Obesity-Related Indices Are Associated with Longitudinal Changes in Lung Function: A Large Taiwanese Population Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. TWB
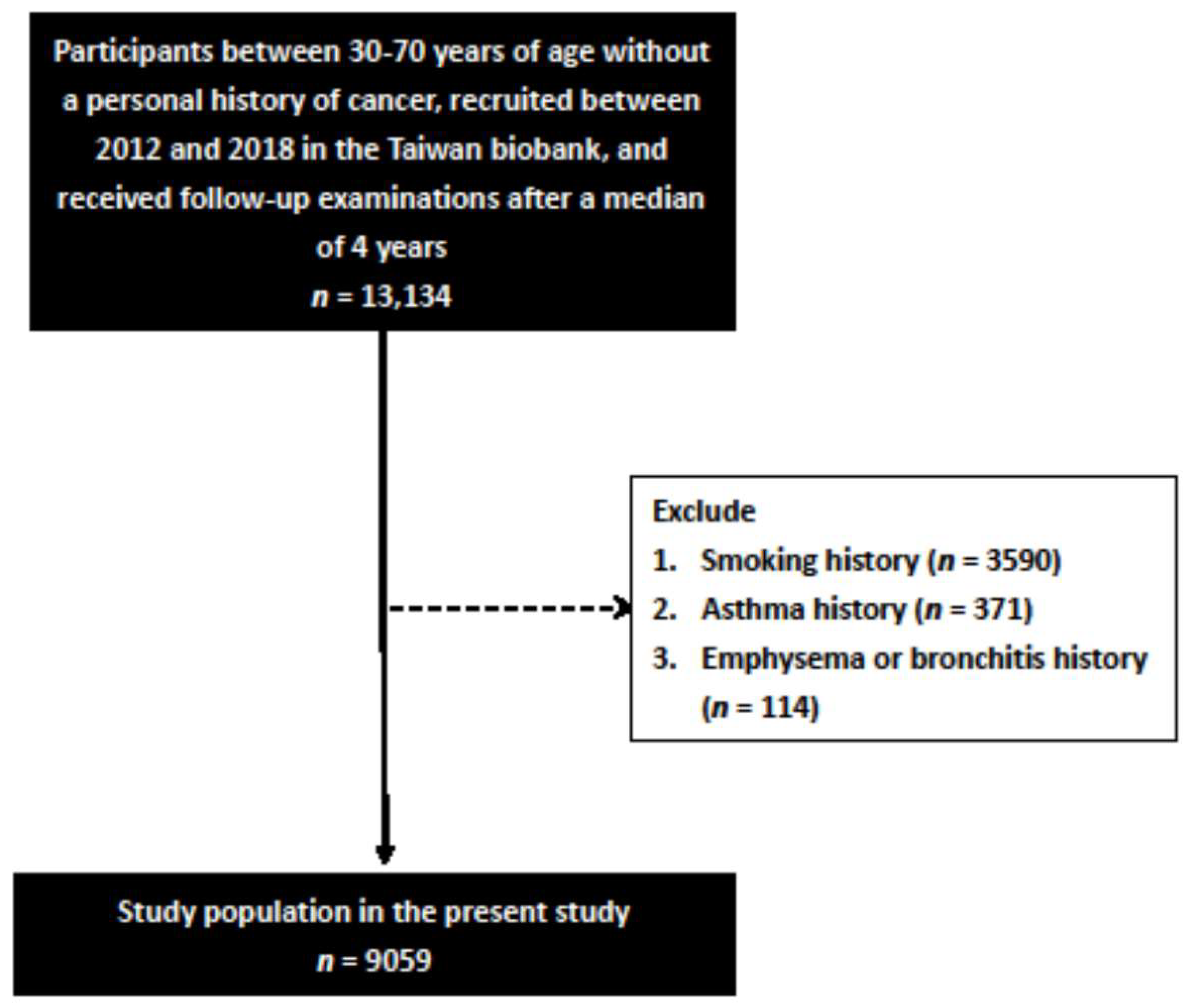
2.2. Study Participants
2.3. Ethics Statement
2.4. Demographic, Medical, and Laboratory Data
2.5. Calculation of Obesity-Related Indices
2.6. Spirometry Measurements
2.7. Statistical Analysis
3. Results
3.1. Comparisons of Clinical Characteristics between the Normal and Obstructive Lung Function Groups
3.2. Associations among the Obesity-Related Indices with Baseline FEV1/FVC
3.3. Associations among the Obesity-Related Indices with ∆FEV1/FVC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Halbert, R.J.; Natoli, J.L.; Gano, A.; Badamgarav, E.; Buist, A.S.; Mannino, D. Global burden of COPD: Systematic review and meta-analysis. Eur. Respir. J. 2006, 28, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.R.; Hill, N.S. Right Ventricular Dysfunction in Chronic Obstructive Pulmonary Disease*: Evaluation and management. Chest 1991, 99, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.B.; Dasenbrook, E.C.; Pitz, M.W.; Murphy, D.J.; Fan, E. Inhaled Corticosteroids in Patients With Stable Chronic Obstructive Pulmonary Disease: A systematic review and meta-analysis. JAMA 2008, 300, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I. Depression in the patient with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2006, 1, 61–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.Y.; Kang, D.; Shin, S.H.; Yoo, K.-H.; Rhee, C.K.; Suh, G.Y.; Kim, H.; Shim, Y.M.; Guallar, E.; Cho, J.; et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: A cohort study. Thorax 2020, 75, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Gunen, H.; Hacievliyagil, S.S.; Kosar, F.; Mutlu, L.C.; Gulbas, G.; Pehlivan, E.; Sahin, I.; Kizkin, O. Factors affecting survival of hospitalised patients with COPD. Eur. Respir. J. 2005, 26, 234–241. [Google Scholar] [CrossRef]
- Fettal, N.; Taleb, A. Pneumothorax secondary to chronic obstructive pulmonary disease. Eur. Respir. J. 2012, 40, P558. [Google Scholar]
- John, M.; Lange, A.; Hoernig, S.; Witt, C.; Anker, S.D. Prevalence of anemia in chronic obstructive pulmonary disease: Comparison to other chronic diseases. Int. J. Cardiol. 2006, 111, 365–370. [Google Scholar] [CrossRef]
- Haider, M.Z.; Anwer, F. Secondary Polycythemia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Papiris, S.; Kotanidou, A.; Malagari, K.; Roussos, C. Clinical review: Severe asthma. Crit. Care 2001, 6, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Mannino, D.M.; Davis, K.J. Lung function decline and outcomes in an elderly population. Thorax 2006, 61, 472–477. [Google Scholar] [CrossRef]
- Cogen, J.; Emerson, J.; Sanders, D.B.; Ren, C.; Schechter, M.S.; Gibson, R.L.; Morgan, W.; Rosenfeld, M.; EPIC Study Group. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr. Pulmonol. 2015, 50, 763–770. [Google Scholar] [CrossRef]
