Cereal Processing By-Products as Rich Sources of Phenolic Compounds and Their Potential Bioactivities
Abstract
1. Introduction
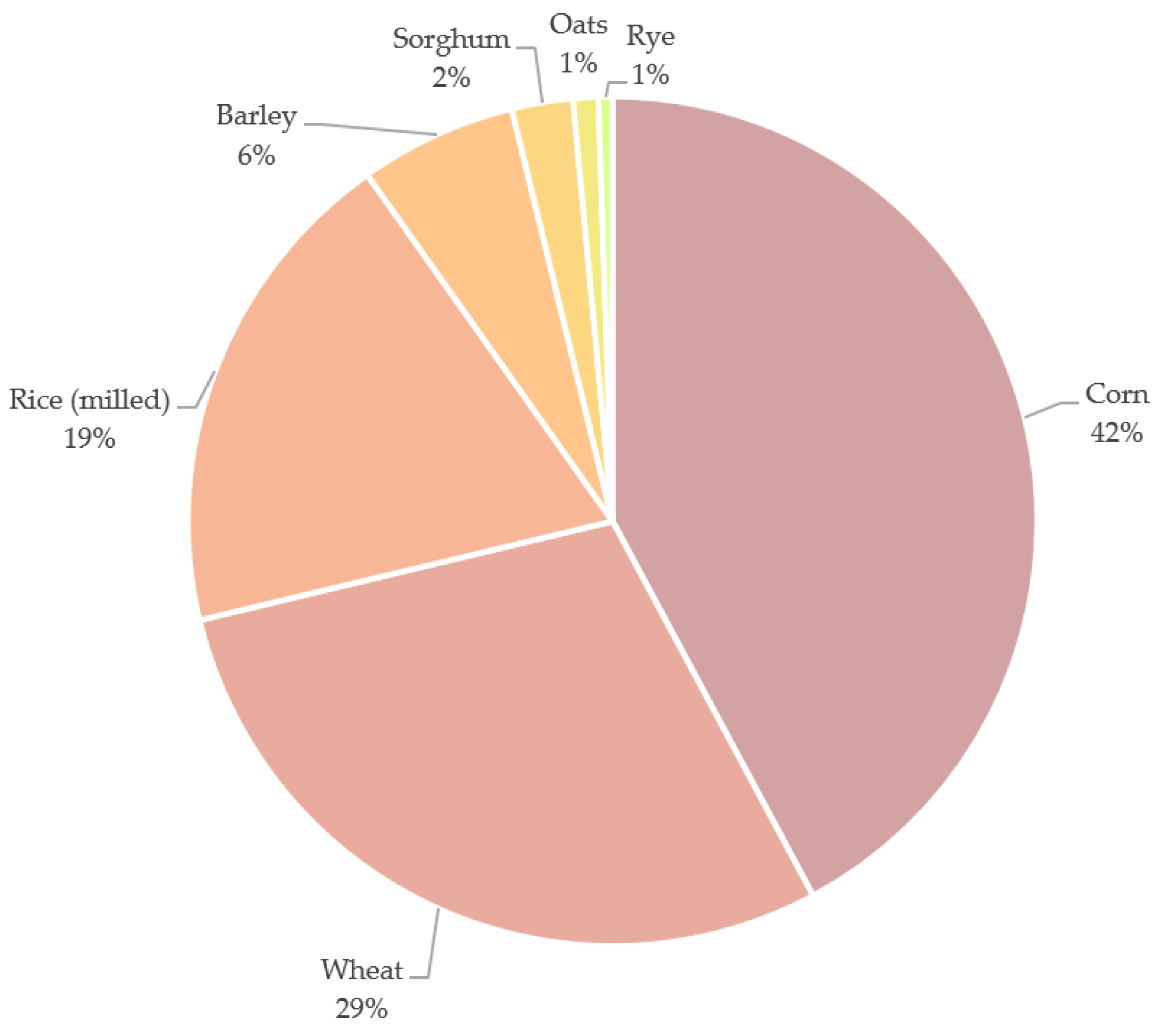
2. Cereal Processing
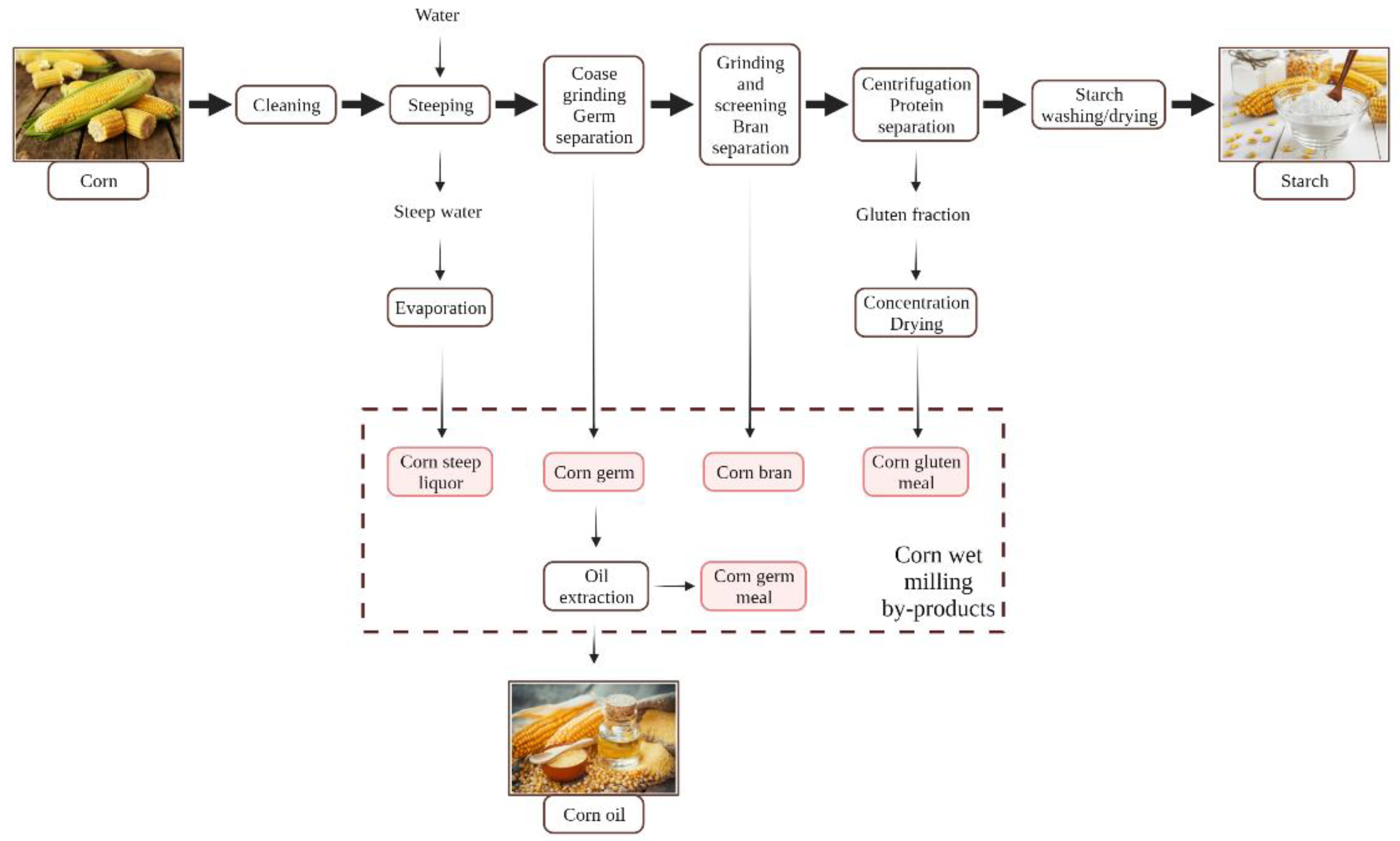
2.1. Corn Processing
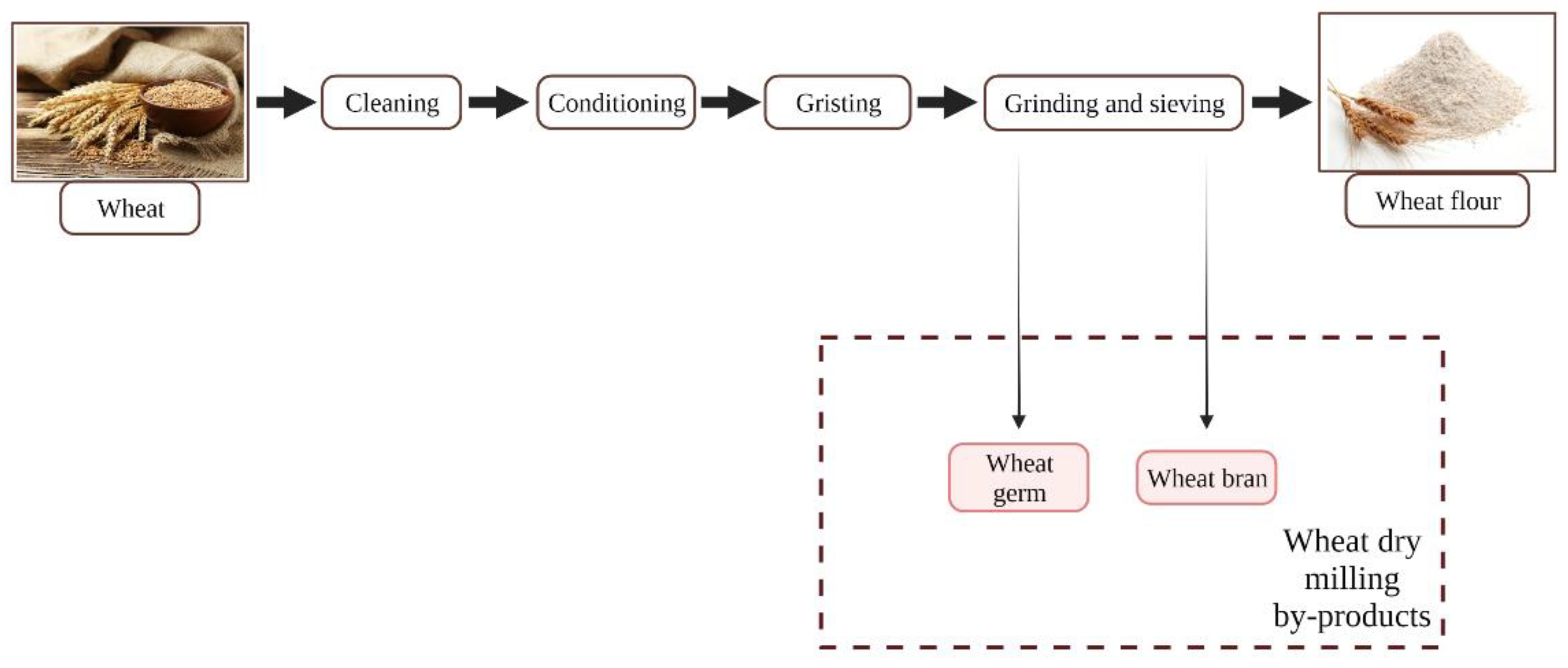
2.2. Wheat Processing
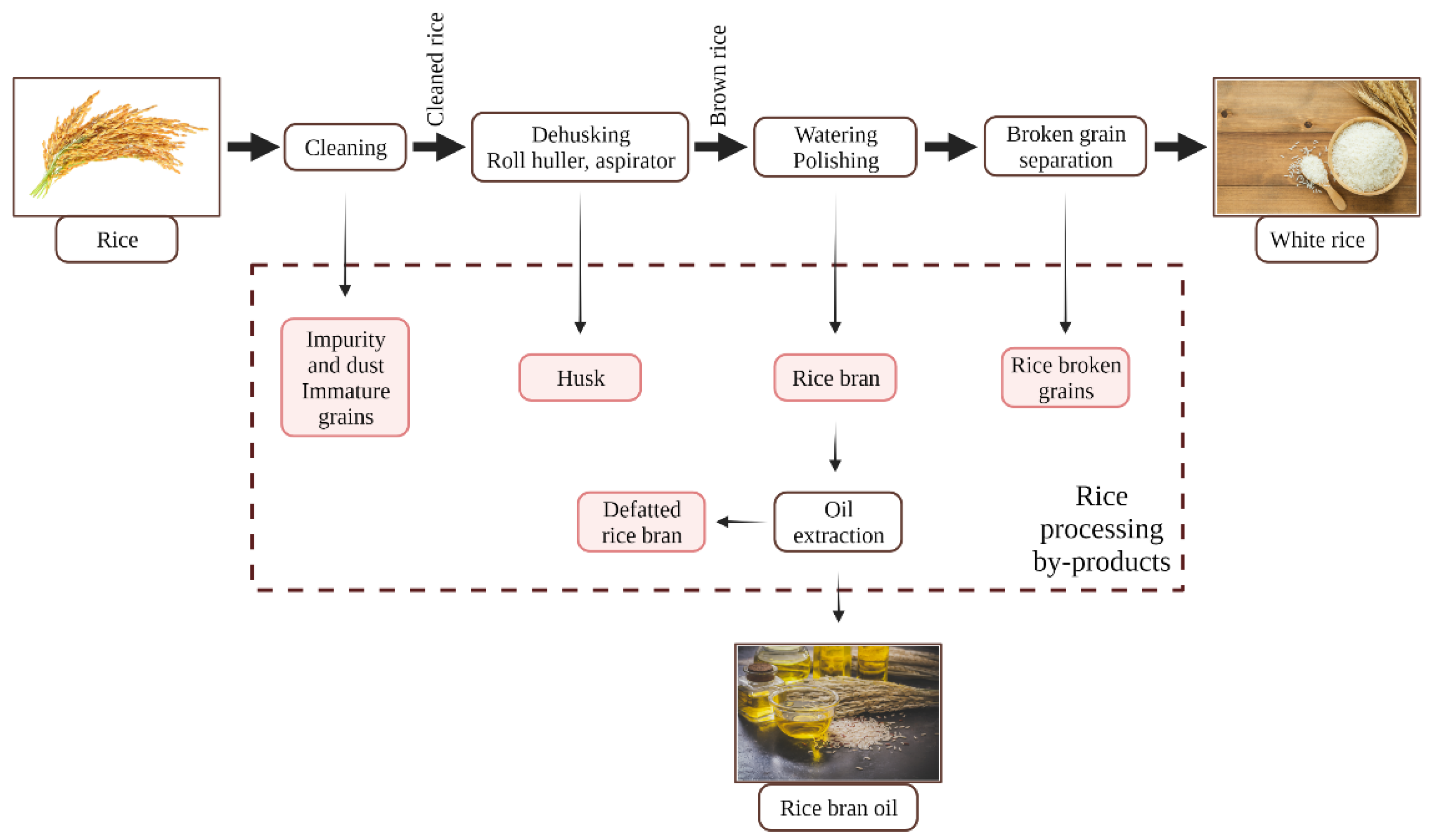
2.3. Rice Processing
