Abstract
Avocados are a nutrient-dense plant-food, but limited trial-derived evidence exists about the effects of avocado intake on family nutritional status. We investigated the impact of two levels of avocado allotment, plus a standard nutrition education intervention on the nutritional status of Hispanic/Latino families. Seventy-two families consisting of at least three members of ≥5 years of age and residing in the same home, free of severe chronic disease, not on specific diets, and self-identified of Hispanic heritage, were randomized to one of two levels of avocado allotment (low = 3/week/family or high = 14/week/family) for 6 months plus 12 bi-weekly nutrition education sessions. The primary outcomes included change in a family’s total energy and macro- and micronutrient intakes. Primary analysis was intention-to-treat with unpaired, two-sided t-tests to assess mean changes between groups at 6 months. At 6 months, the high avocado allotment group had a significant reduction in energy intake, carbohydrate, animal and vegetable protein, saturated and polyunsaturated fat, calcium, magnesium, sodium, potassium, iron, and vitamin D intakes (all p < 0.05). A high allotment of avocados significantly reduced self-reported energy intake by 29% kcal/family/day, compared to a 3% kcal/family/day reduction in families who received a low allotment. Culturally-appropriate plant-food interventions may alter the nutritional status of at-risk families.
Keywords:
avocado; Persea americana; promotora; plant-food; nutrition education; family intervention 1. Introduction
Adopting a healthy dietary pattern results in the consumption of nutrient-dense foods, while reducing the risk of chronic disease [1,2,3,4,5,6]. However, current dietary patterns of Americans are less than optimal. That is, the United States (US) population as a whole does not meet dietary guideline-recommended amounts of vegetables, fruits, and whole grains, and over-consumes refined grains, added sugars, and high-fat and sodium foods [7,8]. Compared to non-Hispanic Whites, Hispanics/Latinos have higher age-adjusted prevalence of obesity (44.8%) [9] while having lower intake of vitamin A and E, folate, magnesium, and potassium, as well as high intake levels of saturated fat and sodium [10]. Of particular relevance, the dietary quality of Hispanics/Latinos and other immigrants worsens as they become acculturated in the US and adopt a Western dietary pattern, which is higher in refined carbohydrates and animal-based fats [11]. This is particularly important since the population of Hispanics/Latinos, which consists of native- and foreign-born individuals immigrating from Latin America, the Caribbean, and Spain, are the second largest ethnic demographic in the US, comprising 18.1% of the population (5.8 million) [12].
Based on their nutrient profile, avocados could be a favorable component of a plant-based eating pattern, with half of a medium sized fruit providing up to 20% of the recommended daily fiber, 10% potassium, 5% magnesium, 15% folate, and 7.5 g of monounsaturated fatty acids (MUFA) [13]. However, there are gaps in our knowledge on the effects of avocado intake on nutritional status. In particular, addressing avocado integration into the dietary pattern of families of Hispanic/Latino heritage could help narrow the diet-related disparities for essential nutrients by highlighting the promotion of a traditionally consumed plant food. Although evidence supports a favorable effect of avocados on the cardiovascular risk profile in adults, with [14,15,16] and without [17,18] metabolic disease, it is important to establish the effects in ethnic populations, such as Hispanics/Latinos, who have different dietary patterns, and are, on average, at increased risk for metabolic diseases that predispose them to cardiovascular disease [19,20].
Avocados are a calorically-dense and contain saturated fat. As such, meticulous attention must be given to the delivery of nutrition education emphasizing how to appropriately incorporate avocados as part of a healthy dietary pattern, i.e., so that avocados do not excessively add to total caloric and/or saturated fat intake, or negatively influence snacking behavior (e.g., chips and guacamole), which could also increase sodium intake. Therefore, we conducted a clinical trial aimed to integrate avocados into the diet of families and measure the impact of this intervention on energy, macro- and micronutrient intakes. Our hypothesis was that the high avocado allotment would lead to an improved family-level nutritional status and improved cardiometabolic risk factors.
2. Materials and Methods
2.1. Study Design and Population
The present cluster, randomized controlled trial was conducted in San Diego County, California. The randomization unit was the family, which was assigned to one of two intervention groups: nutrition education with low avocado allotment (i.e., 3/family/week) or nutrition education with high avocado allotment (i.e., 14/family/week). They were followed for 6 months, with clinical visits at baseline, 3 and 6 months.
Inclusion criteria were: families with 3–8 family members ages > 5 years residing in the same home, willing to participate in the intervention, and self-identified of Hispanic/Latino heritage. Exclusion criteria were: families with members who had clinically severe chronic diseases requiring specific diets, avocado or latex allergy, current high consumers of avocados (i.e., >1 avocado/adult/day and >½ avocado/child/day), unwillingness to eat avocados, presence of pregnant females or females planning to become pregnant and/or intending to move within the next 6 months. Families with members < 5 years of age were included but young children were not counted towards the number of family members expected to participate in the intervention. Each family member was consented/assented into the study individually.
The study protocol and all study materials were approved by the Institutional Review Boards of the University of California San Diego and San Diego State University. This clinical trial was registered under clinicaltrials.gov study identifier NCT02903433.
2.2. Setting, Recruitment, Consent, and Randomization
Recruitment occurred between April 2017 and June 2018 (Figure 1). Electronic medical records at San Ysidro Health (SYH) services, a comprehensive health care system to over 90,000 registered patients in South and Central San Diego County, were queried to search for potential participants. Additional recruitment strategies included telephone calls, flyers, and in-person contacts during clinic health fairs.