- Poulain, M.; Doucet, M.; Major, G.C.; Drapeau, V.; Sériès, F.; Boulet, L.-P.; Tremblay, A.; Maltais, F. The effect of obesity on chronic respiratory diseases: Pathophysiology and therapeutic strategies. Can. Med. Assoc. J. 2006, 174, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, E.N.; Adejumo, A.O.; Azenabor, A.; Ekun, A.O.; Enitan, S.S.; Adebola, O.K.; Ogundahunsi, O.A. Anthropometric parameter that best predict metabolic syndrome in South west Nigeria. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 13, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Ho, C.-C.; Yang, C.-F.; Huang, Y.-C.; Lai, C.-H.; Liaw, Y.-P. Waist-to-hip ratio is a better anthropometric index than body mass index for predicting the risk of type 2 diabetes in Taiwanese population. Nutr. Res. 2010, 30, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Lin, W.-Y.; Lee, L.-T.; Chen, C.-Y.; Lo, H.; Hsia, H.-H.; Liu, I.-L.; Shau, W.-Y.; Lin, R.-S. Four anthropometric indices and cardiovascular risk factors in Taiwan. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1060–1068. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, J.-H.; Chiang, C.W.K.; Hsiung, C.-N.; Wu, P.-E.; Chang, L.-C.; Chu, H.-W.; Chang, J.; Yuan-Tsong, C.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef]
- Fan, C.-T.; Hung, T.-H.; Yeh, C.-K. Taiwan Regulation of Biobanks. J. Law Med. Ethic 2015, 43, 816–826. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D.R.; the Modification of Diet in Renal Disease Study Group. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R. A simple model-based index of abdominal adiposity. J. Clin. Epidemiol. 1991, 44, 955–956. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A Better Index of Body Adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Abdominal volume index. an anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch. Med. Res. 2003, 34, 428–432. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Galesanu, R.G.; Bernard, S.; Marquis, K.; Lacasse, Y.; Poirier, P.; Bourbeau, J.; Maltais, F. Obesity and Chronic Obstructive Pulmonary Disease: Is Fatter Really Better? Can. Respir. J. 2014, 21, 297–301. [Google Scholar] [CrossRef]
- Ijaz, A.; Bashir, I.; Ikhlaq, A.; Ijaz, F.; Aftab, R.K.; Zia, R. Correlation Between Peak Expiratory Flow Rate, Markers of Adiposity, and Anthropometric Measures in Medical Students in Pakistan. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-H.; Zhou, J.-B.; Cai, Y.-H.; Shu, L.-P.; Yang, J.; Wei, W.; Lecube, A. Non-linear association of anthropometric measurements and pulmonary function. Sci. Rep. 2021, 11, 14596. [Google Scholar] [CrossRef]