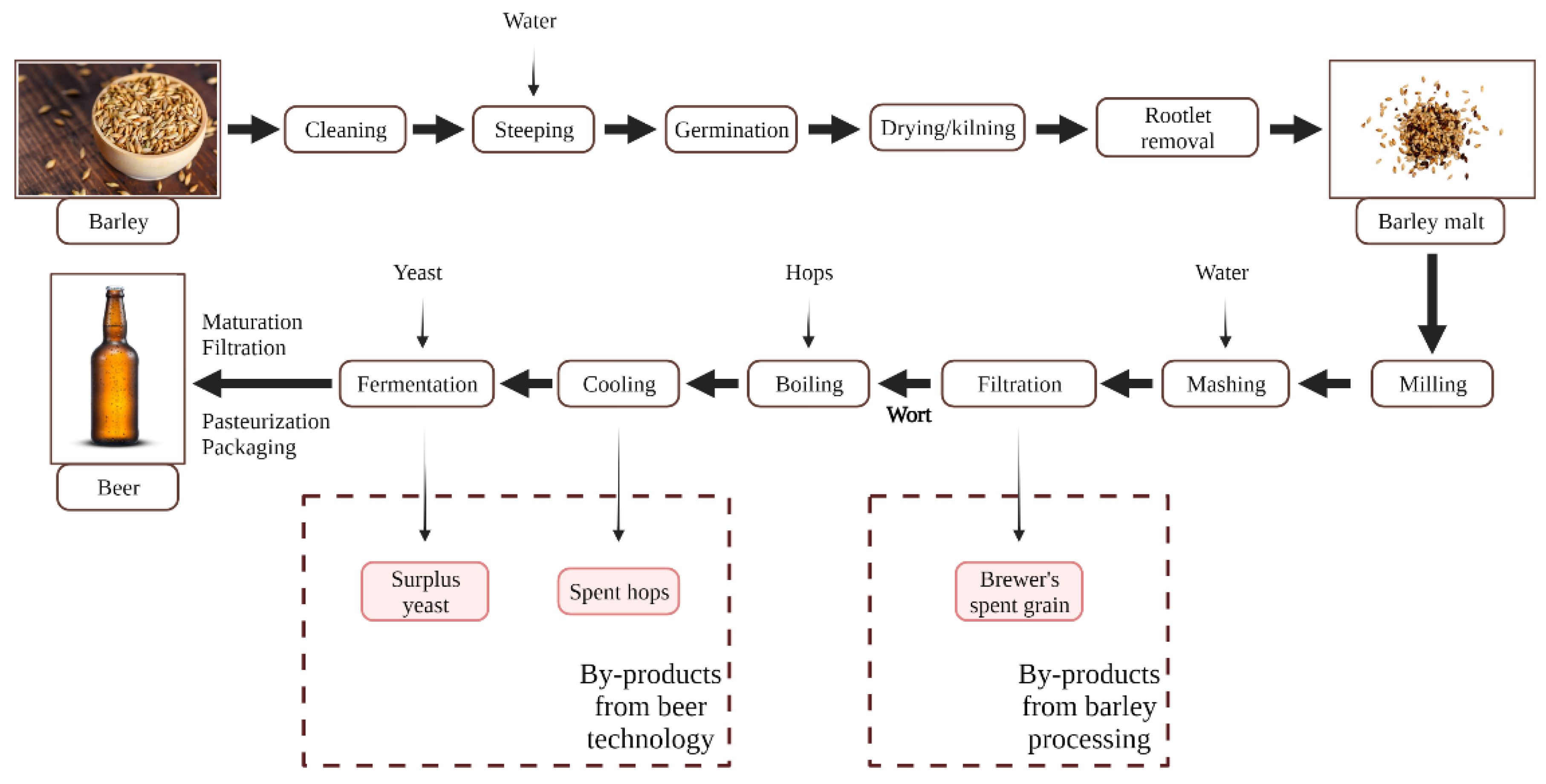
2.4. Barley Processing in Brewing Industry
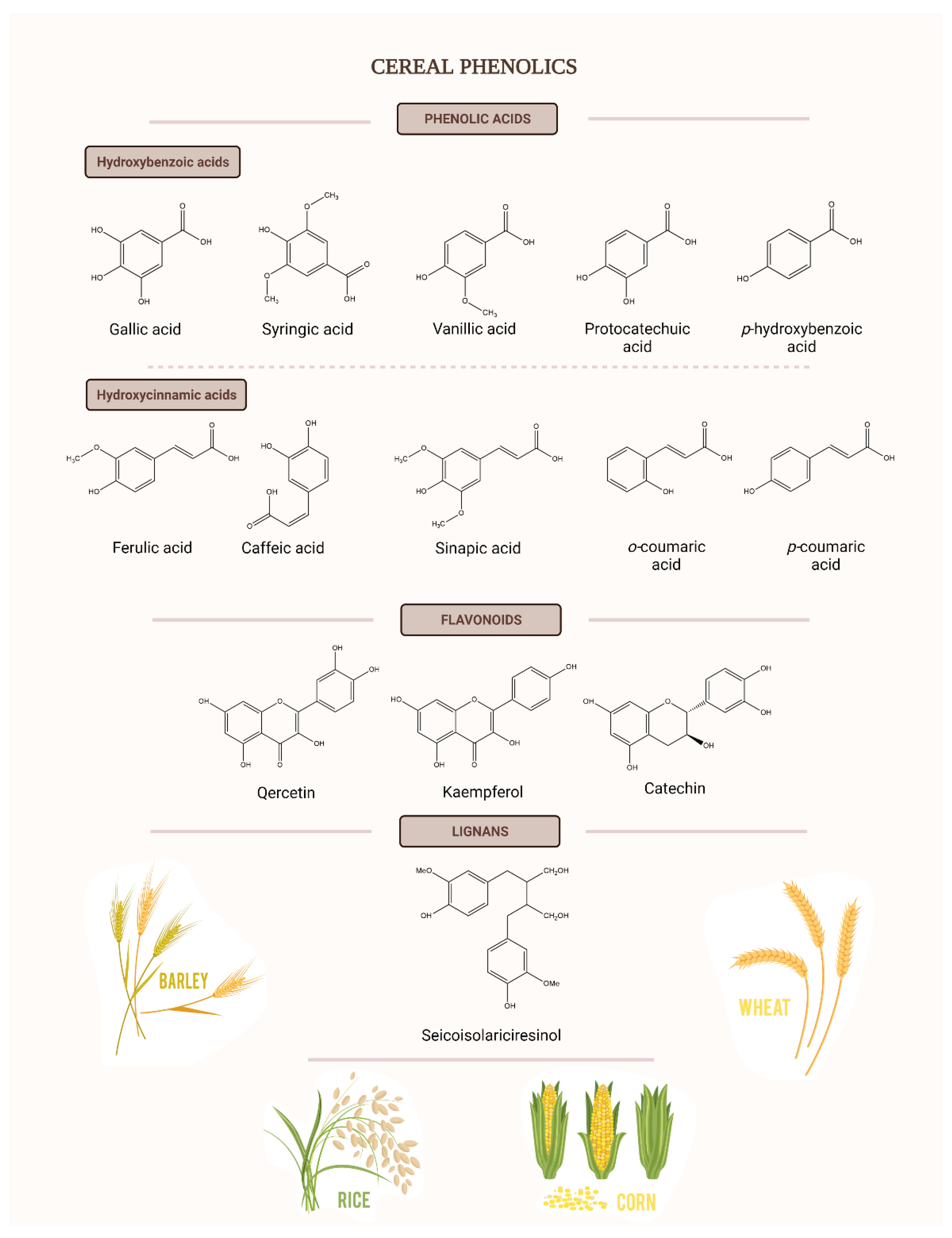
3. Polyphenols in Cereal By-Products
4. Extraction Methods
5. The Potential Health Effects of Extracted Polyphenols
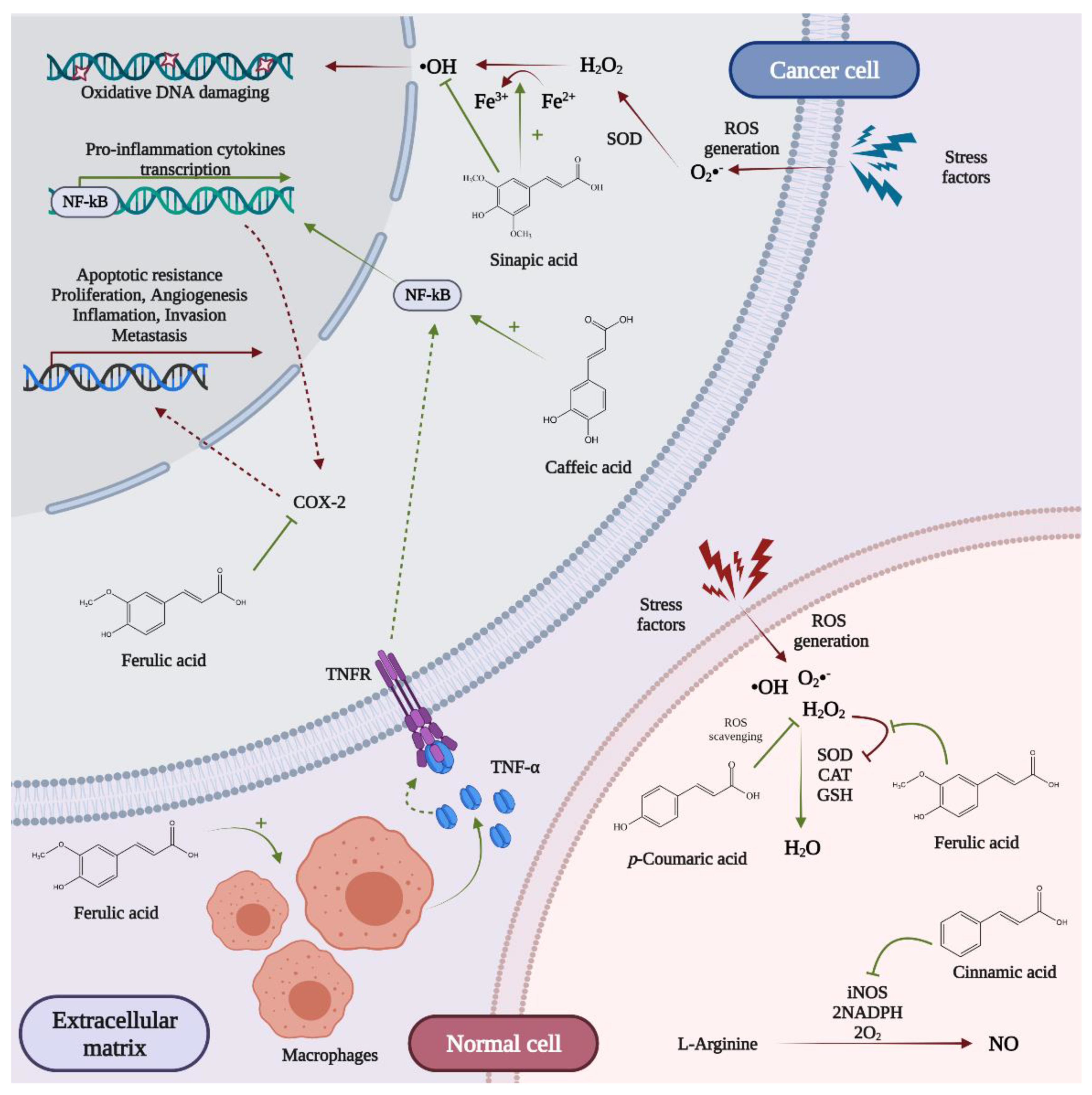
5.1. Antioxidant Activity
5.2. Anti-Carcinogenic Effects
5.2.1. Cell Viability
5.2.2. Cell Proliferation and Apoptosis