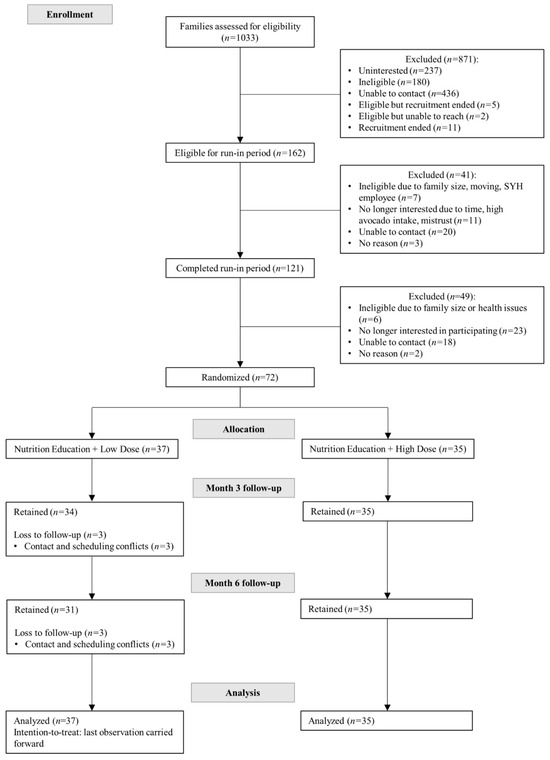
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow diagram of the Effects of Avocado Intake on the Nutritional Status of Families Trial.
A 14-day run in period assessed potential families’ commitment and adherence to study procedures, including participation in a home visit, willingness to schedule avocado delivery and nutrition education sessions, attendance to required clinic and laboratory visits, including all measurements and procedures. During this time, interested families were further screened for eligibility by a trained bilingual/bicultural female promotora during an in-home visit. Promotora is the Spanish term for “community health worker”, a trusted lay community member who receives training to provide elementary health education in the community without being a professional health care worker and who serves as a liaison between the community and traditional health care services. Specifically, families that completed a home visit, completed the questionnaires, and had their blood drawn, demonstrated a commitment and willingness to participate in the study and were scheduled for a baseline clinic visit where the head of the household (i.e., the family member who primarily shops for household groceries and prepares family meals) was identified.
Randomization of the family occurred at the baseline clinic visit using a computer-generated, blocked, randomization sequence. This was achieved with statistical analysis software (SAS) programming, using the RANUNI function procedure within the DATA statement, generating a random set of numbers for a specified range of observations (e.g., 1 to 75). Allocation concealment was accomplished by the randomization sequence only being accessed at the moment of randomization, and only one randomization assignment was visible at a time ensuring no advance notice of each assignment. Study arm assignment was implemented by the study coordinator. All staff members and study researchers, including principal investigators, were blinded to the randomization outcome. Promotoras and participants were unmasked for the intervention assignment due to the need to assist in the distribution of avocados and intervention delivery. During clinic visits, data collection was completed by study personnel who were not involved in delivery of the intervention.
2.3. Intervention
Families in both intervention groups received nutrition education and avocados over a 6-month period. The nutrition education was identical for both groups. Rather, it was standardized using 12 bi-weekly culturally and language appropriate nutrition education materials derived directly from resources provided by the United States Department of Agriculture MyPlate/MiPlato (http://www.choosemyplate.gov/; accessed on 11 March 2016) and aligned with the Dietary Guidelines for Americans (DGA) [7]. These standardized curriculum sessions were delivered by RDN-trained promotoras in participants’ homes with the goal of providing the participating families with tools and tips to improve diet quality and meet nutritional goals, yet not individually counseled on energy restriction or elimination of any foods.
The preparational work of the nutrition education included training of the promotoras by a Registered Dietitian Nutritionist (RDN) on the trial’s specific aims and intervention protocol. Each promotora received a training manual, which included the intervention protocol and materials for the session as well as a nutrition kit, which contained visual aids. The promotora was required to learn and be evaluated by the RDN on all the nutrition education lessons, following the stipulated language and delivery technique, before coming in contact with the participating families. The session design consisted of having at least the head of household of the family (for our purposes, the family member that was responsible for grocery shopping and meal preparation) join the promotora for a 20–30 min nutrition lesson where the promotora would go over the content of the assigned ‘lesson of the day’ pamphlet with the participant. Dialogue and questions were allowed during the session as the objective was for the participant to understand the content discussed. At the end of the session, the participant kept the pamphlet. Families did not receive compensation after each session since they collected compensation halfway through the study and after completion of study activities.
A total of 12 standard nutrition education lessons were specifically chosen to highlight particular MyPlate/MiPlato sections as ‘how to’ sessions including build a great plate, add more vegetables, add more fruit, make half of your grains whole grains, build a healthy meal and snacks for parents and children, choose good sources of protein, liven up meals with fruits and vegetables, make better beverage choices, fun and nutritious child-friendly meals, make good choices at school, work and on holidays. Families were also given a recipe booklet specifically focused on how to incorporate avocados in their diet. The recipe book illustrated a variety of dishes (entrees, sides, desserts) for the families to prepare to avoid monotony, and to encourage inclusion of avocados in new ways.
The dose of avocados in the low allocation group, 3/week, was based on the average reported intake in a survey of selected individuals in the target population (n = 101) and was conducted prior to starting the trial. The rationale for providing 3/week was to standardize the control arm and reduce potential variability that may have occurred if no standard had been provided, but to not increase or decrease families’ usual intake. Alternatively, the “dose” for the “high” intake allocation group (i.e., 14 avocados/week) was designed to allow for a robust increase in daily intake (by allowing for up to 2 avocados/family/day). This substantial increase therefore allowed for a potential increase in family energy intake up to 2625 kcal/week, if the avocados added to the current energy intake of the family. Incorporating a control group without avocado supplementation was considered, but determined to be impractical due to the typical intake by the target population (see above).
Promotoras delivered the allotted avocado amount per study arm to each participating family on a weekly basis throughout the duration of the intervention (6 months). They also provided an avocado care guide so the fruit would gradually mature throughout the week until the next delivery. Participating families were encouraged to not purchase additional avocados.
Study retention strategies involved regular contacts (telephone, email, correspondence, in-person) by study staff and monetary incentives ($100 to each family) provided midway and at the end of the study.
