Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nannochloropsis oceanica Source
2.2. Rats and Diets
2.3. Rat Measurements
3. Results
3.1. Nannochloropsis oceanica
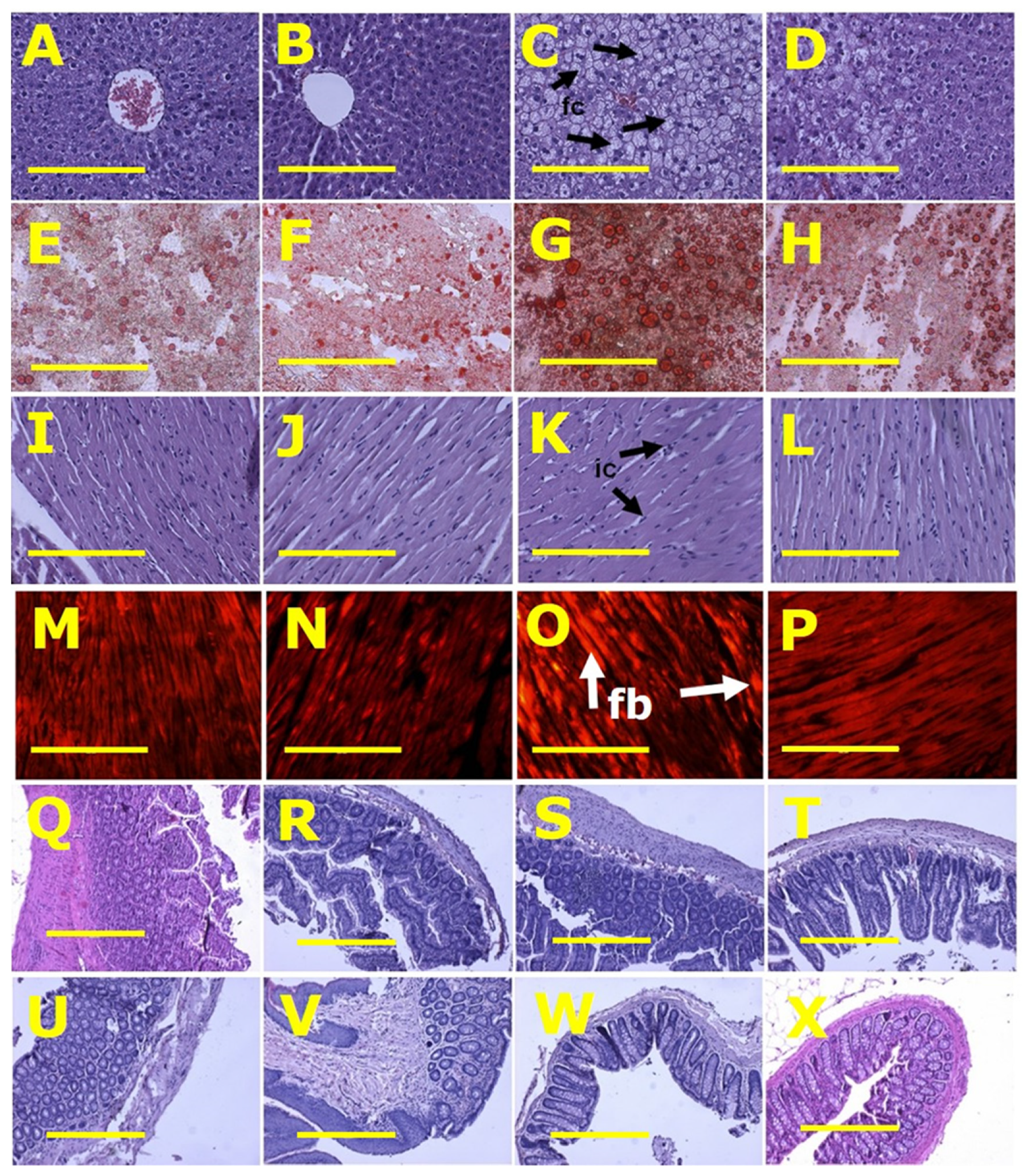
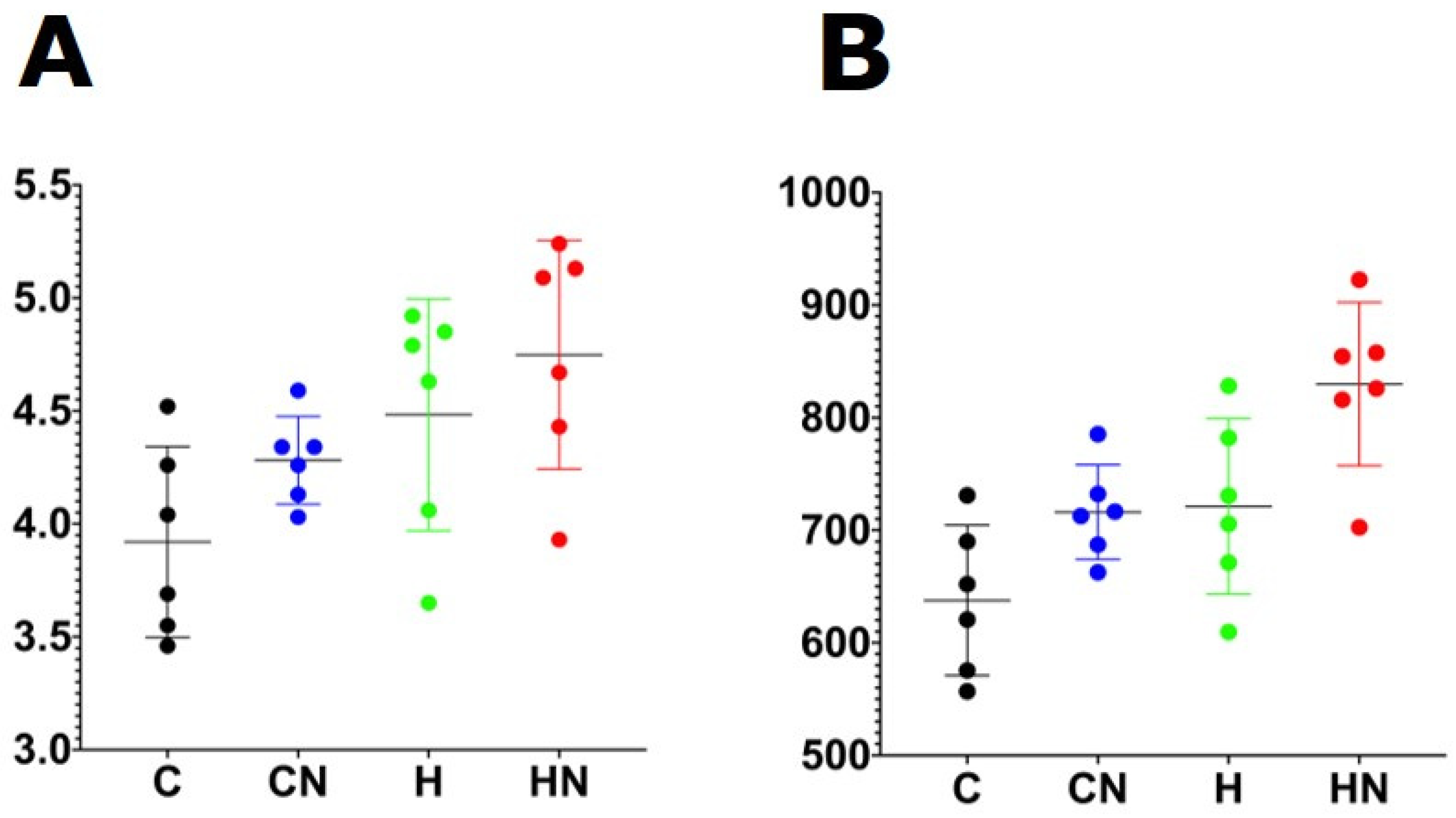
3.2. Physiological Variables
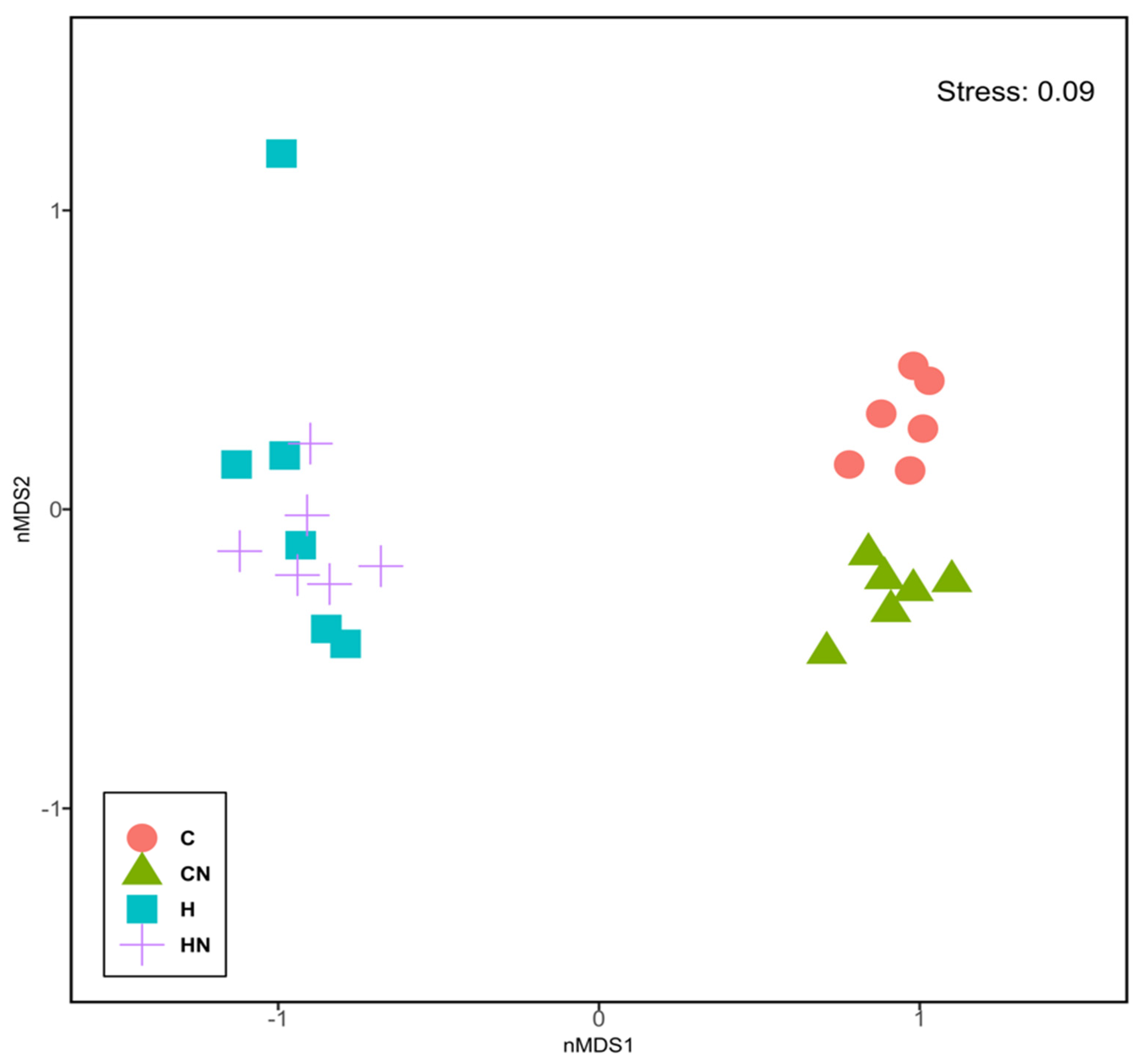
3.3. Gut Structure and Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randrianarison, G.; Ashraf, M.A. Microalgae: A potential plant for energy production. Geol. Ecol. Landsc. 2017, 1, 104–120. [Google Scholar] [CrossRef]
- Chua, E.T.; Schenk, P.M. A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [Green Version]
- Jan, M.; Kazik, P. Nannochloropsis: Biology, Biotechnological Potential and Challenges; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017. [Google Scholar]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass nutrient profiles of the microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef] [PubMed]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Australian Government Department of Health. The Biology of Nannochloropsis oceanica Suda & Miyashita (a Microalga). Available online: http://www.ogtr.gov.au/internet/ogtr/publishing.nsf/Content/5DCF28AD2F3779C4CA257D4E001819B9/$File/Biology%20of%20Nannochloropsis%20oceanica%20(a%20microalga).pdf (accessed on 26 May 2021).
