Optimal Perioperative Nutrition Therapy for Patients Undergoing Pancreaticoduodenectomy: A Systematic Review with a Component Network Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Outcome Measures
2.3. Data Extraction and Quality Assessment
2.4. Statistical Methods
3. Results
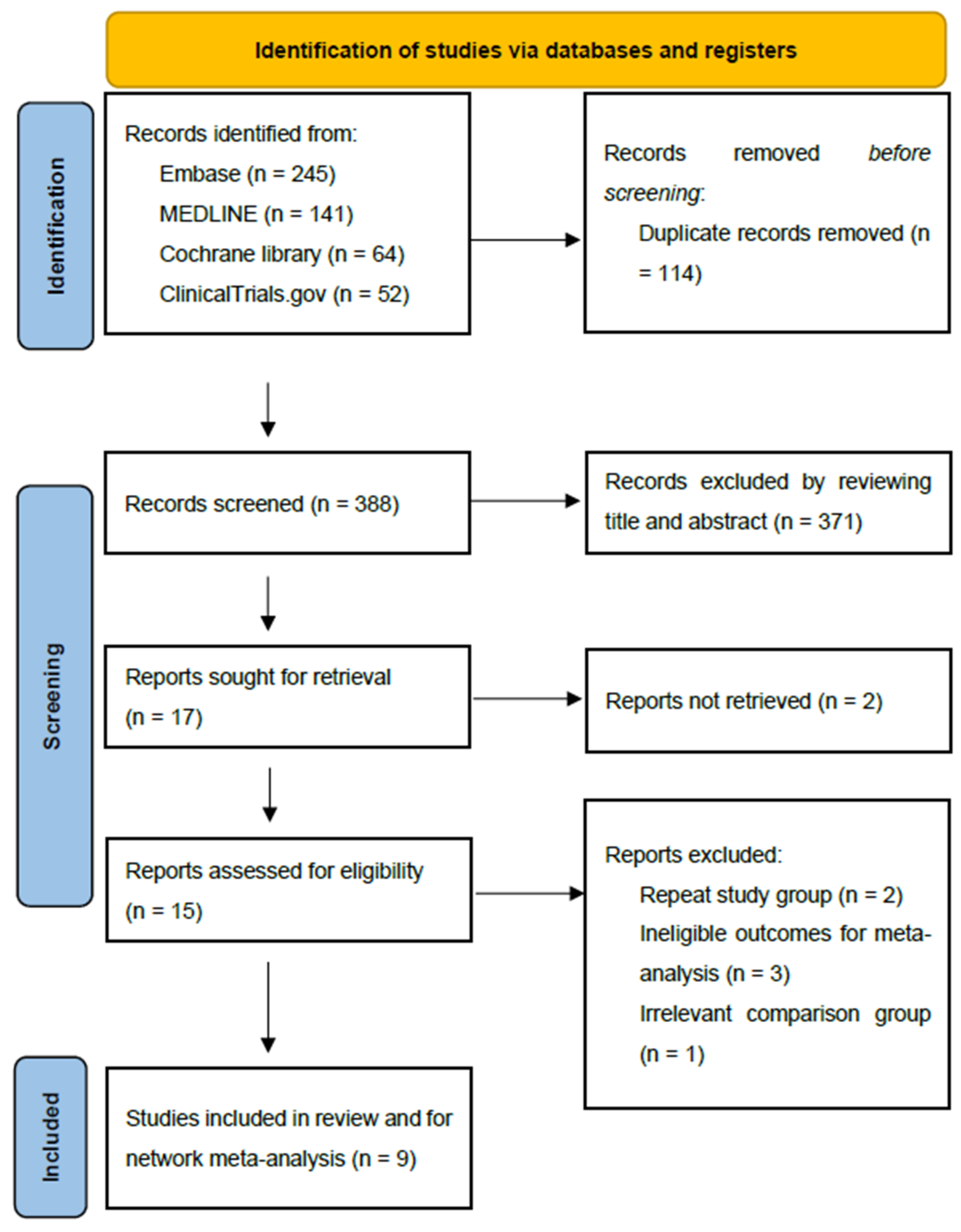
3.1. Study Selection and Study Characteristics
3.2. Risk of Bias within Studies
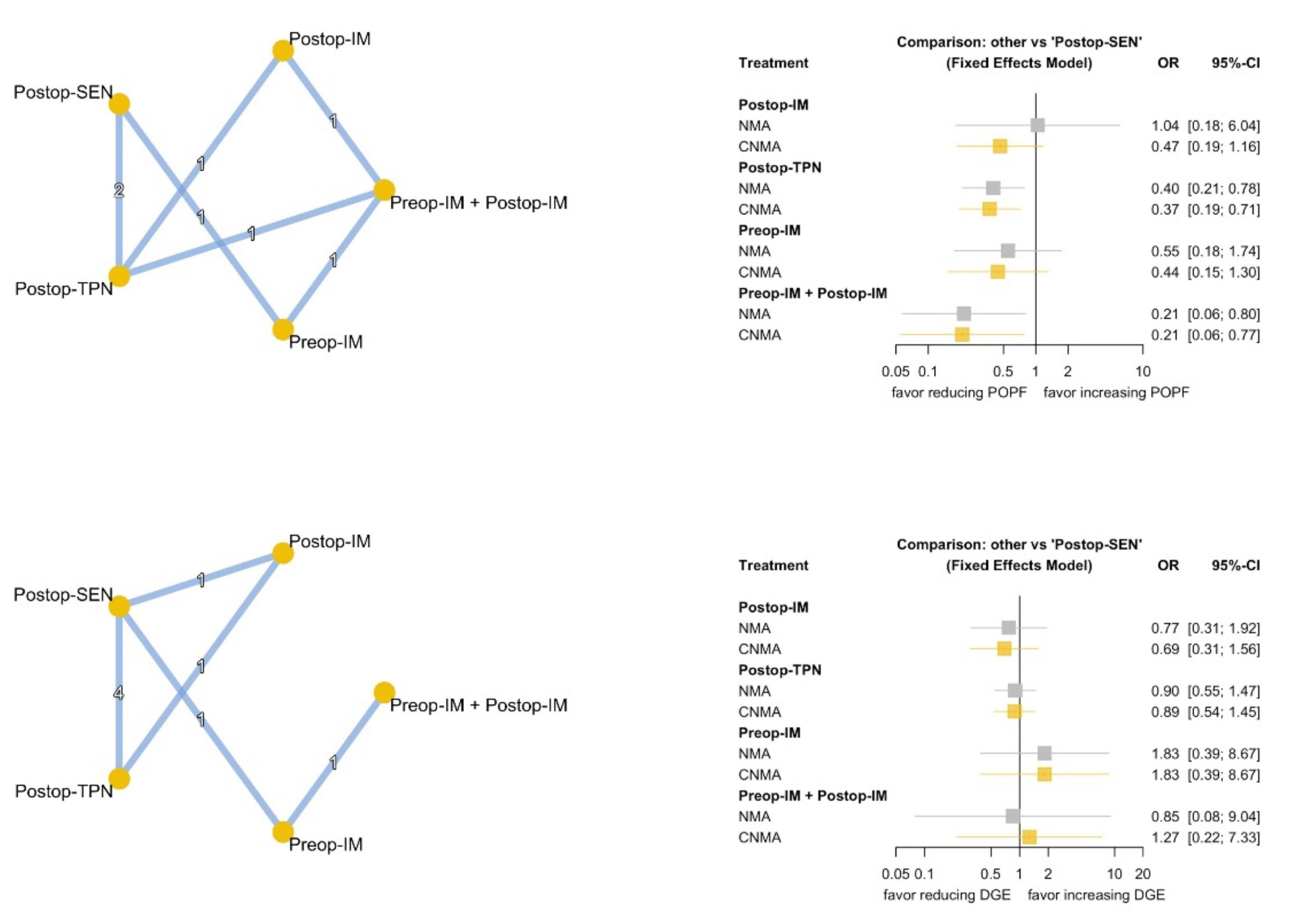
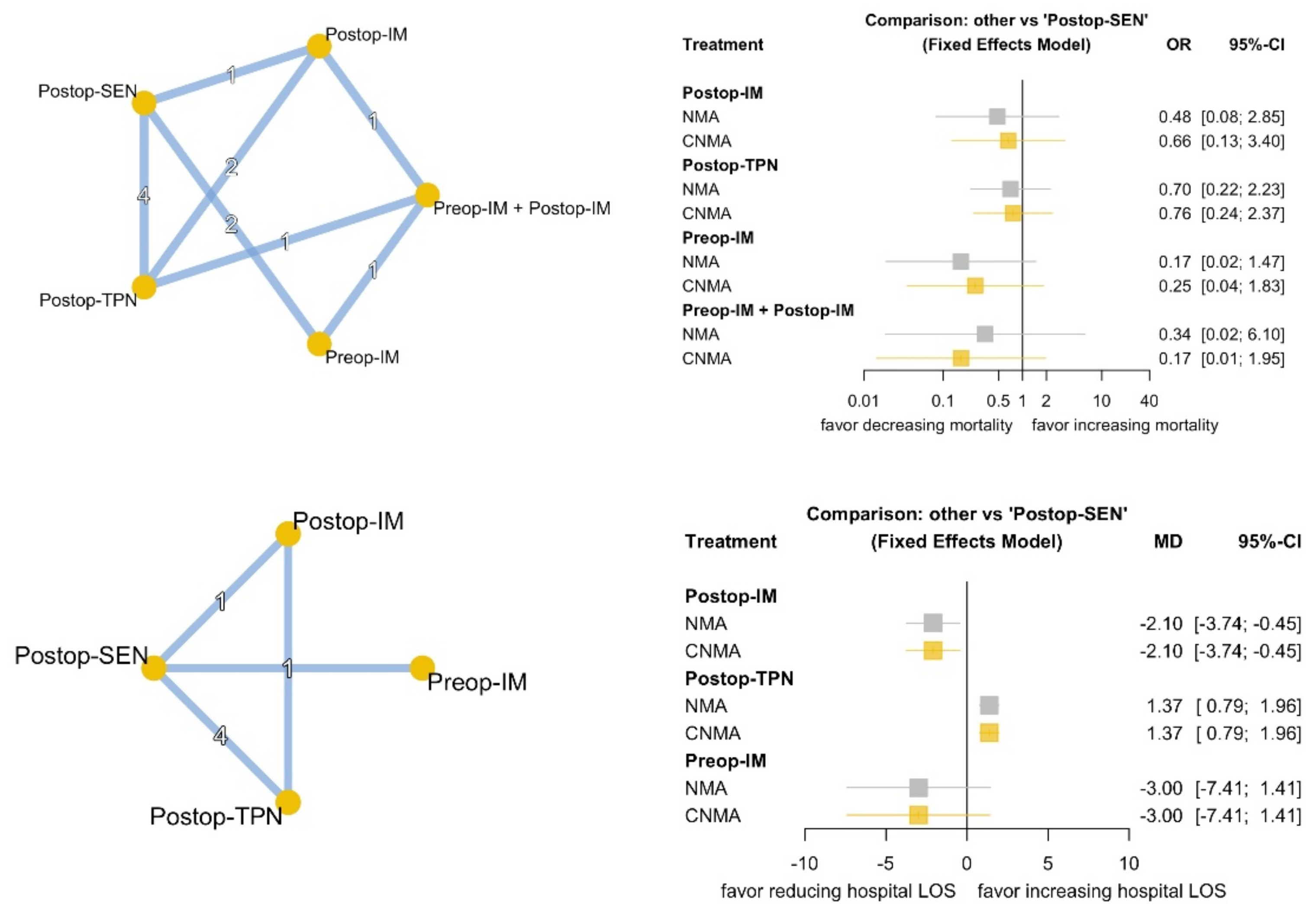
3.3. Primary Outcomes
3.4. Secondary Outcomes
3.5. Relative Ranking of Nutrition Therapy
3.6. Inconsistency and Publication Bias Assessment
3.7. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Cao, H.S.T.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; Mcelhany, A.L.; et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef]
- Sachs, T.E.; Tseng, J.F. Landmark Series in Pancreatic Tumors: Anastomotic Techniques and Route of Reconstruction. Ann. Surg. Oncol. 2021, 28, 2227–2234. [Google Scholar] [CrossRef]
- Gianotti, L.; Besselink, M.G.; Sandini, M.; Hackert, T.; Conlon, K.; Gerritsen, A.; Griffin, O.; Fingerhut, A.; Probst, P.; Abu Hilal, M.; et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2018, 164, 1035–1048. [Google Scholar] [CrossRef]
- Buscemi, S.; Damiano, G.; Palumbo, V.D.; Spinelli, G.; Ficarella, S.; Monte, G.L.; Marrazzo, A.; Monte, A.I.L. Enteral nutrition in pancreaticoduodenectomy: A literature review. Nutrients 2015, 7, 3154–3165. [Google Scholar] [CrossRef]
- Gerritsen, A.; Besselink, M.G.H.; Gouma, D.J.; Steenhagen, E.; Rinkes, I.H.M.B.; Molenaar, I.Q. Systematic review of five feeding routes after pancreatoduodenectomy. Br. J. Surg. 2013, 100, 589–598. [Google Scholar] [CrossRef]