2.4. Measurements
The clinic visits at baseline, 3 and 6 months consisted of a dietary assessment, a participant survey packet, measurements of blood pressure and anthropometrics, measurement of physical activity, plus coordination of a blood draw at a local Laboratory Corporation of America Holdings (LabCorp) site. The participant survey packet asked about family socio-demographic characteristics, dietary and lifestyle factors and behaviors, and avocado consumption behavior at the family level and was only requested to be completed by head of household. Physical activity was measured with the global physical activity questionnaire (GPAQ) [21]. Blood pressure was measured using an automated Omron device three times at least one minute apart after the participant had been sitting for at least 5 min. Anthropometric measurements included weight, height, and waist circumference; height and weight were measured by a calibrated balance beam scale and stadiometer, respectively. Waist and hip circumference were measured with a semi-flexible tape measure, 2 cm above the iliac crest for waist and at the level of the widest circumference over the greater trochanters [22]. Participants visited a local LabCorp laboratory for the blood draw, scheduled within 5 days before or after the clinic visit, for measurement of the following: total lipid profile, fasting glucose, insulin, glycosylated hemoglobin (hemoglobin A1c), red blood cell magnesium, C-reactive protein, and plasma free fatty acids.
Dietary intake was assessed using a validated [23], self-administered, web-based, VioScreen (VioCare, Inc., Princeton, NJ, USA) food frequency questionnaire (FFQ) [24]. This assessment tool has been validated in adults, and a recent pilot in the pediatric population highlighted necessary modifications to tailor the questionnaire in children and adolescents [25]. Viocare Inc worked with the investigative team and incorporated study population-specific consumption foods, as well as avocado (individual level) as intervention food. The web-based FFQ worked as a standard FFQ (paper-based) asking about the amount and frequency of a food item. However, it incorporated additional questions in an interviewer–interviewee format, as well as displaying visual aids of appropriate serving sizes to assist participants’ estimates. Research associates instructed all participants on FFQ usage and supported those who required or desired assistance with the FFQ.
The Avocado Daily Diary was developed for this study, completed by the head of household and returned to the promotora during the bi-weekly home visits or weekly avocado delivery encounters. This instrument was designed by our research team to specifically capture avocado daily consumption of the family (not individually) and determine intervention adherence. A copy of the Avocado Daily Diary has been provided in the Supplemental Material file (Table S1).
The head of household completed all assessments. Non-head of household adult family members completed all assessments except for the participant survey packet. Children and adolescents only completed the GPAQ, dietary assessment, and measurements of blood pressure and anthropometrics.
2.5. Outcomes
The primary outcomes were total energy intake and nutritional status assessed at the family level. Measures included total intake of energy in kilocalories (kcal), macronutrients (carbohydrate, protein, fat, and dietary fiber) and micronutrients (vitamins C, D, E, folate, calcium, magnesium, sodium, potassium, and iron) plus the 2015 Healthy Eating Index (HEI-2015), calculated using the simple HEI scoring algorithm method [26]. We also assessed food group consumption patterns by examining intake of specific food groups (fruits; vegetables; dairy and non-dairy; nuts, animal and vegetable protein sources including red and processed meats; whole and refined grains; sugar; and oils). These measures were derived from the VioScreen FFQ. The values for total intake of energy and each nutrient were summed together per family, as this was the unit of analysis.
Secondary outcomes were cardiometabolic risk indicators assessed in adult participants and included: calculated body mass index (BMI) in adults, waist-to-height ratio in children and adolescents, waist circumference, blood pressure, lipids (total cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL), very low-density lipoproteins (VLDL), and triglycerides), glucose, insulin, hemoglobin A1c, calculated homeostatic model assessment of insulin resistance (HOMA-IR), and c-reactive protein. Several nutritional biomarkers, including plasma free fatty acids and red blood cell (RBC) magnesium, were also measured. Secondary outcomes in adolescents and children included calculated BMI, waist circumference, and blood pressure.
2.6. Intervention Adherence
Intervention adherence was determined using a customized Avocado Daily Diary designed to collect data on daily intake by all family members. Family weekly avocado consumption was calculated based on the number of avocados delivered, consumed, and remaining unconsumed by each family. A continuous adherence value was calculated based on amount consumed by family divided by intervention group intake goal.
2.7. Statistical Analyses
The sample size and power were based on our primary outcome of total intake of energy in kcal/family/day. A sample size of 60 families provided 80% power to detect a 375-kcal difference (equivalent to 1.5 medium avocados/family/day) at an alpha of 0.05 and a standard deviation (SD) of up to 500 kcal/family/day. To allow for up to 15% attrition and a final evaluable sample of 60 families, we aimed to randomize 70 total families.
Descriptive statistics were used to characterize the study population by intervention group. Continuous variables were expressed as mean and SD, while categorical variables were expressed as frequencies and percentages. Normality was evaluated for all continuous variables. For all outcomes, the primary analyses were conducted with an intention-to-treat approach, without covariate adjustment or intervention adherence. The Chi square test was used for comparisons of proportions derived from categorical variables, and 2-sided t-tests were used to assess mean differences in continuous variables between intervention groups. Changes from baseline to month 3 and from baseline to month 6 for the primary secondary outcomes were determined, and mean differences between intervention groups were assessed with 2-sided t-tests. Mean difference and standard deviation (SD or 95% confidence intervals (CI) are presented, where appropriate. Missing outcome data were imputed using the last observation carried forward method. For analyses of macro- and micronutrient intakes, we further adjusted for baseline total energy intake in separate analysis of covariance (ANCOVA) models.