- Brown, R.B.; Wass, T.J.; Thomas-Hall, S.R.; Schenk, P.M. Chromosome-scale genome assembly of two Australian Nannochloropsis oceanica isolates exhibiting superior lipid characteristics. Microbiol. Resour. Announc. 2019, 8, e01288-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Romero, S.; Torrella, J.R.; Pages, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Brown, L. Addressing the insufficient availability of EPA and DHA to meet current and future nutritional demands. Nutrients 2021, 13, 2855. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the anti-inflammatory and antioxidant effects of the microalgae Nannochloropsis gaditana in streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Nasirian, F.; Sarir, H.; Moradi-kor, N. Antihyperglycemic and antihyperlipidemic activities of Nannochloropsis oculata microalgae in streptozotocin-induced diabetic rats. Biomol. Concepts 2019, 10, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Ward, L.C.; Brown, L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1041–1052. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Lyu, M.; Wang, Y.F.; Fan, G.W.; Wang, X.Y.; Xu, S.Y.; Zhu, Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: Current evidence and perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Du Preez, R.; Magnusson, M.; Majzoub, M.E.; Thomas, T.; Praeger, C.; Glasson, C.R.K.; Panchal, S.K.; Brown, L. Brown seaweed Sargassum siliquosum as an intervention for diet-induced obesity in male Wistar rats. Nutrients 2021, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Caulerpa lentillifera (sea grapes) improves cardiovascular and metabolic health of rats with diet-induced metabolic syndrome. Metabolites 2020, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Duong, V.T.; Thomas-Hall, S.R.; Schenk, P.M. Growth and lipid accumulation of microalgae from fluctuating brackish and sea water locations in South East Queensland—Australia. Front. Plant Sci. 2015, 6, 359. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Alsenani, F.; Schenk, P.M. Chapter 1. Microalgae as a sustainable source of nutraceuticals. In Microbial Functional Foods and Nutraceuticals; Gupta, V.K., Treichel, H., Shapaval, V.O., de Oliveira, L.A., Tuohy, M.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–19. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sust. Energ. Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Al-Hoqani, U.; Young, R.; Purton, S. The biotechnological potential of Nannochloropsis. Perspect. Phycol. 