- Poulain, M.; Doucet, M.; Drapeau, V.; Fournier, G.; Tremblay, A.; Poirier, P.; Maltais, F. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chronic Respir. Dis. 2008, 5, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Landbo, C.; Prescott, E.; Lange, P.; Vestbo, J.; Almdal, T.P. Prognostic Value of Nutritional Status in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Cornell, K.; Alam, M.; Lyden, E.; Wood, L.; LeVan, T.D.; Nordgren, T.M.; Bailey, K.; Hanson, C. Saturated Fat Intake Is Associated with Lung Function in Individuals with Airflow Obstruction: Results from NHANES 2007–2012. Nutrients 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Suratt, B.T.; Bates, J.H.T.; Dixon, A.E. Beyond BMI: Obesity and Lung Disease. Chest 2018, 153, 702–709. [Google Scholar] [CrossRef]
- Freeman, A.M.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lazarus, R.; Sparrow, D.; Weiss, S.T. Impaired ventilatory function and elevated insulin levels in nondiabetic males: The Normative Aging Study. Eur. Respir. J. 1998, 12, 635–640. [Google Scholar] [CrossRef]
- Yeh, F.; Dixon, A.E.; Marion, S.; Schaefer, C.; Zhang, Y.; Best, L.G.; Calhoun, D.; Rhoades, E.R.; Lee, E.T. Obesity in Adults Is Associated With Reduced Lung Function in Metabolic Syndrome and Diabetes: The Strong Heart Study. Diabetes Care 2011, 34, 2306–2313. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Ebrahim, S.; Smith, G.D. Associations of measures of lung function with insulin resistance and Type 2 diabetes: Findings from the British Women’s Heart and Health Study. Diabetologia 2004, 47, 195–203. [Google Scholar] [CrossRef]
- Kwon, C.-H.; Rhee, E.-J.; Song, J.-U.; Kim, J.-T.; Kwag, H.J.; Sung, K.-C. Reduced lung function is independently associated with increased risk of type 2 diabetes in Korean men. Cardiovasc. Diabetol. 2012, 11, 38. [Google Scholar] [CrossRef][Green Version]
- Di Filippo, P.; Scaparrotta, A.; Rapino, D.; de Giorgis, T.; Petrosino, M.I.; Attanasi, M.; Di Pillo, S.; Chiarelli, F.; Mohn, A. Insulin resistance and lung function in obese asthmatic pre-pubertal children. J. Pediatr. Endocrinol. Metab. 2018, 31, 45–51. [Google Scholar] [CrossRef]
- Hart, J.E.; Morse, L.R.; Tun, C.G.; Brown, R.D.; Garshick, E. Cross-sectional associations of pulmonary function with systemic inflammation and oxidative stress in individuals with chronic spinal cord injury. J. Spinal Cord Med. 2015, 39, 344–352. [Google Scholar] [CrossRef]
- Stolzmann, K.L.; Gagnon, D.; Brown, R.; Tun, C.G.; Garshick, E. Longitudinal Change in FEV1 and FVC in Chronic Spinal Cord Injury. Am. J. Respir. Crit. Care Med. 2008, 177, 781–786. [Google Scholar] [CrossRef]
- He, H.; Wang, B.; Zhou, M.; Cao, L.; Qiu, W.; Mu, G.; Chen, A.; Yang, S.; Chen, W. Systemic Inflammation Mediates the Associations Between Abdominal Obesity Indices and Lung Function Decline in a Chinese General Population. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, ume 13, 141–150. [Google Scholar] [CrossRef]