As a sensitivity analysis, we performed per protocol adherence analysis, which considered intervention adherence and was applied to both primary and secondary outcomes on participants who completed the study. Family intervention adherence was estimated using the Avocado Daily Diary and calculated based on the number of avocados delivered, consumed, and remaining unconsumed by each family. A continuous adherence value was calculated based on amount consumed by family divided by intervention group intake goal. Complete adherence was defined as complete consumption of avocado allotment per week per family. ANCOVA, with intervention adherence as a covariate, was then used to compare total energy and macro- and micronutrient intakes by intervention groups.
In addition to intervention adherence adjustment, we further adjusted for baseline total energy intake as in the primary analysis. Age-specific subgroup analyses were also performed.
Secondary analyses included energy-adjusted macro- and micronutrients before determining mean difference between baseline and month 3 and baseline and month 6. The energy-adjusted methodology used included nutrient densities expressed as a proportion of energy (i.e., % kcal from fat) for macronutrients carbohydrate, protein, and fat. For micronutrients, food groups, as well as macronutrients, nutrient density was determined as intake (in appropriate units)/1000 kcal.
All p values presented are from 2-tailed analyses; p values of less than 0.05 were considered statistically significant. Analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
3. Results
Between 11 April 2017 and 27 June 2018, a total of 72 families (n = 37 in the low avocado allotment group and n = 35 in the high avocado allotment group) were randomized into this study, resulting in the participation of 231 individuals (Figure 1). Sixty-six families (91.7%) completed the study, with a dropout rate of 16.2% in the low avocado allotment group and 0% in the high avocado allotment group (p = 0.03). The main reasons for dropout were time consuming trial activities, scheduling conflicts, and difficulty contacting families. There were no statistically significant differences in socio-demographic characteristics with those families who remained in the study (Supplemental Table S2).
The average family size was three (SD 0.5; range 3–5 members), and almost half of the enrolled families reported a family annual income < $30,000 (Table 1). All but one head of the household was female (99%). Twenty-five percent of household members were children (mean age 9.3 (SD 2.1); range 5–12 years), 14% were adolescents (mean age 15.8 (SD 1.2); range 13–17 years), and 30% were non-head of household adults. Eighty-three percent of heads of households were born in Mexico and, on average, had lived in the US for 17.3 (SD 12.6) years. The majority were married or cohabitating, homemakers, and their highest educational attainment was an associate’s degree. Study heads of households had a mean age of 45.5 (SD 9.9) and other adults had a mean age of 41.4 years.

Table 1.
Baseline demographic and dietary characteristics of randomized families participating in the Effects of Avocado Intake on the Nutritional Status of Families Trial.
Study heads of households had a mean age of 45.5 (SD 9.9) and other adults had a mean age of 41.4 (SD 19.1; range 18–88 years) (Table 1). Study adults had a BMI of 30.4 (SD 6.4) kg/m2, and blood pressure within normal range (systolic, 118.3 (SD 17.6) mmHg and diastolic, 71.9 (SD 10.3) mmHg) (Table 2). Adolescents and children had a mean waist-height ratio of 0.5 cm (SD 0.1).

Table 2.
Baseline anthropometric and clinical characteristics of randomized families participating in the Effects of Avocado Intake on the Nutritional Status of Families Trial.
At baseline, carbohydrates constituted about half of the families’ daily total kcal, followed by 33% from fat, and 16% from protein (Table 2). This macronutrient distribution was consistent among all subgroups (Supplemental Table S3) and until the end of the trial (Supplemental Table S4). More specifically, in the low vs. high avocado allotment groups, the mean 6-month macronutrient distributions were 49% vs. 49% for carbohydrates, 35% vs. 36% for fat, and 16% vs. 17% for protein, respectively.
A reduction in self-reported family total energy was observed in both intervention groups at 3 and 6 months, with a greater reduction among high avocado allotment families at 6 months. The mean (95% CI) 6-month change in the family’s total energy intake was −259.0 (95% CI −958.0, 440.0) kcal/day for the low avocado allotment group and −2143.1 (95% CI −3286.5, −999.8) kcal/day for the high avocado allotment group (Table 3). This between-group mean difference was significantly different (p = 0.01).

Table 3.
Changes in family nutritional status per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial (n = 72).
Multiple between-group mean family differences were observed at 6-months, including carbohydrate, protein, and fat intakes (p ≤ 0.04 for all) (Table 3). Mean intake of animal and vegetable proteins, as well as MUFA and polyunsaturated fatty acids (PUFA), and saturated fat intakes were reduced in the high avocado allotment families. The mean 6-month differences between intervention groups for these nutrients, with the exception of MUFA intake, were significantly different (p ≤ 0.01 for all). Significant reductions in several micronutrients were also observed in the high avocado allotment families, including vitamin D, calcium, magnesium, potassium, sodium, and iron (p < 0.05 for all). The mean between-group difference for overall family-level HEI-2015 score at 6 months was −7.2 (95% CI −16.6, 2.1), with higher scores favoring the high avocado allotment group, and while suggestive, the difference did not reach statistical significance (p = 0.13). Age-specific subgroup intention-to-treat analysis results are reported in Supplemental Tables S5–S8. The majority of these findings persisted when adjusting macro- and micronutrients for baseline total energy intake.
Regarding differences in particular food groups, there were significant between-group mean differences for dairy, refined grains, chicken and eggs, and red meat food groups at 6 months (p ≤ 0.02 for all) (Table 4). Intake of these food groups were significantly lower in the high avocado allotment families. At 6 months in the high avocado families, heads of households significantly increased their intake of fruit (p = 0.02) and significantly reduced their intake of dairy (p = 0.04) and non-head of household trial adults significantly reduced their consumption of refined grains, chicken and eggs, fish, red meat, and oils (p ≤ 0.02 for all). Adolescents and children in the high avocado allotment families significantly reduced their whole grain intake (p = 0.05) and refined grains (p = 0.03), respectively (Supplemental Tables S9–S12). These results persisted after accounting for baseline total energy intake.

Table 4.
Changes in family food group composition per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial (n = 72).
