The Gluten-Free Diet for Celiac Disease and Beyond
Abstract
1. Introduction
2. Gluten and Celiac Disease
3. Gluten-Free Diet for Celiac Disease
3.1. Efficacy of Gluten-Free Diet in Celiac Disease
3.2. Skepticism of the Gluten-Free Diet
3.3. Challenges of a Gluten-Free Diet
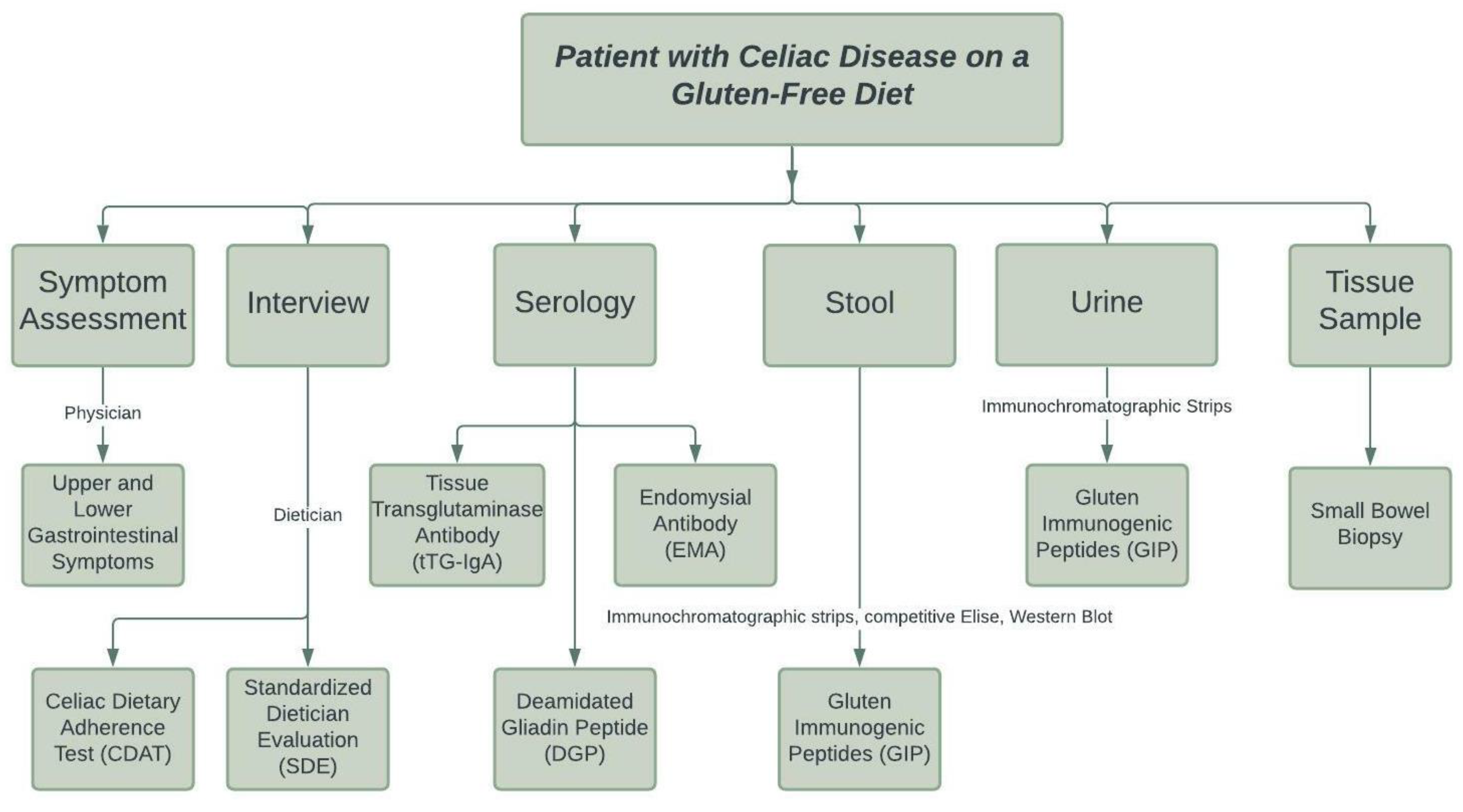
3.4. How to Monitor a Gluten-Free Diet for Celiac Disease
3.4.1. Symptom Assessment
3.4.2. Dietetic Interview
3.4.3. Serology
3.4.4. Stool and Urine Markers
3.4.5. Small Bowel Biopsy and Pathology
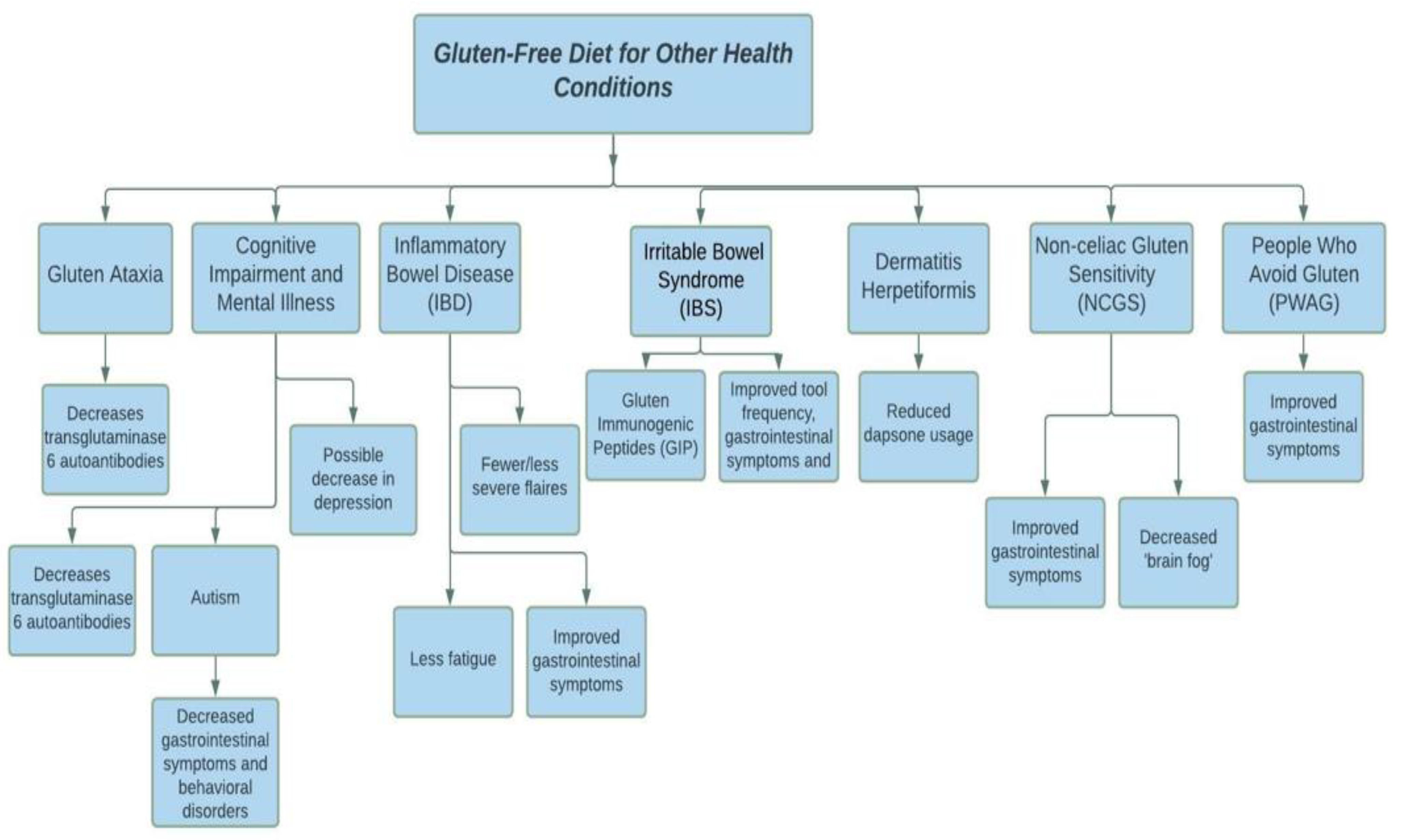
4. Gluten-Free Diet for Other Health Problems
4.1. Gluten Ataxia
4.2. Cognitive Impairment and Neurological and Mental Illnesses
4.3. Inflammatory Bowel Disease and Irritable Bowel Syndrome
4.4. Dermatitis Herpetiformis
4.5. Non-Celiac Gluten Sensitivity (NCGS) and People Who Avoid Gluten
5. Adverse Events of GFD
5.1. Gluten and the Gut Microbiome
5.2. Nutritional Deficiencies
5.3. Cost
5.4. Social and Psychological Impact
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Shurtleff, W.; Huang, H.T.; Aoyagi, A. History of Soybeans and Soyfoods in China and Taiwan, and in Chinese Cookbooks, Restaurants, and Chinese Work with Soyfoods outside China (1024 BCE TO 2014); Soyinfo Center: Lafayette, CA, USA, 2014. [Google Scholar]
- Nature or Nurture? Explaining English Wheat Yields in the Industrial Revolution, c.1770 on JSTOR. Available online: https://www.jstor.org/stable/3874947 (accessed on 26 October 2021).