- Perinel, J.; Mariette, C.; Dousset, B.; Sielezneff, I.; Gainant, A.; Mabrut, J.; Bin Dorel, S.; EL Bechwaty, M.; Delaunay, D.; Bernard, L.; et al. Early Enteral Versus Total Parenteral Nutrition in Patients Undergoing Pancreaticoduodenectomy: A Randomized Multicenter Controlled Trial (Nutri-DPC). Ann. Surg. 2016, 264, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuo, T.; Chen, H.; Wu, C.; Lai, S.; Yang, C.-Y.; Hsu, S.; Ho, T.-W.; Liao, W.; Tien, Y. Randomized trial of oral versus enteral feeding for patients with postoperative pancreatic fistula after pancreatoduodenectomy. Br. J. Surg. 2019, 106, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. ISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777. [Google Scholar] [CrossRef] [Green Version]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 3432, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. 10.4.4.1 Studies with no events in one or more arms. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; Available online: www.training.cochrane.org/handbook (accessed on 9 November 2021).
- Welton, N.J.; Caldwell, D.M.; Adamopoulos, E.; Vedhara, K. Mixed treatment comparison meta-analysis of complex interventions: Psychological interventions in coronary heart disease. Am. J. Epidemiol. 2009, 169, 1158–1165. [Google Scholar] [CrossRef] [Green Version]
- König, J.; Krahn, U.; Binder, H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Stat. Med. 2013, 32, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Schunemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [Green Version]
- Gianotti, L.; Braga, M.; Gentilini, O.; Balzano, G.; Zerbi, A.; Di Carlo, V. Artificial nutrition after pancreaticoduodenectomy. Pancreas 2000, 21, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Furukawa, K.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Effects of perioperative immunonutrition on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy. Surgery 2010, 148, 573–581. [Google Scholar] [CrossRef]