2016, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Garcia-Segovia, P.; Pagan-Moreno, M.J.; Lara, I.F.; Martinez-Monzo, J. Effect of microalgae incorporation on physicochemical and textural properties in wheat bread formulation. Food Sci. Technol. Int. 2017, 23, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez De Marco, E.; Steffolani, M.E.; Martínez, M.; León, A.E. The use of Nannochloropsis sp. as a source of omega-3 fatty acids in dry pasta: Chemical, technological and sensory evaluation. J. Food Sci. Technol. 2018, 53, 499–507. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; El-Feky, A.M.; Ibrahim, N.E.-S.; Sahu, R.K.; El-Sayed, A.E.-K.B. Evaluation of the pancreatoprotective effect of Nannochloropsis oculata extract against streptozotocin-induced diabetes in rats. J. Appl. Pharm. Sci. 2018, 8, 46–58. [Google Scholar] [CrossRef]
- Kagan, M.L.; Matulka, R.A. Safety assessment of the microalgae Nannochloropsis oculata. Toxicol. Rep. 2015, 2, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin attenuates hypertensive vascular remodeling by protecting vascular smooth muscle cells from oxidative stress-induced mitochondrial dysfunction. Oxid. Med. Cell. Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, U.; Derwenskus, F.; Gille, A.; Louis, S.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients 2018, 10, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F. Fatty acids—Nomenclature, structure, isolation and structure determination, biosynthesis and chemical synthesis. In Fatty Acid and Lipid Chemistry; Springer: Boston, MA, USA, 1996; pp. 1–34. [Google Scholar] [CrossRef]
- Lopes, P.A.; Bandarra, N.M.; Martins, S.V.; Martinho, J.; Alfaia, C.M.; Madeira, M.S.; Cardoso, C.; Afonso, C.; Paulo, M.C.; Pinto, R.M.A.; et al. Markers of neuroprotection of combined EPA and DHA provided by fish oil are higher than those of EPA (Nannochloropsis) and DHA (Schizochytrium) from microalgae oils in Wistar rats. Nutr. Metab. 2017, 14, 62. [Google Scholar] [CrossRef] [Green Version]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic acid extraction from Nannochloropsis gaditana using carbon dioxide at supercritical conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Raposo, M.F.; De Morais, A.M.; De Morais, R.M. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Pleite, N.; Mendiola, J.A.; Cifuentes, A.; Herrero, M.; Gilbert-López, B.; Ibáñez, E. Development of green extraction processes for Nannochloropsis gaditana biomass valorization. Electrophoresis 2018, 39, 1875–1883. [Google Scholar] [CrossRef]
- Beacham, T.A.; Bradley, C.; White, D.A.; Bond, P.; Ali, S.T. Lipid productivity and cell wall ultrastructure of six strains of Nannochloropsis: Implications for biofuel production and downstream processing. Algal Res. 2014, 6, 64–69. [Google Scholar] [CrossRef]
