Effects of Coffee Consumption on Insulin Resistance and Sensitivity: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
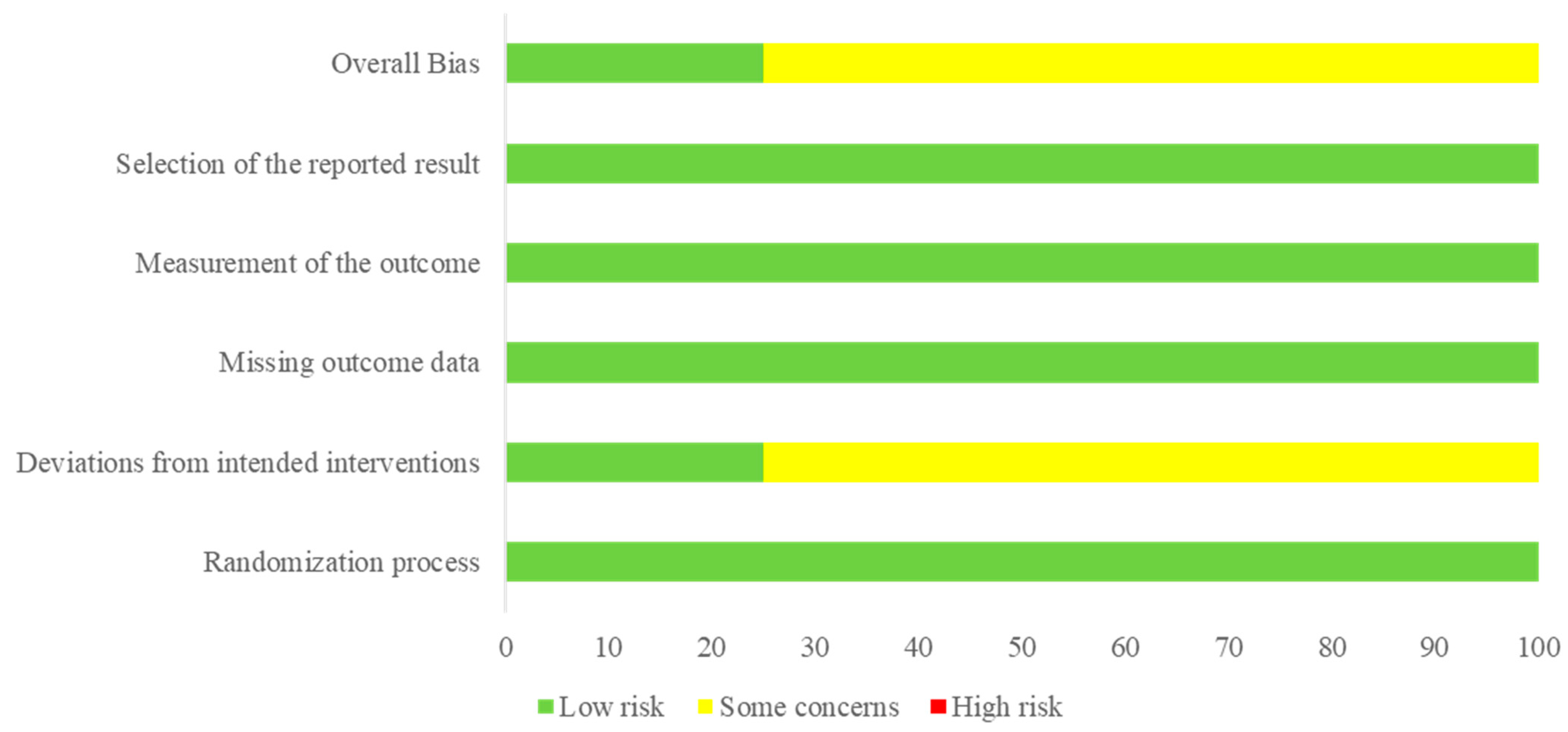
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
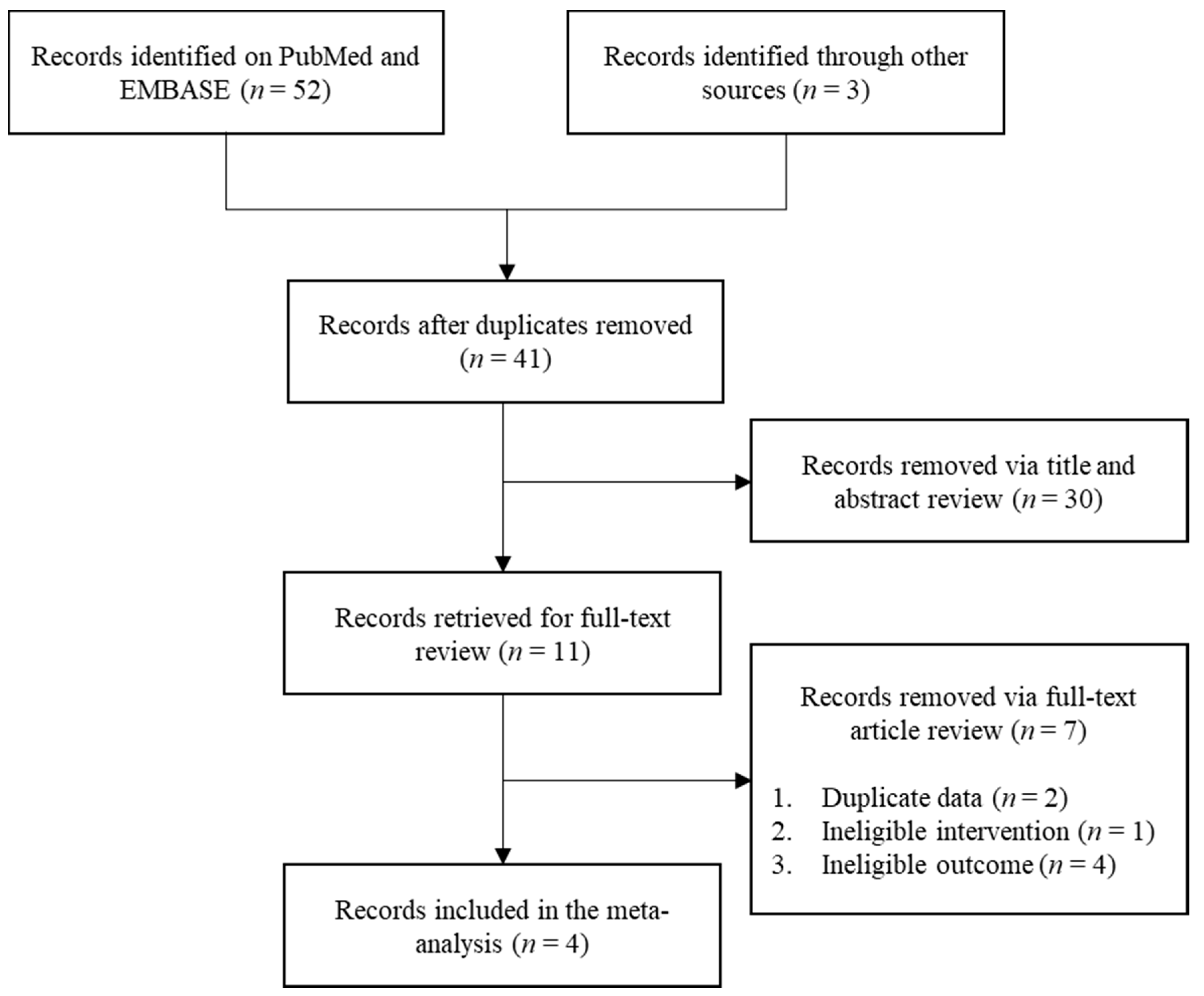
3.1. Selected Studies
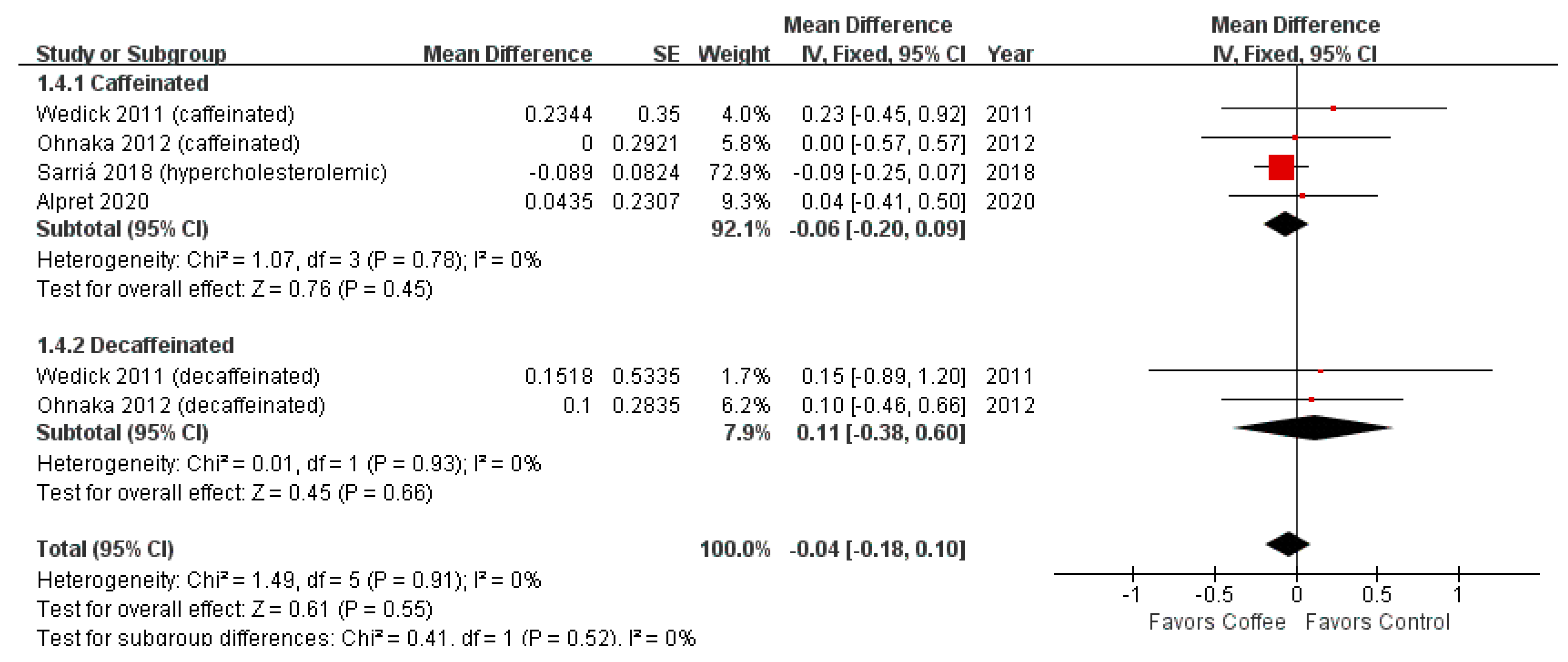
3.2. HOMA-IR
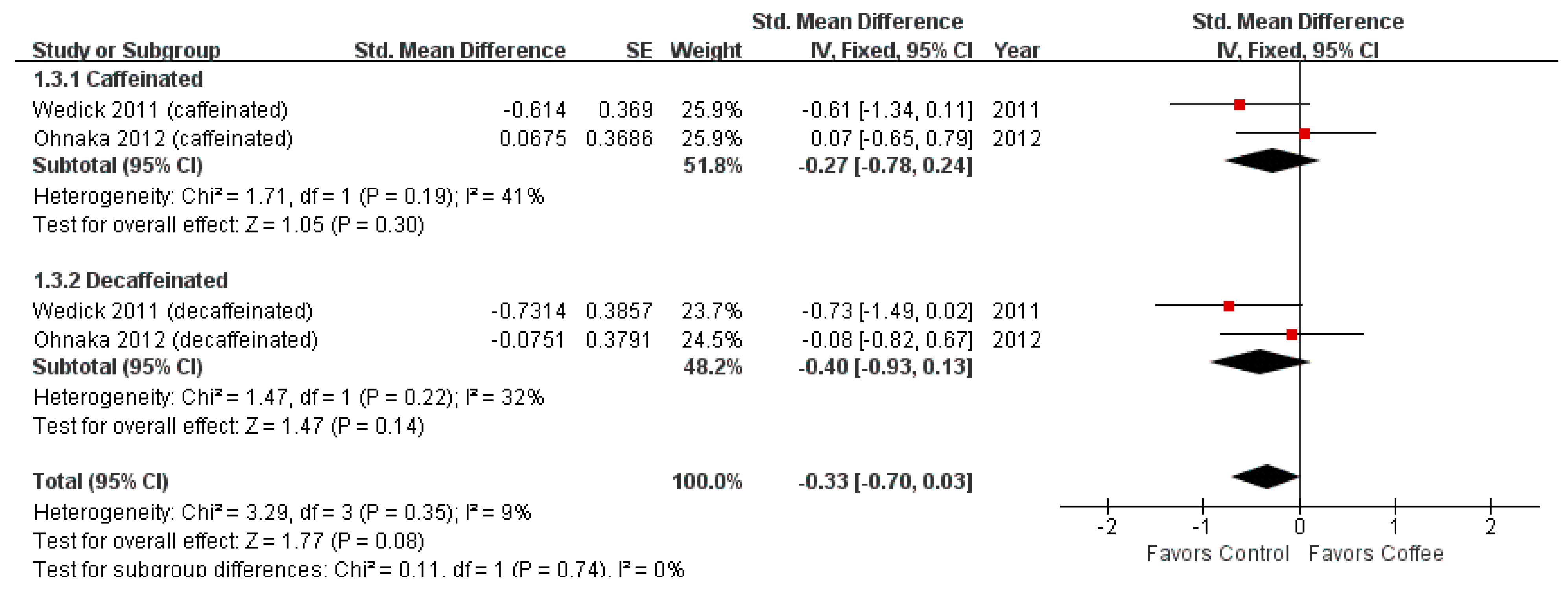
3.3. Matsuda Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Buldak, R.J.; Hejmo, T.; Osowski, M.; Buldak, L.; Kukla, M.; Polaniak, R.; Birkner, E. The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Je, Y.; Giovannucci, E. Coffee consumption and total mortality: A meta-analysis of twenty prospective cohort studies. Br. J. Nutr. 2014, 111, 1162–1173. [Google Scholar] [CrossRef]
- Crippa, A.; Discacciati, A.; Larsson, S.C.; Wolk, A.; Orsini, N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: A dose-response meta-analysis. Am. J. Epidemiol. 2014, 180, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef]
- Je, Y.; Giovannucci, E. Coffee consumption and risk of endometrial cancer: Findings from a large up-to-date meta-analysis. Int. J. Cancer 2012, 131, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Turati, F.; La Vecchia, C.; Tavani, A. Coffee consumption and risk of colorectal cancer: A meta-analysis of case-control studies. Cancer Causes Control 2010, 21, 1949–1959. [Google Scholar] [CrossRef]
- Sang, L.X.; Chang, B.; Li, X.H.; Jiang, M. Consumption of coffee associated with reduced risk of liver cancer: A meta-analysis. BMC Gastroenterol. 2013, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Discacciati, A.; Orsini, N.; Wolk, A. Coffee consumption and risk of nonaggressive, aggressive and fatal prostate cancer--a dose-response meta-analysis. Ann. Oncol. 2014, 25, 584–591. [Google Scholar] [CrossRef]
