C.G.; Zugaib, M. Dietary intake of pregnant women and maternal weight gain after nutritional counseling. Rev. Bras. Ginecol. Obstet. 2011, 33, 87–92. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis: A Global Perspective, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA.
| Variables | Mean (SD) | p-Value 1 | N | % |
|---|---|---|---|---|
| Age (years) | ||||
| <30 | 3320. 87 (483.1) | 133 | 71.89 | |
| ≥30 | 3393.13 (615.02) | 0.39 | 52 | 28.11 |
| Maternal education level | ||||
| ≥Secondary education | 3328.15 (595.13) | 26 | 14.1 | |
| <Secondary education | 3343.31 (512.11) | 0.89 | 159 | 85.9 |
| Family Income | ||||
| >1 Minimum salary | 3371.67 (508.31) | 139 | 75.1 | |
| ≤1 Minimum salary | 3249.07 (560.17) | 0.16 | 46 | 24.9 |
| Antenatal visits | ||||
| ≥7 prenatal appointments | 3376.87 (44.36) | 109 | 58.9 | |
| <7 prenatal appointment | 3307.38 (590.84) | 0.56 | 76 | 41.1 |
| Smoker | ||||
| Yes/ex-smoker | 3375.00 (763.97) | 16 | 8.6 | |
| No | 3337.98 (497.25) | 0.78 | 169 | 91.4 |
| Alcohol consumer | ||||
| No | 3369.55 (495.68) | 161 | 87.0 | |
| Yes | 3250.88 (658.89) | 0.05 | 24 | 13.0 |
| Physical activity | ||||
| No | 3332.63 (520.91) | 169 | 91.4 | |
| Yes | 3431.56 (551.76) | 0.47 | 16 | 8.6 |
| Pregnancy complications (anaemia, urinary tract infection, gestational diabetes, hypertensive disorders) | ||||
| No | 3376.87 (528.30) | 136 | 73.5 | |
| Yes | 3244.12 (499.78) | 0.13 | 49 | 26.5 |
| Pre-gestational Anthropometric status | ||||
| Healthy | 3307.58 (482.85) | 104 | 56.0 | |
| Overweight | 3384.33 (570.25) | 0.32 | 81 | 44.0 |
| Weight gain during pregnancy | ||||
| ≥10 kg | 3424.36 (490.30) | 80 | 56.8 | |
| <10 kg | 3277.81 (540.03) | 0.05 | 105 | 43.2 |
| Total | 3341.18 (522.32) | 185 |