- Zhang, R.; Parniakov, O.; Grimi, N.; Lebovka, N.; Marchal, L.; Vorobiev, E. Emerging techniques for cell disruption and extraction of valuable bio-molecules of microalgae Nannochloropsis sp. Bioprocess Biosyst. Eng. 2019, 42, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.; Regan, F. In-situ lipid and fatty acid extraction methods to recover viable products from Nannochloropsis sp. Sci. Total Environ. 2020, 748, 142464. [Google Scholar] [CrossRef] [PubMed]
- van Baak, M.A.; Mariman, E.C.M. Dietary strategies for weight loss maintenance. Nutrients 2019, 11, 1916. [Google Scholar] [CrossRef] [Green Version]
- Segovia-Siapco, G.; Khayef, G.; Pribis, P.; Oda, K.; Haddad, E.; Sabate, J. Animal protein intake is associated with general adiposity in adolescents: The teen food and development study. Nutrients 2019, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]


| Component | Amount |
|---|---|
| Macronutrients (g/100 g) | |
| Protein | 51.3 |
| Essential amino acids (g/100 g) | |
| Lysine | 3.5 |
| Methionine | 1.1 |
| Isoleucine | 2.1 |
| Leucine | 4.7 |
| Threonine | 2.6 |
| Tryptophan | 0.67 |
| Valine | 2.9 |
| Arginine | 2.9 |
| Histidine | 0.96 |
| Phenylalanine | 2.6 |
| Lipids, moisture, carbohydrates (g/100 g) | |
| Fat | 16.3 |
| Saturated | 3.1 |
| Monounsaturated | 4.9 |
| Polyunsaturated | 7.7 |
| EPA | 6 |
| Omega-6 | 1.7 |
| Trans | <0.01 |
| Moisture | 4 |
| Total sugar | <0.01 |
| Total fibre | 10 |
| Vitamins (mg/100 g) | |
| Vitamin A | 0.025 |
| Vitamin B1 (thiamine) | 7 |
| Vitamin B2 (riboflavin) | 6.2 |
| Vitamin B12 (cyanocobalamin) | 0.17 |
| Vitamin C (ascorbic acid) | 320 |
| Vitamin D | 0.045 |
| Vitamin E | 35 |
| Vitamin K | 0.017 |
| Carotenoids (mg/100 g) | |
| β-carotene | 23.6 |
| Lutein | 248 |
| Violaxanthin | 146 |
| Zeaxanthin | 110 |
| Variables | C | CN | H | HN | p Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| Physiological variables | |||||||
| 0-week body weight, g | 337 ± 1 | 336 ± 1 | 339 ± 1 | 337 ± 1 | 0.19 | 0.19 | 0.66 |
| 8-week body weight, g | 366 ± 7 b | 386 ± 2 b | 445 ± 10 a | 430 ± 5 a | <0.0001 | 0.66 | 0.004 |
| 16-week body weight, g | 388 ± 10 c | 453 ± 6 b | 547 ± 14 a | 528 ± 10 a | <0.0001 | 0.032 | 0.0003 |
| 16-week lean mass, g | 292 ± 15 b | 328 ± 7 a | 299 ± 13 b | 323 ± 6 a | 0.92 | 0.003 | 0.53 |
| 16-week fat mass, g | 75 ± 15 c | 91 ± 12 c | 230 ± 35 a | 172 ± 13 b | <0.0001 | 0.26 | 0.05 |
| 8-week lean/fat mass proportion | 6.2 ± 1.6 a | 5.8 ± 0.4 a | 2.1 ± 0.4 b | 2.8 ± 0.3 b | <0.0001 | 0.82 | 0.42 |
| 16-week lean/fat mass proportion | 3.8 ± 1.0 a | 4.3 ± 0.5 a | 1.5 ± 0.3 b | 2.0 ± 0.2 b | 0.0001 | 0.35 | 1.00 |
| 16-week bone mineral content, g | 11.6 ± 0.3 b | 12.3 ± 0.4 b | 16.6 ± 1.1 a | 15.0 ± 0.5 a | <0.0001 | 0.46 | 0.07 |