- Torres, D.M.; Harrison, S.A. Is it time to write a prescription for coffee? Coffee and liver disease. Gastroenterology 2013, 144, 670–672. [Google Scholar] [CrossRef]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. Adv. Exp. Med. Biol. 2012, 771, 1–11. [Google Scholar] [CrossRef]
- Carlstrom, M.; Larsson, S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Goto, A.; Noma, H.; Iso, H.; Hayashi, K.; Noda, M. Effects of Coffee and Tea Consumption on Glucose Metabolism: A Systematic Review and Network Meta-Analysis. Nutrients 2018, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Pisprasert, V.; Ingram, K.H.; Lopez-Davila, M.F.; Munoz, A.J.; Garvey, W.T. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: Impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care 2013, 36, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.E.G.; Dorea, J.G.; da Costa, T.H.M. Effects of coffee consumption on glucose metabolism: A systematic review of clinical trials. J. Tradit. Complement. Med. 2019, 9, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alperet, D.J.; Rebello, S.A.; Khoo, E.Y.; Tay, Z.; Seah, S.S.; Tai, B.C.; Tai, E.S.; Emady-Azar, S.; Chou, C.J.; Darimont, C.; et al. The effect of coffee consumption on insulin sensitivity and other biological risk factors for type 2 diabetes: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2020, 111, 448–458. [Google Scholar] [CrossRef]