- Aida, T.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery 2014, 155, 124–133. [Google Scholar] [CrossRef]
- Hamza, N.; Darwish, A.; O’Reilly, D.A.; Denton, J.; Sheen, A.J.; Chang, D.; Sherlock, D.J.; Ammori, B.J. Perioperative Enteral Immunonutrition Modulates Systemic and Mucosal Immunity and the Inflammatory Response in Patients with Periampullary Cancer Scheduled for Pancreaticoduodenectomy: A Randomized Clinical Trial. Pancreas 2015, 44, 41–52. [Google Scholar] [CrossRef]
- Gade, J.; Levring, T.; Hillingsø, J.; Hansen, C.P.; Andersen, J.R. The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay After Elective Surgery for Pancreatic Cancer—A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 225–233. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Furukawa, K.; Suzuki, D.; Yoshitomi, H.; Takayashiki, T.; Kuboki, S.; Miyazaki, M.; Ohtsuka, M. Additional effect of perioperative, compared with preoperative, immunonutrition after pancreaticoduodenectomy: A randomized, controlled trial. Int. J. Surg. 2019, 61, 69–75. [Google Scholar] [CrossRef]
- Liu, C.; Du, Z.; Lou, C.; Wu, C.; Yuan, Q.; Wang, J.; Shu, G.; Wang, Y. Enteral nutrition is superior to total parenteral nutrition for pancreatic cancer patients who underwent pancreaticoduodenectomy. Asia Pac. J. Clin. Nutr. 2011, 20, 154–160. [Google Scholar]