- Atack, J.; Margo, R.A. The Impact of Access to Rail Transportation on Agricultural Improvement: The American Midwest as a Test Case, 1850–1860. J. Transp. Land Use 2011, 4, 5–18. [Google Scholar] [CrossRef]
- Fitzgerald, D. World War II and the Quest for Time-Insensitive Foods. Osiris 2020, 35, 291–309. [Google Scholar] [CrossRef]
- Theien, I. Food rationing during World War two: A special case of sustainable consumption? Anthr. Food 2009. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Wen, S.; Wen, N.; Pang, J.; Langen, G.; Brew-Appiah, R.; Mejías, J.; Osorio, C.E.; Yang, M.; Gemini, R.; Moehs, C.; et al. Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc. Natl. Acad. Sci. USA 2012, 109, 20543–20548. [Google Scholar] [CrossRef]
- Shewry, P. What Is Gluten—Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Pan, L.; Lu, Q. Effect of amino and thiol groups of wheat gluten on the quality characteristics of Chinese noodles. J. Food Sci. Technol. 2019, 56, 2825–2835. [Google Scholar] [CrossRef]
- Moreno, M.D.L.; Rodríguez-Herrera, A.; Sousa, C.; Comino, I. Biomarkers to Monitor Gluten-Free Diet Compliance in Celiac Patients. Nutrients 2017, 9, 46. [Google Scholar] [CrossRef]
- Gujral, N. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; de Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 1–20. [Google Scholar] [CrossRef]
- Stazi, A.V.; Trecca, A.; Trinti, B. Osteoporosis in celiac disease and in endocrine and reproductive disorders. World J. Gastroenterol. 2008, 14, 498–505. [Google Scholar] [CrossRef]
- Ndez-Bañares, H.M.; Monzón, H.; Forné, M. A short review of malabsorption and anemia. World J. Gastroenterol. 2009, 15, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef]
- Kreutz, J.M.; Adriaanse, M.P.M.; Van Der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.M.; Liu, E. Celiac Disease: Pathophysiology, Clinical Manifestations, and Associated Autoimmune Conditions. Adv. Pediatr. 2008, 55, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Balaban, D.V.; Dima, A.; Jurcut, C.; Popp, A.; Jinga, M. Celiac crisis, a rare occurrence in adult celiac disease: A systematic review. World J. Clin. Cases 2019, 7, 311–319. [Google Scholar] [CrossRef]
- De Pablo, P.; Cooper, M.S.; Buckley, C.D. Association between bone mineral density and C-reactive protein in a large population-based sample. Arthritis Rheum. 2012, 64, 2624–2631. [Google Scholar] [CrossRef]
- Hardy, R.; Cooper, M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009, 201, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A. The role of dietary calcium in bone health. Proc. Nutr. Soc. 2003, 62, 851–858. [Google Scholar] [CrossRef]
- Van Rijn, J.C.W.; Grote, F.K.; Oostdijk, W.; Wit, J.M. Short stature and the probability of coeliac disease, in the absence of gastrointestinal symptoms. Arch. Dis. Child. 2004, 89, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Garganta, M.D.; Bremer, A.A. Clinical Dilemmas in Evaluating the Short Child. Pediatr. Ann. 2014, 43, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Michaelsson, K.; Ekbom, A.; Montgomery, S.M. Coeliac disease and the risk of fractures—A general population-based cohort study. Aliment. Pharmacol. Ther. 2006, 25, 273–285. [Google Scholar] [CrossRef]
- Melton, L.J.; Beck, T.J.; Amin, S.; Khosla, S.; Achenbach, S.J.; Oberg, A.L.; Riggs, B.L. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporos. Int. 2005, 16, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, T.; Kröger, H.; Janatuinen, E.; Arnala, I.; Kosma, V.-M.; Pikkarainen, P.; Julkunen, R.; Jurvelin, J.; Alhava, E.; Uusitupa, M. Osteoporosis in adult patients with celiac disease. Bone 1999, 24, 249–255. [Google Scholar] [CrossRef]
- Sollid, L.M.; McAdam, S.N.; Molberg, Á.; Quarsten, H.; Arentz-Hansen, H.; Louka, A.S.; Lundin, K.E.A. Genes and envi-ronment in celiac disease. Acta Odontol. Scand. 2001, 59, 183–186. [Google Scholar] [CrossRef]
- Lionetti, E.; Catassi, C. The Role of Environmental Factors in the Development of Celiac Disease: What Is New? Diseases 2015, 3, 282. [Google Scholar] [CrossRef]
- Tian, N.; Leffler, D.A.; Kelly, C.P.; Hansen, J.; Marietta, E.V.; Murray, J.A.; Schuppan, D.; Helmerhorst, E.J. Despite sequence homologies to gluten, salivary proline-rich proteins do not elicit immune responses central to the pathogenesis of celiac disease. Am. J. Physiol. Liver Physiol. 2015, 309, G910–G917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Pérez, S.; Pérez-Andrés, J.; Gutiérrez, S.; Navasa, N.; Martínez-Blanco, H.; Ferrero, M.; Vivas, S.; Vaquero, L.; Iglesias, C.; Casqueiro, J.; et al. The Human Digestive Tract Is Capable of Degrading Gluten from Birth. Int. J. Mol. Sci. 2020, 21, 7696. [Google Scholar] [CrossRef] [PubMed]