| 16-week bone mineral density, g/cm2 | 0.170 ± 0.003 | 0.166 ± 0.002 | 0.181 ± 0.004 | 0.176 ± 0.004 | 0.005 | 0.19 | 0.94 |
| Food intake 0–8 weeks, g/day | 43.2 ± 2.2 a | 40.4 ± 0.8 a | 26.6 ± 1.1 b | 26.0 ± 1.2 b | <0.0001 | 0.21 | 0.42 |
| Food intake 9–16 weeks, g/day | 44.0 ± 1.2 a | 34.9 ± 0.6 b | 23.9 ± 0.9 c | 22.0 ± 1.0 c | <0.0001 | <0.0001 | 0.0009 |
| Water intake 0–8 weeks, g/day | 31.8 ± 1.6 | 36.8 ± 2.5 | 32.4 ± 1.4 | 33.0 ± 1.5 | 0.48 | 0.22 | 0.33 |
| Water intake 9–16 weeks, g/day | 21.7 ± 1.4 c | 36.8 ± 2.4 a | 28.8 ± 1.3 b | 37.0 ± 1.6 a | 0.10 | <0.0001 | 0.12 |
| Energy intake 0–8 weeks, kJ/day | 485 ± 25 b | 454 ± 9 b | 607 ± 19 a | 590 ± 22 a | <0.0001 | 0.24 | 0.73 |
| Energy intake 9–16 weeks, kJ/day | 470 ± 13 b | 392 ± 7 c | 536 ± 15 a | 533 ± 22 a | <0.0001 | 0.0307 | 0.044 |
| 16-week abdominal circumference, cm | 18.7 ± 0.5 c | 21.5 ± 0.2 b | 23.8 ± 0.4 a | 23.4 ± 0.3 a | <0.0001 | 0.0013 | <0.0001 |
| Body mass index, g/cm2 | 0.61 ± 0.03 c | 0.72 ± 0.01 b | 0.81 ± 0.02 a | 0.77 ± 0.02 a | <0.0001 | 0.09 | 0.0008 |
| Retroperitoneal fat, mg/mm | 210 ± 20 c | 256 ± 17 c | 619 ± 69 a | 488 ± 31 b | <0.0001 | 0.24 | 0.018 |
| Epididymal fat, mg/mm | 89 ± 11 b | 90 ± 9 b | 199 ± 39 a | 182 ± 14 a | <0.0001 | 0.67 | 0.63 |
| Omental fat, mg/mm | 139 ± 14 b | 169 ± 16 b | 288 ± 56 a | 278 ± 19 a | <0.0001 | 0.71 | 0.46 |
| Total abdominal fat, mg/mm | 437 ± 42 c | 514 ± 37 c | 1107 ± 57 a | 948 ± 59 b | <0.0001 | 0.47 | 0.042 |
| Visceral adiposity, % | 5.2 ± 0.5 b | 5.2 ± 0.3 b | 9.3 ± 1.1 a | 8.4 ± 0.4 a | <0.0001 | 0.42 | 0.42 |
| Liver wet weight, mg/mm | 261 ± 11 b | 260 ± 6 b | 380 ± 12 a | 370 ± 12 a | <0.0001 | 0.62 | 0.69 |
| Cardiovascular variables | |||||||
| 8-week systolic blood pressure, mmHg | 125 ± 4 b | 126 ± 2 b | 137 ± 3 a | 132 ± 2 a | 0.0019 | 0.46 | 0.27 |
| 16-week systolic blood pressure, mmHg | 123 ± 2 b | 121 ± 2 b | 139 ± 2 a | 135 ± 3 a | <0.0001 | 0.21 | 0.67 |
| Left ventricle + septum, mg/mm | 22.9 ± 1.1 | 23.8 ± 0.7 | 25.2 ± 1.1 | 24.3 ± 0.8 | 0.14 | 1.00 | 0.34 |
| Right ventricle, mg/mm | 4.5 ± 0.7 | 4.3 ± 0.3 | 5.3 ± 0.2 | 5.5 ± 0.4 | 0.03 | 1.00 | 0.65 |
| Left ventricular diastolic stiffness, κ | 22.1 ± 0.8 b | 22.4 ± 0.9 b | 30.1 ± 0.7 a | 30.2 ± 0.8 a | <0.0001 | 0.81 | 0.90 |
| Left ventricular collagen area, % | 6.5 ± 0.6 b | 7.2 ± 0.9 b | 27.4 ± 2.6 a | 26.2 ± 2.1 a | <0.0001 | 0.89 | 0.59 |
| Metabolic variables | |||||||
| Plasma triglycerides, mmol/L | 0.43 ± 0.02 c | 0.71 ± 0.06 b | 1.88 ± 0.31 a | 1.58 ± 0.22 a | <0.0001 | 0.96 | 0.15 |
| Plasma non-esterified fatty acids, mmol/L | 0.38 ± 0.06 b | 0.61 ± 0.06 a | 0.40 ± 0.03 b | 0.71 ± 0.07 a | 0.40 | 0.0005 | 0.58 |
| Plasma total cholesterol, mmol/L | 1.56 ± 0.08 | 1.73 ± 0.06 | 1.57 ± 0.10 | 1.71 ± 0.06 | 0.95 | 0.05 | 0.84 |