| Foods/Food Groups | Dietary Intake Patterns First Trimester * | |||
| Pattern 1 | Pattern 2 | Pattern 3 | Pattern 4 | |
| Cereals, roots, and tubers | 0.621 | |||
| Legumes | 0.637 | |||
| Fruit | 0.861 | |||
| Vegetables | 0.695 | |||
| Milk and dairy products | 0.654 | |||
| Meat and eggs | 0.494 | |||
| Sugars and sweets | 0.507 | |||
| Coffee | 0.727 | |||
| Processed and industrialized foods | 0.461 | |||
| Fats | 0.737 | |||
| Fried Snacks | 0.795 | |||
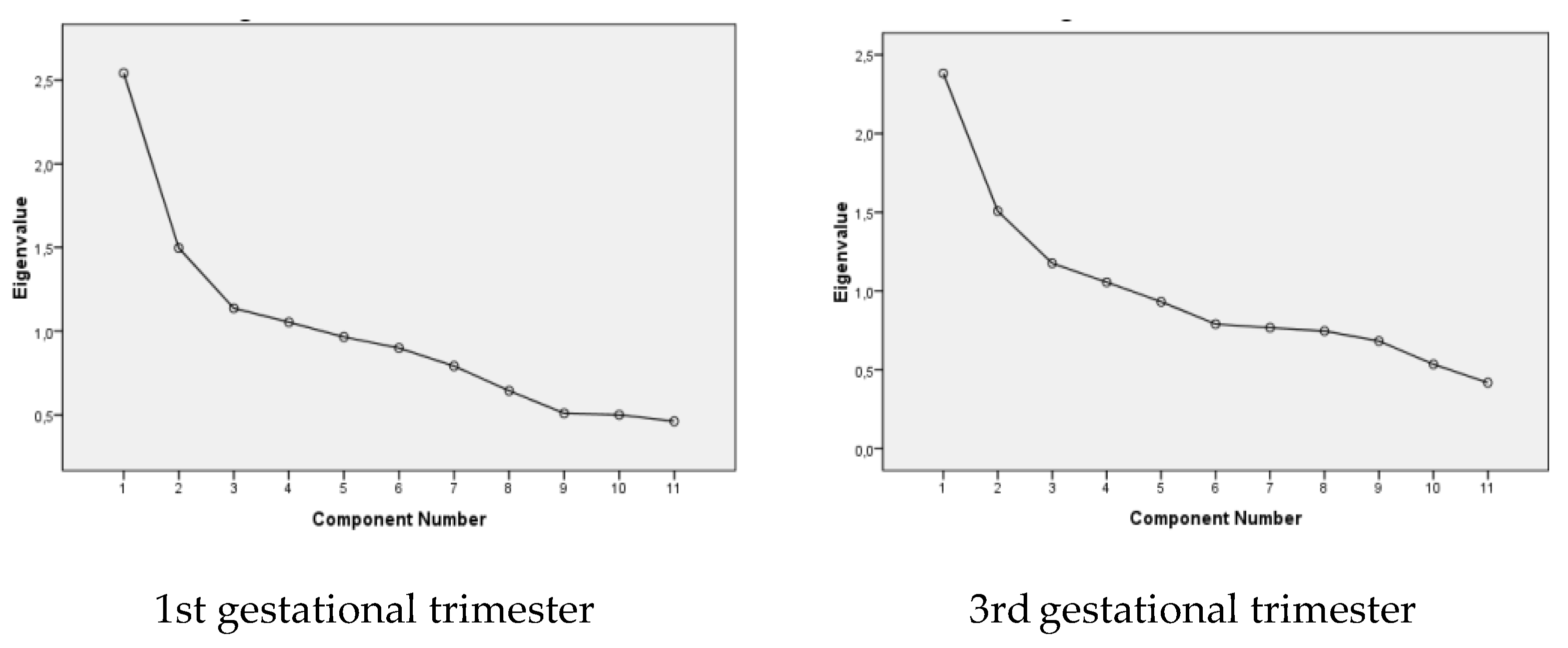
| % Accumulated variance | 19.42 | 30.65 | 41.34 | 50.45 |
| Eigenvalues | 2.54 | 1.49 | 1.13 | 1.05 |
| Cronbach’s Alpha | 0.51 | 0.53 | 0.54 | 0.56 |
| Foods/Food Groups | Dietary Intake Patterns Third Trimester ** | |||
| Pattern 1 | Pattern 2 | Pattern 3 | Pattern 4 | |
| Cereals, roots and tubers | 0.667 | |||
| Legumes | 0.649 | |||
| Fruit | 0.429 | |||
| Vegetables | 0.504 | |||
| Milk and dairy products | 0.498 | |||
| Meat and eggs | 0.599 | |||
| Sugars and sweets | 0.526 | |||
| Coffee | 0.732 | |||
| Processed and industrialized foods | 0.739 | |||
| Fats | 0.722 | |||
| Fried Snacks | 0.43 | |||
| % Accumulated variance | 14.08 | 27.37 | 38.4 | 49.03 |
| Eigenvalues | 2.38 | 1.50 | 1.17 | 1.05 |
| Cronbach’s Alpha | 0.54 | 0.57 | 0.57 | 0.56 |
| Direct Effect | Indirect Effect | Total Effect | ||||
|---|---|---|---|---|---|---|
| Coeff. (g) (CI 95%) | p-Value | Coeff. (g) (CI 95%) | p-Value | Coeff. (g) (CI 95%) | p-Value | |
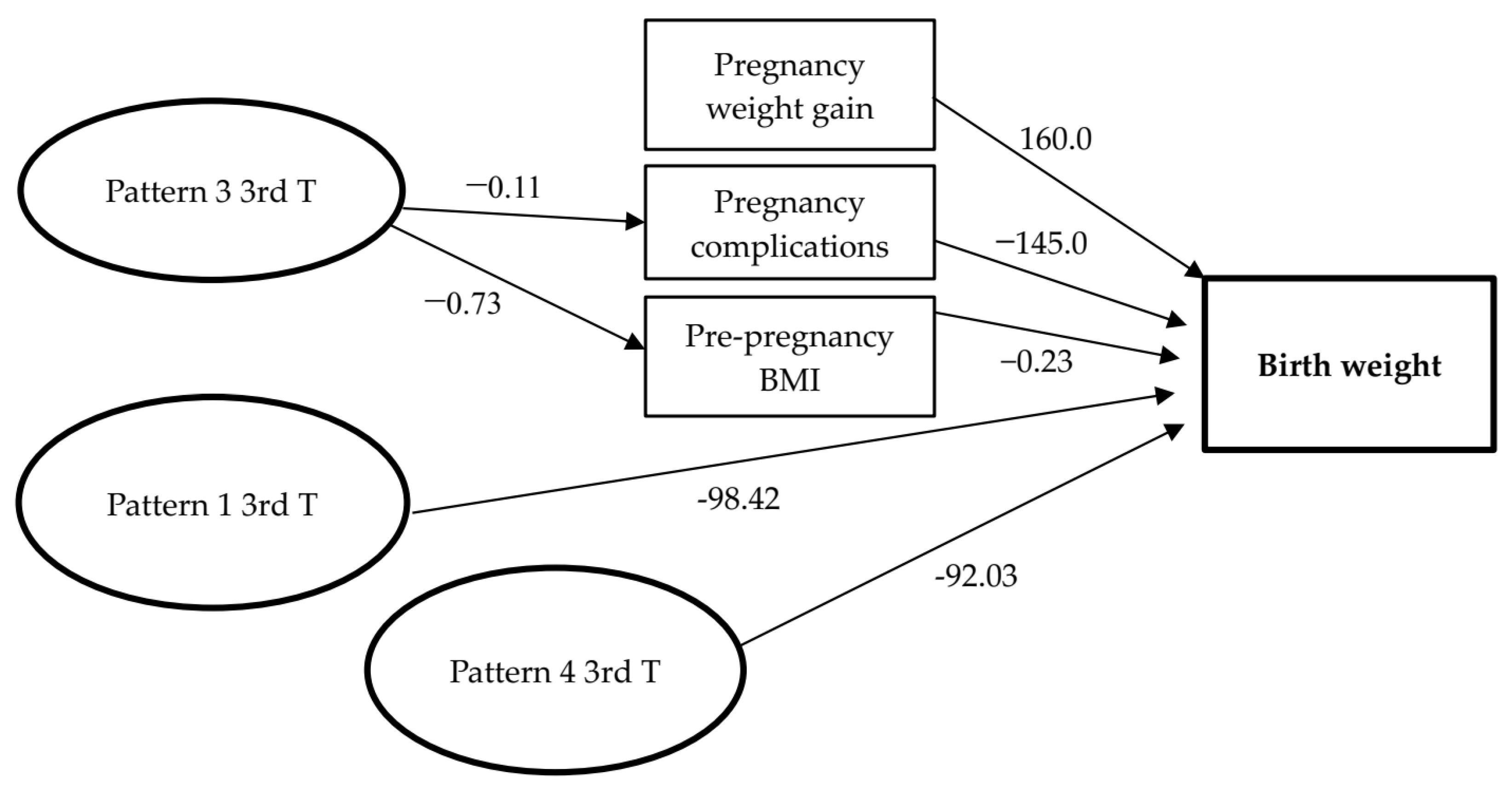
| Birth weight <- Pattern 1 (3rd T*) | −98.42 (−24.26; −172.59) | 0.009 | −98.42 (−39.74; −172.59) | 0.013 | ||
| Birth weight <- Pattern 4 (3rd T*) | −92.03 (−39.88; −165.30) | 0.003 | −92,03 (−38.43; −165.30) | 0.003 | ||