- Camarca, A.; Anderson, R.P.; Mamone, G.; Fierro, O.; Facchiano, A.; Costantini, S.; Zanzi, D.; Sidney, J.; Auricchio, S.; Sette, A.; et al. Intestinal T Cell Responses to Gluten Peptides Are Largely Heterogeneous: Implications for a Peptide-Based Therapy in Celiac Disease. J. Immunol. 2009, 182, 4158–4166. [Google Scholar] [CrossRef] [PubMed]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Clemente, M.G.; Sapone, A.T.A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; Not, T.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.G.; De Virgiliis, S.; Kang, J.S.; Macatagney, R.; Musu, M.P.; Di Pierro, M.R.; Drago, S.; Congia, M.; Fasano, A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003, 52, 218–223. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ráki, M.; Schjetne, K.W.; Stamnaes, J.; Molberg, Ø.; Jahnsen, F.L.; Issekutz, T.B.; Bogen, B.; Sollid, L.M. Surface Expression of Transglutaminase 2 by Dendritic Cells and its Potential Role for Uptake and Presentation of Gluten Peptides to T Cells. Scand. J. Immunol. 2007, 65, 213–220. [Google Scholar] [CrossRef]
- Cecilio, L.A.; Bonatto, M.W. The Prevalence Of Hla Dq2 And Dq8 In Patients With Celiac Disease, In Family and in General Population. Arq. Bras. Cir. Dig. 2015, 28, 183–185. [Google Scholar] [CrossRef]
- Mazzarella, G. Effector and suppressor T cells in celiac disease. World J. Gastroenterol. 2015, 21, 7349–7356. [Google Scholar] [CrossRef]
- La Scaleia, R.; Barba, M.; Di Nardo, G.; Bonamico, M.; Oliva, S.; Nenna, R.; Valitutti, F.; Mennini, M.; Barbato, M.; Montuori, M.; et al. Size and dynamics of mucosal and peripheral IL-17A+ T-cell pools in pediatric age, and their disturbance in celiac disease. Mucosal Immunol. 2012, 5, 513–523. [Google Scholar] [CrossRef][Green Version]
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Corazza, G.R. Coeliac disease. J. Clin. Pathol. 2005, 58, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Bañares, F.F.; Mariné, M.; Rosinach, M.; Carrasco, A.; Esteve, M.; Rodrigo, L. Type 1 Marsh Celiac Disease: Diagnosis and Response. OmniaScience Monogr. 2014, 5, 289–302. [Google Scholar] [CrossRef]
- Kamboj, A.K.; Oxentenko, A.S. Clinical and Histologic Mimickers of Celiac Disease. Clin. Transl. Gastroenterol. 2017, 8, e114. [Google Scholar] [CrossRef] [PubMed]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet in Celiac Disease—Forever and for All? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef]
- Green, P.H.; Fleischauer, A.T.; Bhagat, G.; Goyal, R.; Jabri, B.; Neugut, A.I. Risk of malignancy in patients with celiac disease. Am. J. Med. 2003, 115, 191–195. [Google Scholar] [CrossRef]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non–Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Valitutti, F.; Iorfida, D.; Anania, C.; Trovato, C.M.; Montuori, M.; Cucchiara, S.; Catassi, C. Cereal Consumption among Subjects with Celiac Disease: A Snapshot for Nutritional Considerations. Nutrients 2017, 9, 396. [Google Scholar] [CrossRef]
- Jones, A.L. The Gluten-Free Diet: Fad or Necessity? Diabetes Spectr. 2017, 30, 118–123. [Google Scholar] [CrossRef]
- Wahab, P.J.; Meijer, J.W.; Mulder, C.J. Histologic Follow-up of People With Celiac Disease on a Gluten-Free Diet. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.-T.; Murray, J.A. Mucosal Recovery and Mortality in Adults With Celiac Disease After Treatment With a Gluten-Free Diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.; Elisei, W.; Inchingolo, C.; Monardo, E.; Aiello, F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: A 2-year prospective study. Endoscopy 2006, 38, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kavak, U.S.; Yüce, A.; Koçak, N.; Demir, H.; Saltik, I.N.; Gürakan, F.; Özen, H. Bone Mineral Density in Children With Untreated and Treated Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 434–436. [Google Scholar] [CrossRef]
- Nicoletta, M.; Maria Pia, C.; Maria Teresa, B.; Sergio, O.; Giorgio Giambattista, G.; Paolo, B. Bone Mineral Density in Adult Celiac Patients and the Effect of Gluten-Free Diet from Childhood. Am. J. Gastroenterol. 1990, 85, 51–53. [Google Scholar]
- Barera, G.; Mora, S.; Brambilla, P.; Ricotti, A.; Menni, L.; Beccio, S.; Bianchi, C. Body composition in children with celiac disease and the effects of a gluten-free diet: A prospective case-control study. Am. J. Clin. Nutr. 2000, 72, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, A.G.; Kansu, A.; Girgin, N.; Kucuk, N.O.; Aras, G. Bone Mineral Density and Importance of a Gluten-Free Diet in Patients With Celiac Disease in Childhood. Pediatrics 2001, 108, 89. [Google Scholar] [CrossRef]
- Soliman, A.T.; Laham, M.; Jour, C.; Shaat, M.; Souikey, F.; Itani, M.; Al-Safi, A.; Karmallah, A.; Qudaisat, A.; Alarabi, Z.; et al. Linear growth of children with celiac disease after the first two years on gluten- free diet: A controlled study. Acta Biomed 2019, 90, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kurppa, K.; Collin, P.; Viljamaa, M.; Haimila, K.; Saavalainen, P.; Partanen, J.; Laurila, K.; Huhtala, H.; Paasikivi, K.; Mäki, M.; et al. Diagnosing Mild Enteropathy Celiac Disease: A Randomized, Controlled Clinical Study. Gastroenterology 2009, 136, 816–823. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G. The Symptomatic and Histologic Response to a Gluten-Free Die in Patients With Borderline Enteropathy. J. Clin. Gastroenterol. 2003, 36, 13–17. [Google Scholar]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, A.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef]