| Alanine transaminase, U/L | 34 ± 4 | 31 ± 3 | 38 ± 2 | 35 ± 3 | 0.25 | 0.39 | 1.00 |
| Aspartate transaminase, U/L | 116 ± 2 | 101 ± 6 | 120 ± 12 | 119 ± 12 | 0.30 | 0.45 | 0.51 |
| Liver inflammatory cells, cells/200 µm2 | 12 ± 2 b | 14 ± 2 b | 26 ± 3 a | 29 ± 4 a | <0.0001 | 0.39 | 0.86 |
| Liver fat vacuoles area, fat vacuoles/200 µm2 | 21.2 ± 1.8 c | 22.4 ± 2.3 c | 135.1 ± 12.9 a | 75.0 ± 4.6 b | <0.0001 | 0.0004 | 0.0003 |
| Oral glucose tolerance test | |||||||
| 0-week basal blood glucose, mmol/L | 2.6 ± 0.1 | 2.9 ± 0.3 | 2.6 ± 0.2 | 2.6 ± 0.2 | 0.58 | 0.58 | 0.58 |
| 0-week area under the curve, mmol/L·minutes | 632 ± 30 | 598 ± 19 | 606 ± 19 | 606 ± 19 | 0.70 | 0.46 | 0.46 |
| 8-week basal blood glucose, mmol/L | 2.9 ± 0.2 | 2.8 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 0.001 | 0.7 | 0.68 |
| 8-week 120-min blood glucose, mmol/L | 3.5 ± 0.2 b | 3.8 ± 0.2 b | 5.0 ± 0.1 a | 4.5 ± 0.2 a | <0.0001 | 0.65 | 0.075 |
| 8-week area under the curve, mmol/L·minutes | 530 ± 15 b | 558 ± 17 b | 657 ± 22 a | 640 ± 15 a | <0.0001 | 0.77 | 0.24 |
| 16-week basal blood glucose, mmol/L | 2.8 ± 0.2 | 2.7 ± 0.1 | 3.3 ± 0.2 | 3.0 ± 1.1 | 0.008 | 0.16 | 0.48 |
| 16-week 120-min blood glucose, mmol/L | 3.9 ± 0.2 b | 4.1± 0.2 b | 4.8 ± 0.3 a | 4.8 ± 0.2 a | 0.002 | 0.68 | 0.68 |
| 16-week area under the curve, mmol/L·minutes | 501 ± 21 b | 571 ± 15 a | 617 ± 25 a | 593 ± 16 a | 0.001 | 0.24 | 0.021 |
| Insulin tolerance test | |||||||
| 8-week 120-min blood glucose, mmol/L | 2.9 ± 0.4 b | 3.4 ± 0.4 b | 4.5 ± 0.3 a | 4.3 ± 0.2 a | 0.002 | 0.68 | 0.34 |
| 8-week area under the curve, mmol/L·minutes | 247 ± 58 c | 156 ± 25 c | 408 ± 21 a | 369 ± 22 a | <0.0001 | 0.05 | 0.04 |
| 16-week 120-min blood glucose, mmol/L | 2.7 ± 0.3 b | 3.2 ± 0.3 b | 4.5 ± 0.4 a | 4.1 ± 0.2 a | 0.0001 | 0.87 | 0.16 |
| 16-week area under the curve, mmol/L·minutes | 208 ± 37 c | 307 ± 36 b | 404 ± 54 a | 365 ± 16 a | 0.001 | 0.41 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats. Nutrients 2021, 13, 3991. https://doi.org/10.3390/nu13113991
du Preez R, Majzoub ME, Thomas T, Panchal SK, Brown L. Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats. Nutrients. 2021; 13(11):3991. https://doi.org/10.3390/nu13113991
Chicago/Turabian Styledu Preez, Ryan, Marwan E. Majzoub, Torsten Thomas, Sunil K. Panchal, and Lindsay Brown. 2021. "Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats" Nutrients 13, no. 11: 3991. https://doi.org/10.3390/nu13113991
APA Styledu Preez, R., Majzoub, M. E., Thomas, T., Panchal, S. K., & Brown, L. (2021). Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats. Nutrients, 13(11), 3991. https://doi.org/10.3390/nu13113991