Secondary analysis findings are shown in Supplemental Tables S4 and S13. The mean energy-adjusted 6-month difference following ITT analysis in the proportion of fat between intervention groups was significantly different (p = 0.05) (Supplemental Table S4). Additionally, families randomized to the high avocado allotment group significantly increased their mean energy-adjusted intakes of dietary fiber, MUFA, potassium, vitamin E, and folate, in comparison to those families randomized to the low avocado allotment group following ITT. These findings remained after following per protocol adherence analysis (data not shown). Mean energy-adjusted 6-month differences in cup equivalents of fruit and vegetables between intervention groups was significantly different (p ≤ 0.01) (Supplemental Table S13).
Avocado consumption behavior at baseline and 6 months is presented in Figure 2. There were no significant differences between study groups at baseline and after 6 months (p > 0.05 for all). Suggesting acceptability of the intervention, participating families reported they could afford to buy avocados, readily add them to their diet, and include them as part of family gatherings.
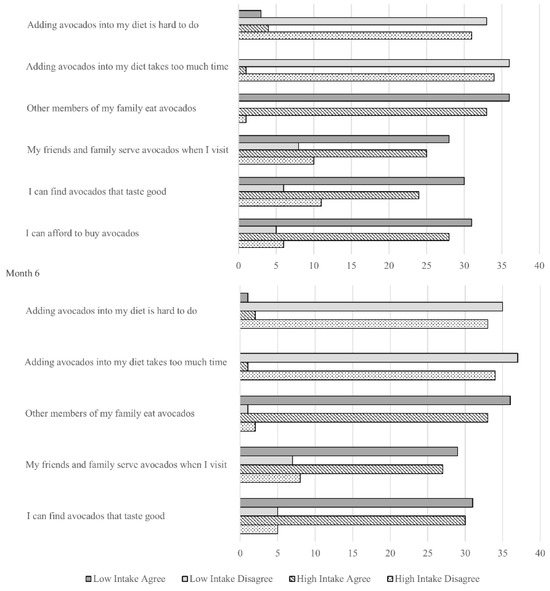
Figure 2.
Avocado consumption behavior at baseline and 6 months in the Effects of Avocado Intake on the Nutritional Status of Families Trial. The question: “I can afford to buy avocados” was not applicable at 6 months since the study team supplied families with avocados as part of the intervention. All between-group differences p-values > 0.05.
At 6 months, BMI remained unchanged in both study groups, while waist circumference in both study groups increased modestly (Table 5). There were few significant changes in blood pressure except that systolic blood pressure increased slightly in adults in the high avocado allotment group. Specifically, the mean 6-month between-group difference was 5.9 (95% CI 1.9, 9.8) mmHg (p = 0.004), which was mostly due to the decrease seen in the low avocado allotment group (mean −5.5 (95% CI −8.5, −2.6) mmHg).

Table 5.
Changes in family members’ body mass index, waist circumference, and blood pressure, per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial (n = 72 families).
Lipids and glycemia biomarkers were not significantly different in the high avocado allotment group at 6 months (Table 6). Free fatty acids and RBC magnesium marginally increased in the high allotment adult group after 6 months, with a significant mean between-group difference in RBC magnesium of 0.36 (95% CI 0.09, 0.62) mg/dL (p = 0.010) for those tested (n = 33).

Table 6.
Changes in free fatty acids, magnesium, lipids, glycemia markers, and c-reactive protein in adults, per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial (n = 113).
Greater than 80% adherence was met by 95% and 83% of low and high avocado allotment families at month 6, respectively. A total of 61 families (85%) had ≥80% continuous adherence to the intervention protocol throughout the duration of the study, corresponding to 92% and 77% for low and high avocado allotment families, respectively. Per protocol analyses suggested larger differential changes in family total energy and macronutrient intakes at 6 months, with these differences being statistically significant (p < 0.05 for all; Table 7). With the exception of sodium, mean differences in micronutrient intake at 6 months (calcium, potassium, magnesium, iron, sodium, and vitamin D) remained statistically significant after following per protocol adherence analyses. Mean differences in family food group intakes at 6 months persisted following per protocol adherence analyses (Table 8). The per protocol adherence analyses of secondary outcomes provided similar results to the intention-to-treat analyses, with no additional findings (data not shown). Age-specific per protocol adherence analyses nutrient and food group results were also conducted. The findings were similar to the intention-to-treat analyses (data not shown). Finally, there were no material differences in these results after adjustment for baseline total energy intake. There were no harms or unintended effects reported by study participants.

Table 7.
Changes in family nutritional status per protocol adherence analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial.

Table 8.
Changes in family food group composition, per protocol adherence analysis, in the Effects of Avocado Intake on the Nutritional Status of Families Trial.
4. Discussion
To our knowledge, this is the first randomized controlled trial to test the effect of a single plant-food intervention on family energy intake, without dietary eliminations or restrictions or counseling on energy intake. We found that a high allotment of avocados (14/family/week) significantly reduced self-reported energy intake by 29% kcal/family/day, compared to a 3% kcal/family/day reduction in families who received a low (3 avocados/family/week) allotment. The findings have potentially important implications for Hispanics/Latinos who have a high burden of obesity, placing them at higher risk for metabolic and cardiovascular diseases [20,27]. Notably, although the lack of change in BMI or waist circumference in either group reduces our confidence in the self-reported values for daily energy, and while the higher allotment of avocados may have modified the intake of specific micronutrient and foods, there is no evidence the resulting changes adversely affected energy intake or body weight.