- Park, J.S.; Chung, H.K.; Hwang, H.K.; Kim, J.K.; Yoon, D.S. Postoperative nutritional effects of early enteral feeding compared with total parental nutrition in pancreaticoduodectomy patients: A prosepective, randomized study. J. Korean Med. Sci. 2012, 27, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Arnold, M.A.; Chang, D.C.; Coleman, J.; Hodgin, M.B.; Sauter, P.K.; Hruban, R.H.; Riall, T.S. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. Off. J. Soc. Surg Aliment. Tract. 2006, 10, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Van Hilst, J.; de Rooij, T.; Bosscha, K.; Brinkman, D.J.; van Dieren, S.; Dijkgraaf, M.G. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): A multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 199–207. [Google Scholar] [CrossRef]
- Pappas, S.; Krzywda, E.; McDowell, N. Nutrition and pancreaticoduodenectomy. Nutr. Clin. Pract. 2010, 25, 234–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 2002, 21, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Domagala, P.; Hartog, H.; Eijck, C.; Groot Koerkamp, B. Current evidence of nutritional therapy in pancreatoduodenectomy: Systematic review of randomized controlled trials. Ann. Gastroenterol. Surg. 2019, 3, 620–629. [Google Scholar] [CrossRef]
- Guan, H.; Chen, S.; Huang, Q. Effects of Enteral Immunonutrition in Patients Undergoing Pancreaticoduodenectomy: A Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2019, 74, 53–61. [Google Scholar] [CrossRef]
- Yang, F.-A.; Chen, Y.-C.; Tiong, C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2798. [Google Scholar] [CrossRef]

| Author, Year | Country | Study Type | Patient | Treatment vs. Control | n | Age, Mean ± SD | Male, n (%) | Outcome | Brand of IM |
|---|---|---|---|---|---|---|---|---|---|
| Immunonutrition vs. other | |||||||||
| Gianotti, 2000 [16] | Italy | RCT | PD or PPPD for pancreatic head or periampullary lesion | postop IM vs. SEN vs. postop TPN | 71/73/68 | 61.1 ± 11.9/ 59.8 ± 12.2/ 60.2 ± 10.4 | 44 (62.0%)/ 47 (64.4%)/ 43 (63.2%) | mortality, complications, infectious complications, noninfectious complications, POPF, DGE, hospital LOS | IMPACT |