- Ohnaka, K.; Ikeda, M.; Maki, T.; Okada, T.; Shimazoe, T.; Adachi, M.; Nomura, M.; Takayanagi, R.; Kono, S. Effects of 16-week consumption of caffeinated and decaffeinated instant coffee on glucose metabolism in a randomized controlled trial. J. Nutr. Metab. 2012, 2012, 207426. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur. J. Nutr. 2018, 57, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Wedick, N.M.; Brennan, A.M.; Sun, Q.; Hu, F.B.; Mantzoros, C.S.; van Dam, R.M. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: A randomized controlled trial. Nutr. J. 2011, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonora, E.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; Raffaelli, A.; et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002, 25, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Anderwald, C.; Anderwald-Stadler, M.; Promintzer, M.; Prager, G.; Mandl, M.; Nowotny, P.; Bischof, M.G.; Wolzt, M.; Ludvik, B.; Kästenbauer, T.; et al. The Clamp-Like Index: A novel and highly sensitive insulin sensitivity index to calculate hyperinsulinemic clamp glucose infusion rates from oral glucose tolerance tests in nondiabetic subjects. Diabetes Care 2007, 30, 2374–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkura, T.; Shiochi, H.; Fujioka, Y.; Sumi, K.; Yamamoto, N.; Matsuzawa, K.; Izawa, S.; Kinoshita, H.; Ohkura, H.; Kato, M.; et al. 20/(fasting C-peptide × fasting plasma glucose) is a simple and effective index of insulin resistance in patients with type 2 diabetes mellitus: A preliminary report. Cardiovasc. Diabetol. 2013, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Curtin, F.; Altman, D.G.; Elbourne, D. Meta-analysis combining parallel and cross-over clinical trials. I: Continuous Outcomes. Stat. Med. 2002, 21, 2131–2144. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Nutr. J. 2021, 20, 35. [Google Scholar] [CrossRef]