Interestingly, although we did not observe significant increases in either MUFA or dietary fiber intakes in analyses unadjusted for changes in energy intake, energy-adjusted secondary analyses demonstrated significant changes in MUFA and fiber (plus potassium and folate), as would be hypothesized based on the nutrient profile of avocados. It has been previously demonstrated that fat and dietary fiber induce a ceiling effect on satiety [28,29]. Fats and some dietary fibers appear to impact total energy intake by affecting gastrointestinal functions, including slowing gastric emptying by adding bulk and viscosity, regulating glucose and insulin reactions, prolonging nutrient absorption, and modifying appetite–satiety gastrointestinal peptide hormones [29,30,31]. Specifically, one medium size Haas avocado (~136 g without skin and seed) contains 21 g of fat (13 g oleic acid—MUFA) and 9.2 g dietary fiber, and has both a medium energy density of 1.7 kcal/g and a viscose water, dietary fiber, and fruit oil matrix that appears to enhance satiety [14,28]. In this regard, and among overweight and moderately obese adults, the addition of half an avocado to a lunch meal increased satiety for over 5 h, and inhibited desire to continue eating, compared to a meal without avocado [28]. Similarly, the work of Zhu et al., among overweight and obese adults, found the replacement of carbohydrate in a high-carbohydrate meal with fat- and fiber-rich whole avocado without increasing energy suppressed hunger and improved satiety, while increasing satisfaction (satiety) feelings for over 6 h, compared to a control meal (low-fat, high-carbohydrate) [32]. Interestingly, Zhu et al. reported that satiety induced by whole avocado replacement was mostly due to impact on responses to gastrointestinal peptide YY hormone [32].
Given the reported reductions in total energy intake at the family and individual level, the reported decreases in macro- and micronutrients were expected. However, high avocado intake families consumed significantly less protein of animal origin (31.9% reduction, equivalent to a decrease of ~8 ounces, per family) primarily from chicken and eggs, and red and processed meats, which are usually higher in fat and saturated fat (31.6% reduction per family). These two nutrients tend to be high in Western dietary patterns and current guidelines advise reduced intake of these sources of macronutrients [7]. Although reduced, the macronutrient distribution was consistent at the three time points, at both family and individual levels, and between groups (49% carbohydrate, 35% fat, 17% protein), and similar to those observed in Mexican-heritage adults in the Hispanic Community Health Study/Study of Latinos, the largest community-based cohort of Hispanics/Latino in the US (50% carbohydrate, 32% fat, 18% protein) [33].
Correspondingly, and in general, the families who had a high avocado allocation reported decreased calcium, magnesium, potassium, iron, sodium, and vitamin D consumption. Because avocados contain potassium and magnesium [14], we did not anticipate this change. This effect could be associated with overall reduced caloric intake, leading to general reductions in nutrients. Nevertheless, the findings are contrary to results observed in a cross-sectional analysis in the National Health And Nutrition Examination Survey (NHANES), where, compared to non-consumers, avocado consumers (average intake half avocado/day) had significantly higher intakes of MUFA, dietary fiber, magnesium, and potassium (p < 0.0001), and no significant differences in total energy intake and sodium [34].
At baseline, participating families were, on average, consuming almost two times more sodium than recommended by the DGA [7]. We observed a significant between-group difference of −2664.3 mg sodium/family/day (p = 0.05) at 6 months, with a greater reduction in high avocado intake families. High avocado intake families reduced their sodium intake by 24.7%, equivalent to ~0.6 teaspoons as a family.
We acknowledge the apparent incongruity regarding the absence of change in both BMI and waist circumference in the context of significant reductions in self-reported total energy intake over 6 months, a finding that is seemingly implausible. These results are in contrast to existing cross-sectional and longitudinal evidence on frequent consumption of avocado being associated with significantly lower BMI, body weight, and waist circumference compared to non-consumption [34,35]. Several explanations for the discrepancy must be considered, including possible measurement errors by study personnel, underreporting of dietary intake, failure to consider specific ethnic foods or beverages unaccounted for by the FFQ, and/or the inexactitude of the dietary intake tool. Notably, if the discrepancy was due to underreporting of energy generally, or of ethnicity-specific foods, our results suggest underreporting differed by intervention group, which is improbable. Regardless, our laboratory and anthropometric results suggest the provision of a higher allotment of avocados did not result in adverse changes in cardiometabolic risk factors. Several 24-h food recalls were considered in addition to the FFQs, and we acknowledge these may have provided better estimates of energy intake. However, the use of 24-h food recalls was not feasible since the majority of the study families did not have internet connection to be able to complete them at home and we did not have the necessary staff to go to their homes and collect that data. Additionally, families had complicated daily schedules that did not allow them to come into the clinic to complete these food recalls.
Although between-group differences in lipids did not reach statistical significance, there was a greater reduction in total cholesterol, VLDL lipoprotein cholesterol, and triglycerides in the high avocado allocation group, whereas there were greater mean reductions in LDL cholesterol in the low avocado allocation group. These findings contrast a recent meta-analysis by Peou et al., that examined the effect of diets enriched with avocado on plasma lipoproteins and found that avocado-enriched diets where dietary fats are substituted (vs. added) to free diets, significantly decreased total cholesterol, LDL, and triglyceride levels [36]. A possible explanation for these findings may be the design of the intervention; i.e., we supplemented existing diets and emphasized healthy dietary guidelines, without enforcing replacement of particular nutrients.
Our study had several strengths, including being a relatively long-term, large, cluster randomized controlled trial of families. The cultural appropriateness of the intervention design and engagement of experienced bicultural and bilingual educators, promotoras, in the home, are additional strengths. On the other hand, there are limitations. First, the use of self-reported data to evaluate dietary and nutrient intake changes could be a source of information bias. Second, we acknowledge incorporating an additional dietary data collection method would have strengthened the results, yet this was not feasible in our participants, as discussed above. Third, and related, social desirability bias, recall bias, and other potential reporting biases may have affected the findings. However, it seems reasonable to presume that these biases, if they existed, would be non-differential between groups because both intervention groups completed identical recall assessments and received identical advice on a healthful diet and adherence to MyPlate and DGA. Furthermore, while underreporting bias could be contributing to the inconsistency between the energy intake and anthropometric findings, some energy reduction still occurred since the study groups demonstrated separation by avocado intake level at both 3 and 6 months, with a greater effect between 3 and 6 months. Although we cannot rule out possible non-random error or be confident the FFQ provided an accurate assessment, an allotment of a higher number of avocados did not result in adverse changes in the different cardiometabolic outcome measures and risk factors. Fourth, we recognize our findings are limited to families of Hispanic/Latino heritage. Fifth, attrition was only observed in the low avocado group (16.2% dropout rate), which may have impacted statistical power in the primary analysis. In analyses where we removed those lost to follow-up and where we adjusted for intervention adherence, the primary results persisted.