| Suzuki, 2010 [17] | Japan | RCT | PD or PPPD | preop IM + postop IM vs. postop IM vs. postop TPN | 10/10/10 | 62.0 ± 4.0/ 61.0 ± 3.0/ 66.0 ± 3.0 | 7 (70%)/ 7 (70%)/ 4 (40%) | mortality, infectious complications, noninfectious complications, POPF | IMPACT |
| Aida, 2014 [18] | Japan | RCT | PD or PPPD | preop IM vs. SEN | 25/25 | 66.4 ± 1.5/ 65.1 ± 1.9 | 20 (80%)/ 16 (64%) | mortality, complications, infectious complications, noninfectious complications, POPF, DGE | IMPACT |
| Hamza, 2015 [19] | United Kingdom | RCT | PD for periampullary cancer | preop IM + postop IM vs. preop EN + SEN | 17/20 | 63.3 ± 3.2/ 66.8 ± 2.0 | 9 (52.9%)/ 11 (55%) | infectious complications, noninfectious complications | IMPACT |
| Gade, 2016 [20] | Denmark | RCT | PD and other surgery for pancreatic cancer | preop IM vs. SEN | 19/16 | 66.8 ± 8.9/ 67.5 ± 7.5 | 12 (63.2%)/ 6 (37.5%) | mortality, hospital LOS | IMPACT |
| Miyauchi, 2019 [21] | Japan | RCT | PD or PPPD | preop IM + postop IM vs. preop IM | 30/30 | 67.8 ± 9.3/ 67.6 ± 7.5 | 16 (53.3%)/ 18 (60%) | mortality, complications, infectious complications, noninfectious complications, POPF, DGE | IMPACT |
| EN vs. TPN | |||||||||
| Liu, 2011 [22] | China | RCT | PD for pancreatic cancer | SEN vs. postop TPN | 28/30 | 59.7 ± 11.2/ 60.5 ± 11.9 | 16 (57.1%)/ 17 (56.7%) | mortality, POPF, DGE, hospital LOS | - |
| Park, 2012 [23] | Korea | RCT | PD or PPPD | SEN vs. postop TPN | 18/20 | 62.7 ± 10.3/ 61.3 ± 13.2 | 7 (38.9%)/ 12 (60%) | mortality, complications, infectious complications, noninfectious complications, POPF, DGE, hospital LOS | - |