- Hischenhuber, C.; Crevel, R.; Jarry, B.; Maki, M.; Moneret-Vautrin, D.A.; Romano, A.; Troncone, R.; Ward, R. Review article: Safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment. Pharmacol. Ther. 2006, 23, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Lähdeaho, M.-L.; Mäki, M.; Laurila, K.; Huhtala, H.; Kaukinen, K. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Rossini, M.; Ratsch, I.M.; Bearzi, I.; Santinelli, A.; Castagnani, R.; Pisani, E.; Coppa, G.V.; Giorgi, P.L. Dose dependent effects of protracted ingestion of small amounts of gliadin in coeliac disease children: A clinical and jejunal morphometric study. Gut 1993, 34, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Lerner, B.A.; Vo, L.T.P.; Yates, S.; Rundle, A.G.; Green, P.H.; Lebwohl, B. Detection of Gluten in Gluten-Free Labeled Restaurant Food: Analysis of Crowd-Sourced Data. Am. J. Gastroenterol. 2019, 114, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Thorell, L.; Kaukinen, K.; Mäki, M. The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment. Pharmacol. Ther. 2004, 19, 1277–1283. [Google Scholar] [CrossRef]
- Stevens, L.; Rashid, M. Gluten-Free and Regular Foods: A Cost Comparison. Can. J. Diet. Pr. Res. 2008, 69, 147–150. [Google Scholar] [CrossRef]
- Lee, A.R.; Ng, D.L.; Zivin, J.; Green, P.H.R. Economic burden of a gluten-free diet. J. Hum. Nutr. Diet. 2007, 20, 423–430. [Google Scholar] [CrossRef]
- Singh, J.; Whelan, K. Limited availability and higher cost of gluten-free foods. J. Hum. Nutr. Diet. 2011, 24, 479–486. [Google Scholar] [CrossRef]
- Mustalahti, K.; Lohiniemi, S.; Collin, P.; Vuolteenaho, N.; Laippala, P.; Mäki, M. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff. Clin. Pract. 2002, 5, 105–113. [Google Scholar]
- Plugis, N.M.; Khosla, C. Therapeutic approaches for celiac disease. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Bebb, J.R.; Lawson, A.; Knight, T.; Long, R.G. Long-term follow-up of coeliac disease—What do coeliac patients want? Aliment. Pharmacol. Ther. 2006, 23, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Kurppa, K.; Lauronen, O.; Collin, P.; Ukkola, A.; Laurila, K.; Huhtala, H.; Mäki, M.; Kaukinen, K. Factors Associated with Dietary Adherence in Celiac Disease: A Nationwide Study. Digestion 2012, 86, 309–314. [Google Scholar] [CrossRef]
- Galli, G.; Carabotti, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Conti, L. Relationship between Persistent Gastrointestinal Symptoms and Duodenal Histological Findings after Adequate Gluten-Free Diet: A Gray Area of Celiac Disease Management in Adult Patients. Nutrients 2021, 13, 600. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; George, J.E.; Jamma, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A Validated Disease-Specific Symptom Index for Adults With Celiac Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1328–1334.e3. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; George, J.B.E.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A Simple Validated Gluten-Free Diet Adherence Survey for Adults With Celiac Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536.e2. [Google Scholar] [CrossRef] [PubMed]
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Małgorzewicz, S. Celiac Dietary Adherence Test and Standardized Dietician Evaluation in Assessment of Adherence to a Gluten-Free Diet in Patients with Celiac Disease. Nutrients 2020, 12, 2300. [Google Scholar] [CrossRef]
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients 2021, 13, 2274. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.D. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology 2005, 128, S25–S32. [Google Scholar] [CrossRef]
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The diagnostic accuracy of serologic tests for celiac disease: A systematic review. Gastroenterology 2005, 128, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.A.; Okunogbe, A.; Ewing, B.; Grant, S.; Newberry, S.J.; Motala, A.; Shanman, R.; Mejia, N.; Arifkhanova, A.; Shekelle, P.; et al. Diagnosis of Celiac Disease. Diagnosis Celiac Dis. 2016, 162, 1. [Google Scholar]
- Vitoria, J.C.; Arrieta, A.; Arranz, C.; Ayesta, A.; Sojo, A.; Maruri, N.; García-Masdevall, M.D. Antibodies to Gliadin, Endomysium, and Tissue Transglutaminase for the Diagnosis of Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Holm, K.; Hallstrom, O.; Collin, P.; Viander, M.; Savilahti, E.; Lipsanen, V.; Koskimies, S. Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet 1991, 338, 1350–1353. [Google Scholar] [CrossRef]
- Adriaanse, M.; Leffler, D.A. Serum Markers in the Clinical Management of Celiac Disease. Dig. Dis. 2015, 33, 236–243. [Google Scholar] [CrossRef]