| Birth weight <- Weight gain | 160.0 (36.47; 243.56) | 0.004 | 160,0 (34.4; 243.56) | 0.04 | ||
| Birth weight <- Pregnancy Complications <- Pattern 3 (3rd T*) | −145 (−21.39; −211.45) | 0.008 | −0.11 (−0.04; 0.17) | 0.001 | −145.11 (−23.3; −211.0) | 0.04 |
| Birth weight <- PP BMI <- Pattern 3 (3rd T*) | −0.73 (−0.09; −0.14) | 0.004 | −0.73 (−3.6; 27.71) | 0.13 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Mota Santana, J.; de Oliveira Queiroz, V.A.; Pereira, M.; Paixão, E.S.; Brito, S.M.; dos Santos, D.B.; Oliveira, A.M. Associations between Maternal Dietary Patterns and Infant Birth Weight in the NISAMI Cohort: A Structural Equation Modeling Analysis. Nutrients 2021, 13, 4054. https://doi.org/10.3390/nu13114054
da Mota Santana J, de Oliveira Queiroz VA, Pereira M, Paixão ES, Brito SM, dos Santos DB, Oliveira AM. Associations between Maternal Dietary Patterns and Infant Birth Weight in the NISAMI Cohort: A Structural Equation Modeling Analysis. Nutrients. 2021; 13(11):4054. https://doi.org/10.3390/nu13114054
Chicago/Turabian Styleda Mota Santana, Jerusa, Valterlinda Alves de Oliveira Queiroz, Marcos Pereira, Enny S. Paixão, Sheila Monteiro Brito, Djanilson Barbosa dos Santos, and Ana Marlucia Oliveira. 2021. "Associations between Maternal Dietary Patterns and Infant Birth Weight in the NISAMI Cohort: A Structural Equation Modeling Analysis" Nutrients 13, no. 11: 4054. https://doi.org/10.3390/nu13114054
APA Styleda Mota Santana, J., de Oliveira Queiroz, V. A., Pereira, M., Paixão, E. S., Brito, S. M., dos Santos, D. B., & Oliveira, A. M. (2021). Associations between Maternal Dietary Patterns and Infant Birth Weight in the NISAMI Cohort: A Structural Equation Modeling Analysis. Nutrients, 13(11), 4054. https://doi.org/10.3390/nu13114054