- Sadeghimakki, R.; McCarthy, H.D. Interactive effects of adiposity and insulin resistance on the impaired lung function in asthmatic adults: Cross-sectional analysis of NHANES data. Ann. Hum. Biol. 2019, 46, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sorlí-Aguilar, M.; Martín-Luján, F.; Flores-Mateo, G.; Jardí-Piñana, C.; Aparicio-Llopis, E.; Basora-Gallisà, J.; Solà-Alberich, R. Adiposity markers and lung function in smokers: A cross-sectional study in a Mediterranean population. BMC Pulm. Med. 2016, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Sparrow, D.; Weiss, S.T. Effects of Obesity and Fat Distribution on Ventilatory Function: The normative aging study. Chest 1997, 111, 891–898. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zamel, N.; Dubarry, L.; Hoffstein, V. Airflow Limitation in Morbidly Obese, Nonsmoking Men. Ann. Intern. Med. 1990, 112, 828–832. [Google Scholar] [CrossRef]
- Chlif, M.; Keochkerian, D.; Mourlhon, C.; Choquet, D.; Ahmaidi, S. Noninvasive assessment of the tension–time index of inspiratory muscles at rest in obese male subjects. Int. J. Obes. 2005, 29, 1478–1483. [Google Scholar] [CrossRef]
- Biring, M.S.; Lewis, M.I.; Liu, J.T.; Mohsenifar, Z. Pulmonary Physiologic Changes of Morbid Obesity. Am. J. Med. Sci. 1999, 318, 293–297. [Google Scholar] [CrossRef]
- Chen, M.; Huang, C.; Feng, W.; Li, Y.; Wu, Y. C-reactive protein mediates the association between leisure-time physical activity and lung function in middle-aged and older adults. BMC Public Health 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.C.; Blaha, M.J.; Blumenthal, R.S. Relation of C-Reactive Protein to Abdominal Adiposity. Am. J. Cardiol. 2010, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Kern, J.A.; Miller, Y.E. Senescence in Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2012, 9, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.; Larsson, L.-G.; Rönmark, E.; Jonsson, A.-C.; Larsson, K.; Lundbäck, B. Decline in FEV1 in Relation to Incident Chronic Obstructive Pulmonary Disease in a Cohort with Respiratory Symptoms. COPD: J. Chronic Obstr. Pulm. Dis. 2007, 4, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, C.; Li, B.; Shi, G.; Li, H.; Zhang, J.; Gu, Y.; Zhou, J.; Song, Y.; Bai, C. Risk factors for FEV1 decline in mild COPD and high-risk populations. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 435–442. [Google Scholar] [CrossRef]
- Ishikawa, C.; Barbieri, M.A.; Bettiol, H.; Bazo, G.; Ferraro, A.A.; Vianna, E.O. Comparison of body composition parameters in the study of the association between body composition and pulmonary function. BMC Pulm. Med. 2021, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, B.; Jacobs, D.R., Jr.; Apostol, G.G.; Smith, L.J.; Jensen, R.L.; Crapo, R.O.; Barr, R.G.; Lewis, C.E.; Williams, O.D. Longitudinal association of body mass index with lung function: The CARDIA Study. Respir. Res. 2008, 9, 31. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Normal (n = 6016) | Obstructive (n = 3043) | p |
|---|---|---|---|
| Age (year) | 50.9 ± 10.1 | 51.2 ± 10.5 | 0.275 |
| Male gender (%) | 20.9 | 18.2 | 0.003 |
| DM (%) | 4.5 | 3.8 | 0.115 |
| Hypertension (%) | 11.2 | 11.3 | 0.867 |
| Regular exercise habits (%) | 49.7 | 48.8 | 0.406 |
| SBP (mmHg) | 115.6 ± 17.5 | 116.7 ± 17.9 | 0.005 |