- Lewis, N.R.; Scott, B.B. Meta-analysis: Deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment. Pharmacol. Ther. 2010, 31, 73–81. [Google Scholar] [CrossRef]
- Barbato, M.; Maiella, G.; Di Camillo, C.; Guida, S.; Valitutti, F.; Lastrucci, G.; Mainiero, F.; Cucchiara, S. The anti-deamidated gliadin peptide antibodies unmask celiac disease in small children with chronic diarrhoea. Dig. Liver Dis. 2011, 43, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Monzani, A.; Rapa, A.; Fonio, P.; Tognato, E.; Panigati, L.; Oderda, G. Use of Deamidated Gliadin Peptide Antibodies to Monitor Diet Compliance in Childhood Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 55–60. [Google Scholar] [CrossRef]
- Liu, E.; Li, M.; Emery, L.; Taki, I.; Barriga, K.; Tiberti, C.; Eisenbarth, G.S.; Rewers, M.J.; Hoffenberg, E.J. Natural History of Antibodies to Deamidated Gliadin Peptides and Transglutaminase in Early Childhood Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 293–300. [Google Scholar] [CrossRef]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, D.R. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: A Meta-analysis. Gastroenterology 2017, 153, 689–701. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; Vivas, S.; Síglez, M.; Caminero, A.; Nistal, E.; Casqueiro, J.; Rodríguez-Herrera, A.; Cebolla, Á.; Sousa, C. Monitoring of gluten-free diet compliance in celiac patients by assessment of gliadin 33-mer equivalent epitopes in feces. Am. J. Clin. Nutr. 2012, 95, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Fischbach, M.A. Community Health Care: Therapeutic Opportunities in the Human Microbiome. Sci. Transl. Med. 2011, 3, 78ps12. [Google Scholar] [CrossRef]
- Morón, B.; Cebolla, Á.; Manyani, H.; Álvarez-Maqueda, M.; Megías, M.; Thomas, M.D.C.; López, M.C.; Sousa, C. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am. J. Clin. Nutr. 2008, 87, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Segura, V.; Ortigosa, L.; Espín, B.; Castillejo, G.; Garrote, J.A.; Sierra, C.; Millán-Jiménez, A.; Ribes-Koninckx, C.; Román, E.; et al. Prospective longitudinal study: Use of faecal gluten immunogenic peptides to monitor children diagnosed with coeliac disease during transition to a gluten-free diet. Aliment. Pharmacol. Ther. 2019, 49, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, Á.; Moreno, M.D.L.; Coto, L.; Sousa, C. Gluten Immunogenic Peptides as Standard for the Evaluation of Potential Harmful Prolamin Content in Food and Human Specimen. Nutrients 2018, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Baviera, L.C.B.; Aliaga, E.D.; Ortigosa, L.; Litwin, N.; Peña-Quintana, L.; Méndez, V.; González, M.V.; López-Manzanares, J.M.; Méndez, E.; Koninckx, C.R. Celiac Disease Screening by Immunochromatographic Visual Assays: Results of a Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Fernández-Bañares, F.; Esteve, M.; Ortigosa, L.; Castillejo, G.; Fambuena, B.; Ribes-Koninckx, C.; Sierra, C.; Rodríguez-Herrera, A.; Salazar, J.C.; et al. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am. J. Gastroenterol. 2016, 111, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.D.L.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2015, 66, 250–257. [Google Scholar] [CrossRef]
- Leonard, M.M.; Weir, D.C.; DeGroote, M.; Mitchell, P.D.; Singh, P.; Silvester, J.; Leichtner, A.M.; Fasano, A. Value of IgA tTG in Predicting Mucosal Recovery in Children With Celiac Disease on a Gluten-Free Diet. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 286–291. [Google Scholar] [CrossRef]
- Pais, W.P.; Duerksen, D.R.; Pettigrew, N.M.; Bernstein, C.N. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest. Endosc. 2008, 67, 1082–1087. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Mearin, M.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Unalp-Arida, A.; Ruhl, C.E.; Brantner, T.L.; Everhart, J.E.; Murray, J.A. Less Hidden Celiac Disease But Increased Gluten Avoidance Without a Diagnosis in the United States. Mayo Clin. Proc. 2016, 92, 30–38. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Aeschlimann, P.; Sanders, D.S.; Mäki, M.; Kaukinen, K.; Grunewald, R.A.; Bandmann, O.; Woodroofe, N.; Haddock, G.; Aeschlimann, D. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 2013, 80, 1740–1745. [Google Scholar] [CrossRef]
- Dipper, C.R.; Maitra, S.; Thomas, R.; Lamb, C.A.; Mclean-Tooke, A.P.C.; Ward, R.; Smith, D.; Spickett, G.; Mansfield, J.C. Anti-tissue transglutaminase antibodies in the follow-up of adult coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Gibson, A.; Davies-Jones, G.; Lobo, A.; Stephenson, T.; Milford-Ward, A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet 1996, 347, 369–371. [Google Scholar] [CrossRef]
- Buie, T. The Relationship of Autism and Gluten. Clin. Ther. 2013, 35, 578–583. [Google Scholar] [CrossRef]
- Lau, N.M.; Green, P.H.R.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS ONE 2013, 8, e66155. [Google Scholar] [CrossRef]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-Free Diet in Children with Autism Spectrum Disorders: A Randomized, Controlled, Single-Blinded Trial. J. Autism Dev. Disord. 2019, 50, 482–490. [Google Scholar] [CrossRef]