5.2.3. DNA Damaging
5.2.4. Inflammation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EU | European Union |
| FAO | Food and Agriculture Organization |
| dCGM | Dry Corn Germ Meal |
| CE | Catechin Equivalent |
| dWB | Dry Wheat Bran |
| dWG | Dry Wheat Germ |
| dRB | Dry Rice Bran |
| GAE | Gallic Acid Equivalent |
| dRG | Dry Rice Germ |
| RDA | Recommended Dietary Allowance |
| BSG | Brewer’s spent Grain |
| SC-CO2 | Supercritical Carbon Dioxide |
| UAE | Ultrasound Assisted Extraction |
| MAE | Microwave Assisted Extraction |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| CAT | Catalase |
| NO | Nitric Oxide |
| iNOS | Nitric Oxide Synthase |
| LPS | Lipopolysaccharide |
| AP | Apurinic/Apirimidinic |
| NF-kB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| TNFα | Tumor Necrosis Factor alpha |
| COX-2 | Cyclooxygenase-isoform 2 |
References
- Cattaneo, A.; Sánchez, M.V.; Torero, M.; Vos, R. Reducing Food Loss and Waste: Five Challenges for Policy and Research. Food Policy 2021, 98, 101974. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the Valorisation and Functionalization of By-Products and Wastes from Cereal-Based Processing Industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun Kim, S.; Wong, J.W.C. Sustainable Processing of Food Waste for Production of Bio-Based Products for Circular Bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Ubando, A.; Felix, C.; Chen, W. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Varjani, S.; Pant, D.; Sauer, M.; Chang, J.S. Circular Bioeconomy Approaches for Sustainability. Bioresour. Technol. 2020, 318, 124084. [Google Scholar] [CrossRef]
- Belc, N.; Mustatea, G.; Apostol, L.; Iorga, S.; Vlǎduţ, V.N.; Mosoiu, C. Cereal Supply Chain Waste in the Context of Circular Economy. E3S Web Conf. 2019, 112, 03031. [Google Scholar] [CrossRef]
- FAO. Cereal Supply and Demand Brief|World Food Situation|Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 9 August 2021).