| DBP (mmHg) | 71.0 ± 10.6 | 71.6 ± 10.6 | 0.007 |
| Body height (cm) | 159.5 ± 7.5 | 159.3 ± 7.3 | 0.220 |
| Body weight (kg) | 60.6 ± 11.0 | 59.6 ± 10.5 | <0.001 |
| Waist circumference (cm) | 81.9 ± 9.5 | 81.1 ± 9.3 | <0.001 |
| Hip circumference (cm) | 95.3 ± 6.7 | 94.9 ± 6.6 | 0.007 |
| Laboratory parameters | |||
| Fasting glucose (mg/dL) | 94.9 ± 17.7 | 94.3 ± 17.3 | 0.139 |
| Hemoglobin (g/dL) | 13.4 ± 1.5 | 13.4 ± 1.4 | 0.037 |
| Triglyceride (mg/dL) | 106.8 ± 74.5 | 104.2 ± 69.1 | 0.105 |
| Total cholesterol (mg/dL) | 196.4 ± 35.3 | 195.9 ± 34.9 | 0.568 |
| HDL-cholesterol (mg/dL) | 56.1 ± 13.1 | 56.1 ± 13.0 | 0.853 |
| LDL-cholesterol (mg/dL) | 121.7 ± 31.3 | 121.0 ± 30.9 | 0.560 |
| eGFR (mL/min/1.73 m2) | 110.7 ± 25.5 | 111.5 ± 24.7 | 0.153 |
| Uric acid (mg/dL) | 5.24 ± 1.31 | 5.16 ± 1.30 | 0.006 |
| Lung function | |||
| FVC (L, baseline) | 2.68 ± 0.71 | 2.58 ± 0.73 | <0.001 |
| FVC (L, follow-up) | 2.48 ± 0.69 | 2.44 ± 0.70 | 0.003 |
| FEV1 (L, baseline) | 2.25 ± 0.61 | 1.31 ± 0.53 | <0.001 |
| FEV1 (L, follow-up) | 2.17 ± 0.63 | 2.10 ± 0.66 | <0.001 |
| FEV1/FVC (%, baseline) | 84.02 ± 6.21 | 50.55 ± 13.65 | <0.001 |
| FEV1/FVC (%, follow-up) | 87.86 ± 9.63 | 86.31 ± 12.18 | <0.001 |
| Obesity-related indices | |||
| BMI (kg/m2) | 23.8 ± 3.5 | 23.4 ± 3.4 | <0.001 |
| WHR (%) | 85.8 ± 6.7 | 85.3 ± 6.8 | 0.001 |
| WHtR (%) | 51.4 ± 6.0 | 51.0 ± 5.9 | 0.001 |
| LAP | 29.0 ± 27.5 | 27.6 ± 25.4 | 0.022 |
| BRI | 6.6 ± 1.8 | 6.4 ± 1.8 | <0.001 |
| CI | 1.222 ± 0.082 | 1.219 ± 0.084 | 0.037 |
| BAI | 29.4 ± 4.0 | 29.3 ± 3.9 | 0.185 |
| AVI | 13.7 ± 3.2 | 13.5 ± 3.1 | <0.001 |
| Characteristics | Univariable | |
|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | |
| Age (per 1 year) | −0.053 (−0.090, −0.016) | 0.005 |
| Male (vs. female) | 1.154 (0.208, 2.100) | 0.017 |
| DM | 1.174 (−0.697, 3.044) | 0.219 |
| Hypertension | −0.341 (−1.539, 0.857) | 0.577 |
| Regular exercise habits | −0.078 (−0.835, 0.680) | 0.841 |
| SBP (per 1 mmHg) | −0.044 (−0.066, −0.023) | <0.001 |
| DBP (per 1 mmHg) | −0.063 (−0.099, −0.028) | 0.001 |
| Laboratory parameters | ||
| Fasting glucose (per 1 mg/dL) | 0.013 (−0.008, 0.035) | 0.230 |
| Hemoglobin (per 1 g/dL) | 0.227 (−0.033, 0.488) | 0.088 |
| Triglyceride (per 1 mg/dL) | 0.005 (0, 0.010) | 0.069 |
| Total cholesterol (per 1 mg/dL) | −0.004 (−0.014, 0.007) | 0.510 |
| HDL-cholesterol (per 1 mg/dL) | −0.006 (−0.035, 0.023) | 0.675 |
| LDL-cholesterol (per 1 mg/dL) | 7.781 × 10−5 (−0.012, 0.012) | 0.990 |
| eGFR (per 1 mL/min/1.73 m2) | −0.001 (−0.016, 0.014) | 0.854 |
| Uric acid (per 1 mg/dL) | 0.396 (0.106, 0.686) | 0.007 |
| Obesity-Related Indices | Multivariable | Adjusted R Square | |
|---|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | ||
| BMI (per 1 kg/m2) | 0.303 (0.180, 0.426) | <0.001 | 0.6% |
| WHR (per 1%) | 0.123 (0.059, 0.186) | <0.001 | 0.5% |
| WHtR (per 1%) | 0.190 (0.120, 0.261) | <0.001 | 0.7% |
| LAP (per 1) | 0.245 (0.092, 0.397) | 0.002 | 0.5% |