| Perinel, 2016 [6] | France | RCT | PD or PPPD | SEN vs. postop TPN | 103/101 | 65.46 ± 11.25/ 64.02 ± 9.9 | 39 (37.9%)/ 40 (39.6%) | mortality, complications, infectious complications, noninfectious complications, POPF, DGE, hospital LOS | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Hung, Y.-L.; Hsu, C.-C.; Hu, C.-H.; Huang, R.-Y.; Sung, C.-M.; Li, Y.-R.; Kou, H.-W.; Chen, M.-Y.; Chang, S.-C.; et al. Optimal Perioperative Nutrition Therapy for Patients Undergoing Pancreaticoduodenectomy: A Systematic Review with a Component Network Meta-Analysis. Nutrients 2021, 13, 4049. https://doi.org/10.3390/nu13114049
Wang S-Y, Hung Y-L, Hsu C-C, Hu C-H, Huang R-Y, Sung C-M, Li Y-R, Kou H-W, Chen M-Y, Chang S-C, et al. Optimal Perioperative Nutrition Therapy for Patients Undergoing Pancreaticoduodenectomy: A Systematic Review with a Component Network Meta-Analysis. Nutrients. 2021; 13(11):4049. https://doi.org/10.3390/nu13114049
Chicago/Turabian StyleWang, Shang-Yu, Yu-Liang Hung, Chih-Chieh Hsu, Chia-Hsiang Hu, Ruo-Yi Huang, Chang-Mu Sung, Yan-Rong Li, Hao-Wei Kou, Ming-Yang Chen, Shih-Chun Chang, and et al. 2021. "Optimal Perioperative Nutrition Therapy for Patients Undergoing Pancreaticoduodenectomy: A Systematic Review with a Component Network Meta-Analysis" Nutrients 13, no. 11: 4049. https://doi.org/10.3390/nu13114049
APA StyleWang, S.-Y., Hung, Y.-L., Hsu, C.-C., Hu, C.-H., Huang, R.-Y., Sung, C.-M., Li, Y.-R., Kou, H.-W., Chen, M.-Y., Chang, S.-C., Lee, C.-W., Tsai, C.-Y., Liu, K.-H., Hsu, J.-T., Yeh, C.-N., Yeh, T.-S., Hwang, T.-L., Jan, Y.-Y., & Chen, M.-F. (2021). Optimal Perioperative Nutrition Therapy for Patients Undergoing Pancreaticoduodenectomy: A Systematic Review with a Component Network Meta-Analysis. Nutrients, 13(11), 4049. https://doi.org/10.3390/nu13114049