- Peters, S.L.; Biesiekierski, J.; Yelland, G.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, H.M.; Demmer, R.T.; Murray, J.A.; Green, P.H.; Lebwohl, B. Depression and insomnia among individuals with celiac disease or on a gluten-free diet in the USA. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Ohrnberger, J.; Fichera, E.; Sutton, M. The relationship between physical and mental health: A mediation analysis. Soc. Sci. Med. 2017, 195, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, S.; Chikhi, S.; Maltais, D. The benefits of physical activities on cognitive and mental health in healthy and pathological aging. Geriatrie et psychologie neuropsychiatrie du vieillissement 2018, 16, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ergün, C.; Urhan, M.; Ayer, A. A review on the relationship between gluten and schizophrenia: Is gluten the cause? Nutr. Neurosci. 2017, 21, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Levinta, A.; Mukovozov, I.; Tsoutsoulas, C. Use of a Gluten-Free Diet in Schizophrenia: A Systematic Review. Adv. Nutr. 2018, 9, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, U.; Ullrich, R.; Riecken, E.; Schulzke, J. Celiac disease–like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology 2001, 121, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a Gluten-Free Diet in Subjects With Irritable Bowel Syndrome-Diarrhea Unaware of Their HLA-DQ2/8 Genotype. Clin. Gastroenterol. Hepatol. 2015, 14, 696–703.e1. [Google Scholar] [CrossRef]
- Celiac disease, non-celiac gluten sensitivity and inflammatory bowel disease. Minerva Gastroenterol. Dietol. 2015, 61, 267–271. Available online: https://www.minervamedica.it/en/journals/gastroenterology/article.php?cod=R08Y2015N04A0267 (accessed on 8 September 2021).
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a Gluten-free Diet and Improvement of Clinical Symptoms in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef]
- Lindberg, E.; Magnusson, K.-E.; Tysk, C.; Jarnerot, G. Antibody (IgG, IgA, and IgM) to baker’s yeast (Saccharomyces cerevisiae), yeast mannan, gliadin, ovalbumin and betalactoglobulin in monozygotic twins with inflammatory bowel disease. Gut 1992, 33, 909–913. [Google Scholar] [CrossRef]
- Reunala, T.; Blomqvist, K.; Tarpila, S.; Halme, H.; Kangas, K. Gluten-free diet in dermatitis herpetiformis. Br. J. Dermatol. 1977, 97, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.; Riches, D.; Seah, P.; Hoffbrand, A. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet 1973, 301, 288–291. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Vanga, R.R.; Leffler, D.A.; Villafuerte-Galvez, J.; Pallav, K.; Hansen, J.; Mukherjee, R.; Dennis, M.; Kelly, C.P. Celiac Disease or Non-Celiac Gluten Sensitivity? An Approach to Clinical Differential Diagnosis. Am. J. Gastroenterol. 2014, 109, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.; Pawlicka, M.; Mroczek, A.; Bałabuszek, K.; Nieradko-Iwanicka, B. Non-Celiac Gluten Sensitivity: A Review. Medicina 2019, 55, 222. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac Gluten Sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Yelland, G.W. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J. Gastroenterol. Hepatol. 2017, 32, 90–93. [Google Scholar] [CrossRef]
- Truswell, A.S. Cereal grains and coronary heart disease. Eur. J. Clin. Nutr. 2002, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Flight, I.; Clifton, P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Koh-Banerjee, P.; Hu, F.B.; Franz, M.; Sampson, L.; Grønbaek, M.; Rimm, E.B. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004, 80, 1492–1499. [Google Scholar] [CrossRef]
- Tang, G.; Wang, D.; Long, J.; Yang, F.; Si, L. Meta-Analysis of the Association Between Whole Grain Intake and Coronary Heart Disease Risk. Am. J. Cardiol. 2015, 115, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Brienesse, S.C.; Walker, M.M.; Boyle, A.; Talley, N.J. Effect of the gluten-free diet on cardiovascular risk factors in patients with coeliac disease: A systematic review. J. Gastroenterol. Hepatol. 2017, 33, 781–791. [Google Scholar] [CrossRef]
- Heikkilä, K.; Koskinen, O.; Agarwal, A.; Tikkinen, K.; Mäki, M.; Kaukinen, K. Associations of coeliac disease with coronary heart disease and cerebrovascular disease: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 816–831. [Google Scholar] [CrossRef]
- Kim, H.-S.; Demyen, M.F.; Mathew, J.; Kothari, N.; Feurdean, M.; Ahlawat, S.K. Obesity, Metabolic Syndrome, and Cardiovascular Risk in Gluten-Free Followers Without Celiac Disease in the United States: Results from the National Health and Nutrition Examination Survey 2009–2014. Dig. Dis. Sci. 2017, 62, 2440–2448. [Google Scholar] [CrossRef]