| BRI (per 1) | 0.565 (0.336, 0.793) | <0.001 | 0.6% |
| CI (per 0.1) | 0.694 (0.210, 1.178) | 0.005 | 0.4% |
| BAI (per 1) | 0.263 (0.152, 0.374) | <0.001 | 0.6% |
| AVI (per 1) | 0.296 (0.161, 0.431) | <0.001 | 0.6% |
| Characteristics | Univariable | |
|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | |
| Age (per 1 year) | 0.020 (−0.020, 0.061) | 0.325 |
| Male (vs. female) | −1.717 (−2.761, −0.674) | 0.001 |
| DM | −0.707 (−2.771, 1.357) | 0.502 |
| Hypertension | 0.347 (−0.975, 1.669) | 0.607 |
| Regular exercise habits | −0.207 (−1.043, 0.629) | 0.627 |
| SBP (per 1 mmHg) | 0.073 (0.050, 0.097) | <0.001 |
| DBP (per 1 mmHg) | 0.113 (0.073, 0.152) | <0.001 |
| Laboratory parameters | ||
| Fasting glucose (per 1 mg/dL) | −0.012 (−0.036, 0.012) | 0.317 |
| Hemoglobin (per 1 g/dL) | −0.246 (−0.534, 0.042) | 0.094 |
| Triglyceride (per 1 mg/dL) | −0.004 (−0.010, 0.002) | 0.169 |
| Total cholesterol (per 1 mg/dL) | 0.012 (0, 0.024) | 0.045 |
| HDL-cholesterol (per 1 mg/dL) | −0.021 (−0.053, 0.011) | 0.202 |
| LDL-cholesterol (per 1 mg/dL) | 0.006 (−0.008, 0.019) | 0.401 |
| eGFR (per 1 mL/min/1.73 m2) | 0.034 (0.018, 0.051) | <0.001 |
| Uric acid (per 1 mg/dL) | −0.248 (−0.568, 0.072) | 0.128 |
| Obesity-Related Indices | Multivariable | Adjusted R Square | |
|---|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | ||
| BMI (per 1 kg/m2) | −0.280 (−0.409, −0.151) | <0.001 | 1.0% |
| WHR (per 1%) | −0.123 (−0.192, −0.055) | <0.001 | 0.8% |
| WHtR (per 1%) | −0.190 (−0.265, −0.115) | <0.001 | 1.1% |
| LAP (per 1) | −0.284 (−0.448, −0.120) | 0.001 | 0.9% |
| BRI (per 1) | −0.567 (−0.808, −0.325) | <0.001 | 1.0% |
| CI (per 0.1) | −0.855 (−1.385, −0.326) | 0.002 | 0.9% |
| BAI (per 1) | −0.272 (−0.392, −0.153) | <0.001 | 1.0% |
| AVI (per 1) | −0.306 (−0.449, −0.163) | <0.001 | 1.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-E.; Chen, S.-C.; Geng, J.-H.; Wu, D.-W.; Wu, P.-Y.; Huang, J.-C. Obesity-Related Indices Are Associated with Longitudinal Changes in Lung Function: A Large Taiwanese Population Follow-Up Study. Nutrients 2021, 13, 4055. https://doi.org/10.3390/nu13114055
Hsu Y-E, Chen S-C, Geng J-H, Wu D-W, Wu P-Y, Huang J-C. Obesity-Related Indices Are Associated with Longitudinal Changes in Lung Function: A Large Taiwanese Population Follow-Up Study. Nutrients. 2021; 13(11):4055. https://doi.org/10.3390/nu13114055
Chicago/Turabian StyleHsu, Yu-En, Szu-Chia Chen, Jiun-Hung Geng, Da-Wei Wu, Pei-Yu Wu, and Jiun-Chi Huang. 2021. "Obesity-Related Indices Are Associated with Longitudinal Changes in Lung Function: A Large Taiwanese Population Follow-Up Study" Nutrients 13, no. 11: 4055. https://doi.org/10.3390/nu13114055
APA StyleHsu, Y.-E., Chen, S.-C., Geng, J.-H., Wu, D.-W., Wu, P.-Y., & Huang, J.-C. (2021). Obesity-Related Indices Are Associated with Longitudinal Changes in Lung Function: A Large Taiwanese Population Follow-Up Study. Nutrients, 13(11), 4055. https://doi.org/10.3390/nu13114055