5. Conclusions
This trial demonstrated that a differential allotment of avocados may impact overall self-reported caloric consumption, as well as macro- and micronutrient nutrient intake, including saturated fat and sodium, and food groups, including dairy, refined grains, and red and processed meats. Our trial results may help provide a strategy to support existing public health efforts to reduce saturated fat and sodium, which are commonly consumed in excess. It also demonstrates a high degree of adherence to the incorporation of a single, nutrient-dense plant food. These observations should be interpreted with caution since there were no statistically significant between-group differences in BMI, waist circumference, or cardiometabolic biomarkers. However, when combined with nutrition education, a higher level of avocados may be incorporated into a healthful diet for families who are of Hispanic/Latino heritage, seemingly without adverse cardiometabolic effects. Testing of a culturally appropriate plant-food on energy intake, by bicultural and bilingual community health workers, should be extended to other populations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13114021/s1, Supplemental Table S1. Avocado Daily Diary used as an intervention adherence tool in the Effects of Avocado Intake on the Nutritional Status of Families Trial by subgroup; Supplemental Table S2. Socio-demographic characteristics in families that completed and dropped out from the Effects of Avocado Intake on the Nutritional Status of Families Trial study; Supplemental Table S3. Baseline dietary intake of randomized families in the Effects of Avocado Intake on the Nutritional Status of Families Trial by subgroup; Supplemental Table S4. Changes in family energy-adjusted nutrients and food groups intake per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S5. Changes in the nutritional status of heads of households, per intention-to-treat analysis, in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S6. Changes in the nutritional status of non-head of household adults, per intention-to-treat analysis, in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S7. Changes in the nutritional status of adolescents, per intention-to-treat analysis, in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S8. Changes in the nutritional status of children, per intention-to-treat analysis, in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S9. Changes in the food group composition of heads of households, per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S10. Changes in food group composition of other adults, per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S11. Changes in food group composition of adolescents, per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S12. Changes in food group composition of children, per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial; Supplemental Table S13. Changes in family energy-adjusted food group composition per intention-to-treat analysis in the Effects of Avocado Intake on the Nutritional Status of Families Trial.
Author Contributions
Conceptualization, R.D.B., C.A.M.A. and M.A.A.; methodology, L.S.P., R.D.B., C.A.M.A. and M.A.A.; software, L.S.P. and J.O.D.; validation, L.S.P., R.D.B. and J.O.D.; formal analysis, L.S.P.; investigation, L.S.P., R.D.B., C.A.M.A. and M.A.A.; resources, M.A.A.; data curation, L.S.P. and J.O.D.; writing—original draft preparation, L.S.P. and R.D.B.; writing—L.S.P., R.D.B., C.A.M.A., J.O.D. and M.A.A.; visualization, L.S.P.; supervision, L.S.P. and M.A.A.; project administration, L.S.P., J.O.D. and M.A.A.; funding acquisition, M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hass Avocado Board. Additionally, this work was supported by the National Heart, Lung, and Blood Institute (T32 HL079891-11, PI: Matthew Allison); the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK007703-26, PI: Frank Hu and Christopher Duggan); and the Harvard Chan Yerby Fellowship at Harvard T.H. Chan School of Public Health. The trial was registered at Clinical Trial Registry website: http://www.clinicaltrials.gov (accessed on 10 November 2021); under study number: NCT02903433.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of California San Diego and San Diego State (protocol #160584, approved 05/05/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent or verbal assent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval. Proposals should be directed to mallison@health.ucsd.edu.
Acknowledgments
The authors would like to thank the participants and staff of the Effects of Avocado Intake on the Nutritional Status of Families trial, for their participation, commitment and valuable contributions.
Conflicts of Interest
The authors declare no potential conflict of interest. All authors report the grant from the Hass Avocado Board, and L.S. Pacheco reports the grants from the National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and Harvard Chan Yerby Fellowship at Harvard T.H. Chan School of Public Health, during the conduct of the study and/or manuscript development. The Hass Avocado Board funded the trial and provided all the trial’s avocados at no cost to study participants. funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Correction Statement
This article has been republished with a minor correction to resolve spelling errors. This change does not affect the scientific content of the article.
References
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the incidence of type 2 diabetes with the mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- The DASH Diet. Dietary Approaches to Stop Hypertension. Lippincotts Prim. Care Pract. 1998, 2, 536–538. [Google Scholar]
- Hinderliter, A.L.; Babyak, M.A.; Sherwood, A.; Blumenthal, J.A. The DASH diet and insulin sensitivity. Curr. Hypertens. Rep. 2011, 13, 67–73. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L.; Li, D. Cardiovascular Disease Mortality and Cancer Incidence in Vegetarians: A Meta-Analysis and Systematic Review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 7 February 2019).
- U.S. Department of Agriculture and Agricultural Research Service. Average Intakes by Age-Sex Group, Healthy U.S.-Style Food Patterns, Which Vary Ased on Age, Sex, and Activity Level, for Recommended Intakes and Limits. What We Eat in America, NHANES 2007–2010. 2010. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/0910/Table_1_NIN_GEN_09.pdf (accessed on 21 April 2021).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016 Key Findings Data; National Health and Nutrition Examination Survey: Hyattsville, MD, USA, 2017. Available online: https://www.cdc.gov/nchs/data/databriefs/db288_table.pdf#1 (accessed on 22 January 2020).