- Lee, S.; Min, J.Y.; Min, K.B. Caffeine and Caffeine Metabolites in Relation to Insulin Resistance and Beta Cell Function in U.S. Adults. Nutrients 2020, 12, 1783. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Pasman, W.J.; Verhoef, P. Effects of coffee consumption on fasting blood glucose and insulin concentrations: Randomized controlled trials in healthy volunteers. Diabetes Care 2004, 27, 2990–2992. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, T.; Comi, R.; Sluss, P.; Keisari, R.; Manwar, S.; Kim, J.; Larson, R.; Baron, J.A. Metabolic and hormonal effects of caffeine: Randomized, double-blind, placebo-controlled crossover trial. Metabolism 2007, 56, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Thong, F.S.; Derave, W.; Kiens, B.; Graham, T.E.; Ursø, B.; Wojtaszewski, J.F.; Hansen, B.F.; Richter, E.A. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes 2002, 51, 583–590. [Google Scholar] [CrossRef]
- Arnlov, J.; Vessby, B.; Riserus, U. Coffee consumption and insulin sensitivity. JAMA 2004, 291, 1199–1201. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA 2005, 294, 97–104. [Google Scholar] [CrossRef] [PubMed]

| PICO | Keywords |
|---|---|
| Population | Not defined |
| Intervention | Coffee |
| Comparison | Not defined |
| Outcome | Insulin sensitivity, insulin resistance, homeostasis model assessment (HOMA), quantitative insulin sensitivity check index (QUICKI), Matsuda, McAuley, Belfiore, Cederholm, Avignon, Stumvoll, Gutt |