- Deora, V.; Aylward, N.; Sokoro, A.; El-Matary, W. Serum Vitamins and Minerals at Diagnosis and Follow-up in Children With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 185–189. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Sue, A.; Dehlsen, K.; Ooi, C.Y. Paediatric Patients with Coeliac Disease on a Gluten-Free Diet: Nutritional Adequacy and Macro- and Micronutrient Imbalances. Curr. Gastroenterol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2012, 6, 39–51. [Google Scholar] [CrossRef]
- Missbach, B.; Schwingshackl, L.; Billmann, A.; Mystek, A.; Hickelsberger, M.; Bauer, G.; Koenig, J. Gluten-free food database: The nutritional quality and cost of packaged gluten-free foods. PeerJ 2015, 3, e1337. [Google Scholar] [CrossRef] [PubMed]
- Zarkadas, M.; Cranney, A.; Case, S.; Molloy, M.; Switzer, C.; Graham, I.D.; Butzner, J.D.; Rashid, M.; Warren, R.E.; Burrows, V. The impact of a gluten-free diet on adults with coeliac disease: Results of a national survey. J. Hum. Nutr. Diet. 2006, 19, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Weiten, D.; Graff, L.A.; Walker, J.R.; Duerksen, D.R. Living gluten-free: Adherence, knowledge, lifestyle adaptations and feelings towards a gluten-free diet. J. Hum. Nutr. Diet. 2015, 29, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Bohnhoff, M.; Miller, C.P. Enhanced Susceptibility to Salmonella Infection in Streptomycin-Treated Mice. J. Infect. Dis. 1962, 111, 117–127. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef]
- Golfetto, L.; De Senna, F.D.; Hermes, J.; Beserra, B.T.S.; França, F.D.S.; Martinello, F. Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq. Gastroenterol. 2014, 51, 139–143. [Google Scholar] [CrossRef]
- Wacklin, P.; Laurikka, P.; Lindfors, K.; Collin, P.; Salmi, T.; Lähdeaho, M.-L.; Saavalainen, P.; Mäki, M.; Mättö, J.; Kurppa, K.; et al. Altered Duodenal Microbiota Composition in Celiac Disease Patients Suffering From Persistent Symptoms on a Long-Term Gluten-Free Diet. Am. J. Gastroenterol. 2014, 109, 1933–1941. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the effect of probiotics supplementation on intestinal microbiota of food allergic mice. Am. J. Transl. Res. 2017, 9, 376–385. [Google Scholar] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2016, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Viti, M.G.; Montouri, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The Gluten-Free Diet: A Nutritional Risk Factor for Adolescents with Celiac Disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Summo, C.; Clodoveo, M.L.; Pasqualone, A. Evaluation of the nutritional quality of the lipid fraction of gluten-free biscuits. Eur. Food Res. Technol. 2007, 227, 135–139. [Google Scholar] [CrossRef]
- MacCulloch, K.; Rashid, M. Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr. Child Heal. 2014, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S.; et al. Management of Type 1 Diabetes With a Very Low–Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef]
- Rosenfalck, A.M.; Almdal, T.; Viggers, L.; Madsbad, S.; Hilsted, J. A low-fat diet improves peripheral insulin sensitivity in patients with Type 1 diabetes. Diabet. Med. 2006, 23, 384–392. [Google Scholar] [CrossRef]
- Patton, S.R. Adherence to Diet in Youth with Type 1 Diabetes. J. Am. Diet. Assoc. 2011, 111, 550–555. [Google Scholar] [CrossRef]
- Camarca, M.E.; Mozzillo, E.; Nugnes, R.; Zito, E.; Falco, M.; Fattorusso, V.; Mobilia, S.; Buono, P.; Valerio, G.; Troncone, R.; et al. Celiac disease in type 1 diabetes mellitus. Ital. J. Pediatrics 2012, 38, 10. [Google Scholar] [CrossRef] [PubMed]
- Scarff, J.R. Orthorexia Nervosa: An Obsession With Healthy Eating. Fed. Pract. 2017, 34, 36–39. [Google Scholar] [PubMed]
- Wolf, R.L.; Lebwohl, B.; Lee, A.R.; Zybert, P.; Reilly, N.R.; Cadenhead, J.; Amengual, C.; Green, P.H.R. Hypervigilance to a Gluten-Free Diet and Decreased Quality of Life in Teenagers and Adults with Celiac Disease. Dig. Dis. Sci. 2018, 63, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
| Type | Intraepithelial Lymphocytes/100 Enterocytes (Duodenum) | Crypt | Villous Architecture | Lesion |
|---|---|---|---|---|
| 0 | <30 | Normal | Normal | Pre-infiltrative |
| 1 | >30 | Normal | Normal | Infiltrative |
| 2 | >30 | Hyperplasia | Normal | Infiltrative-hyperplastic |
| 3a | >30 | Hyperplasia | Mild atrophy | Flat destructive |
| 3b | >30 | Hyperplasia | Marked atrophy | Flat destructive |
| 3c | >30 | Hyperplasia | Complete atrophy | Flat destructive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. https://doi.org/10.3390/nu13113993
Aljada B, Zohni A, El-Matary W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients. 2021; 13(11):3993. https://doi.org/10.3390/nu13113993
Chicago/Turabian StyleAljada, Bara, Ahmed Zohni, and Wael El-Matary. 2021. "The Gluten-Free Diet for Celiac Disease and Beyond" Nutrients 13, no. 11: 3993. https://doi.org/10.3390/nu13113993
APA StyleAljada, B., Zohni, A., & El-Matary, W. (2021). The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients, 13(11), 3993. https://doi.org/10.3390/nu13113993