- U.S. Department of Agriculture ARS. Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Race/Ethnicity and Age, What We Eat in America, NHANES 2017–2018. 2020. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/Table_2_NIN_RAC_17.pdf (accessed on 20 April 2021).
- Dixon, L.B. Differences in Energy, Nutrient, and Food Intakes in a US Sample of Mexican-American Women and Men: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2000, 152, 548–557. [Google Scholar] [CrossRef]
- U.S. Census Bureau. Profile America Facts for Features: 2018 Hispanic Heritage Month; Washington, DC, USA, 2018. Available online: https://www.census.gov/content/dam/Census/newsroom/facts-for-features/2018/hispanic-heritage-fff.pdf (accessed on 21 April 2021).
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Carranza-Madrigal, J.; Herrera-Abarca, J.E.; Alvizouri-Munoz, M.; Alvarado-Jimenez, M.R.; Chavez-Carbajal, F. Effects of a vegetarian diet vs. a vegetarian diet enriched with avocado in hypercholesterolemic patients. Arch. Med. Res. 1997, 28, 537–541. [Google Scholar]
- Lerman-Garber, I.; Ichazo-Cerro, S.; Zamora-González, J.; Cardoso-Saldaña, G.; Posadas-Romero, C. Effect of a high-monounsaturated fat diet enriched with avocado in NIDDM patients. Diabetes Care 1994, 17, 311–315. [Google Scholar] [CrossRef]
- Colquhoun, D.M.; Moores, D.; Somerset, S.M.; Humphries, J.A. Comparison of the effects on lipoproteins and apolipoproteins of a diet high in monounsaturated fatty acids, enriched with avocado, and a high-carbohydrate diet. Am. J. Clin. Nutr. 1992, 56, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Alvizouri-Munoz, M.; Carranza-Madrigal, J.; Herrera-Abarca, J.E.; Chavez-Carbajal, F.; Amezcua-Gastelum, J.L. Effects of avocado as a source of monounsaturated fatty acids on plasma lipid levels. Arch. Med. Res. 1992, 23, 163–167. [Google Scholar] [PubMed]
- Wang, L.; Bordi, P.L.; Fleming, J.A.; Hill, A.M.; Kris-Etherton, P.M. Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: A randomized, controlled trial. J. Am. Heart Assoc. 2015, 4, e001355. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital Signs: Leading Causes of Death, Prevalence of Diseases and Risk Factors, and Use of Health Services Among Hispanics in the United States—2009–2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 469–478. [Google Scholar]
- Rodriguez, C.J.; Allison, M.; Daviglus, M.L.; Isasi, C.R.; Keller, C.; Leira, E.C.; Palaniappan, L.; Piña, I.L.; Ramirez, S.M.; Rodriguez, B.; et al. Status of Cardiovascular Disease and Stroke in Hispanics/Latinos in the United States. A Science Advisory from the American Heart Association. Circulation 2014, 130, 593–625. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Physical Activity Questionnaire (GPAQ). Available online: http://www.who.int/chp/steps/GPAQ/en/index.html (accessed on 20 July 2019).
- WHO Expert Committee on Physical Status. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J. Acad. Nutr. Diet. 2014, 114, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Viocare, I. VioScreen—VIOCARE®. Available online: https://www.viocare.com/vioscreen.html (accessed on 20 July 2019).
- Deierlein, A.L.; Bihuniak, J.D.; Nagi, E.; Litvak, J.; Victoria, C.; Braune, T.; Weiss, R.; Parekh, N. Development of a technology-assisted food frequency questionnaire for elementary and middle school children: Findings from a pilot study. Nutrients 2019, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. HEI Scoring Algorithm. Available online: https://epi.grants.cancer.gov/hei/hei-scoring-method.html (accessed on 29 October 2020).
- Heiss, G.; Snyder, M.L.; Teng, Y.; Schneiderman, N.; Llabre, M.M.; Cowie, C.; Carnethon, M.; Kaplan, R.; Giachello, A.; Gallo, L.C.; et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care 2014, 37, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Haddad, E.; Oda, K.; Sabaté, J. A randomized 3×3 crossover study to evaluate the effect of Hass avocado intake on post-ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr. J. 2013, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B. Dietary Fiber and Energy Regulation. J. Nutr. 2000, 130, 272S–275S. [Google Scholar] [CrossRef]
- Gentilcore, D.; Chaikomin, R.; Jones, K.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Maljaars, J.; Romeyn, E.A.; Haddeman, E.; Peters, H.P.; Masclee, A.A. Effect of fat saturation on satiety, hormone release, and food intake. Am. J. Clin. Nutr. 2009, 89, 1019–1024. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Y.; Edirisinghe, I.; Park, E.; Burton-Freeman, B. Using the avocado to test the satiety effects of a fat-fiber combination in place of carbohydrate energy in a breakfast meal in overweight and obese men and women: A randomized clinical trial. Nutrients 2019, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Siega-Riz, A.M.; Sotres-Alvarez, D.; Ayala, G.X.; Ginsberg, M.; Himes, J.H.; Liu, K.; Loria, C.M.; Mossavar-Rahmani, Y.; Rock, C.L.; Rodriguez, B.; et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am. J. Clin. Nutr. 2014, 99, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni Iii, V.L.; Dreher, M.; Davenport, A.J. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: Results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutr. J. 2013, 12, 1. Available online: http://www.nutritionj.com/content/12/1/1 (accessed on 7 March 2017). [CrossRef] [PubMed]
- Heskey, C.; Oda, K.; Sabaté, J. Avocado intake, and longitudinal weight and body mass index changes in an adult cohort. Nutrients 2019, 11, 691. [Google Scholar] [CrossRef]
- Peou, S.; Milliard-Hasting, B.; Shah, S.A. Impact of avocado-enriched diets on plasma lipoproteins: A meta-analysis. J. Clin. Lipidol. 2016, 10, 161–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).