| Author (Year) | Country | Design | Duration | Sample Size | Population | Intervention | Control | Outcome |
|---|---|---|---|---|---|---|---|---|
| Alperet (2020) [19] | Singapore | Parallel | 24 weeks | 126 | Non-diabetic, non-smokers, aged 35–69 years, overweight (BMI 22.5–35.4 kg/m2), habitual coffee drinkers (≥1 cup/day), not insulin sensitive (HOMA-IR ≥ 1.30), not-having other illnesses that could affect study outcomes | Instant coffee beverage (73.7% of a nondairy creamer) four cups per day. Contained 30 kcal per cup with 0.96 g/100 g of caffeine. Sweeteners (caloric or artificial) or milk was permitted | Coffee-like placebo beverage four cups per day. Contained 30 kcal per cup | HOMA-IR |
| Sarriá (2018) [21] | Spain | Crossover | 8 weeks per period | 52 | Men and women aged 18–45 years, BMI < 25 kg/m2, non-smokers, non-vegetarian, non-pregnant women, not-having vitamins or dietary supplements, not-having taken antibiotics 6 months before, not suffering chronic disorders, apart from hypercholesterolemia | 2 g serving of the coffee blend dissolved in 200 mL of hot water, without milk or sugar three times per day. The daily consumption of hydroxycinnamic acids and methylxanthines was 510.6 and 123 mg (121.2 mg was caffeine), respectively | Control drink consisting of water or an isotonic caffeine- and polyphenol-free drink three times per day | HOMA-IR |
| Ohnaka (2012) [20] | Japan | Parallel | 16 weeks | 45 | Men aged 40–64 years, BMI 25–30 kg/m2, fasting plasma glucose 100–140 mg/dL | One cup/glass of coffee using one spoonful (1.2–1.3 g) of instant coffee five times per day (caffeinated or decaffeinated). With mineral water one 500 mL bottle. Either hot or ice coffee was permitted, but coffee was drunk without sugar, milk, or any other additives | Two 500-mL bottles per day | HOMA-IR, Matsuda index |
| Wedick (2011) [22] | United States | Parallel | 8 weeks | 45 | Non-diabetic, regular coffee consumers (≥2 cups/day), non-smokers, aged ≥ 18 years, overweight (BMI 25–35 kg/m2), but otherwise healthy | 2 g portions of instant coffee with 6 ounces of boiling water five times per day (caffeinated (345 mg caffeine per day) or decaffeinated). A non-caloric sweetener or a non-dairy creamer was permitted | 6 ounce glass of water five times per day | HOMA-IR, Matsuda index |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.-M.; Joo, M.-J.; Lee, Y.-S.; Kim, M.-G. Effects of Coffee Consumption on Insulin Resistance and Sensitivity: A Meta-Analysis. Nutrients 2021, 13, 3976. https://doi.org/10.3390/nu13113976
Moon S-M, Joo M-J, Lee Y-S, Kim M-G. Effects of Coffee Consumption on Insulin Resistance and Sensitivity: A Meta-Analysis. Nutrients. 2021; 13(11):3976. https://doi.org/10.3390/nu13113976
Chicago/Turabian StyleMoon, Su-Min, Min-Jin Joo, Young-Seo Lee, and Myeong-Gyu Kim. 2021. "Effects of Coffee Consumption on Insulin Resistance and Sensitivity: A Meta-Analysis" Nutrients 13, no. 11: 3976. https://doi.org/10.3390/nu13113976
APA StyleMoon, S.-M., Joo, M.-J., Lee, Y.-S., & Kim, M.-G. (2021). Effects of Coffee Consumption on Insulin Resistance and Sensitivity: A Meta-Analysis. Nutrients, 13(11), 3976. https://doi.org/10.3390/nu13113976