- Grain Production Worldwide by Type, 2020/21|Statista. Available online: https://www.statista.com/statistics/263977/world-grain-production-by-type/ (accessed on 2 September 2021).
- Singh, A.; Karmakar, S.; Jacob, B.S.; Bhattacharya, P.; Kumar, S.P.J.; Banerjee, R. Enzymatic Polishing of Cereal Grains for Improved Nutrient Retainment. J. Food Sci. Technol. 2015, 52, 3147. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, S.; Xiao, Z. Technologies for Enhancement of Bioactive Components and Potential Health Benefits of Cereal and Cereal-Based Foods: Research Advances and Application Challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Dapčević-Hadnađev, T.; Hadnađev, M.; Pojić, M. The Healthy Components of Cereal By-Products and Their Functional Properties. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–61. [Google Scholar] [CrossRef]
- Tan, J.P.; Jahim, J.M.; Wu, T.Y.; Harun, S.; Mumtaz, T. Use of Corn Steep Liquor as an Economical Nitrogen Source for Biosuccinic Acid Production by Actinobacillus Succinogenes. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012058. [Google Scholar] [CrossRef]
- Albuquerque, C.; Rabello, C.; Santos, M.; Lima, M.; Silva, E.; Lima, T.; Ventura, D.; Dutra, W., Jr. Chemical Composition and Metabolizable Energy Values of Corn Germ Meal Obtained by Wet Milling for Layers. Brazilian J. Poult. Sci. 2014, 16, 107–112. [Google Scholar] [CrossRef]
- Smuda, S.S.; Mohsen, S.M.; Olsen, K.; Aly, M.H. Bioactive Compounds and Antioxidant Activities of Some Cereal Milling By-Products. J. Food Sci. Technol. 2018, 55, 1134. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Inglett, G.; Liu, S. Utilisation of Corn (Zea Mays) Bran and Corn Fiber in the Production of Food Components. J. Sci. Food Agric. 2010, 90, 915–924. [Google Scholar] [CrossRef]
- Hu, R.; Dunmire, K.M.; Truelock, C.N.; Paulk, C.B.; Aldrich, G.; Li, Y. Antioxidant Performances of Corn Gluten Meal and DDGS Protein Hydrolysates in Food, Pet Food, and Feed Systems. J. Agric. Food Res. 2020, 2, 100030. [Google Scholar] [CrossRef]
- Najera, G.; Patricia, S. A Compositional Breakage Equation for First Break Roller Milling of Wheat; University of Manchester—CEAS: Manchester, UK, 2014. [Google Scholar]
- Rosenfelder, P.; Eklund, M.; Mosenthin, R. Nutritive Value of Wheat and Wheat By-Products in Pig Nutrition: A Review. Anim. Feed Sci. Technol. 2013, 185, 107–125. [Google Scholar] [CrossRef]
- Brandolini, A.; Hidalgo, A. Wheat Germ: Not Only a by-Product. Int. J. Food Sci. Nutr. 2012, 63, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat Bran-Based Biorefinery 1: Composition of Wheat Bran and Strategies of Functionalization. LWT Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice Bran: Nutritional Values and Its Emerging Potential for Development of Functional Food—A Review. Bioact. Carbohydrates Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Moongngarm, A.; Daomukda, N.; Khumpika, S. Chemical Compositions, Phytochemicals, and Antioxidant Capacity of Rice Bran, Rice Bran Layer, and Rice Germ. APCBEE Procedia 2012, 2, 73–79. [Google Scholar] [CrossRef]
- Bodie, A.R.; Micciche, A.C.; Atungulu, G.G.; Rothrock, M.J.J.; Ricke, S.C. Current Trends of Rice Milling Byproducts for Agricultural Applications and Alternative Food Production Systems. Front. Sustain. Food Syst. 2019, 3, 47. [Google Scholar] [CrossRef]
- Choi, O.-K.; Yun, S.-K.; Hwang, S.-Y. The Chemical Components of Korean Rice Germ. J. Korean Soc. Food Cult. 2000, 15, 253–258. [Google Scholar]
- Rondanelli, M.; Miccono, A.; Peroni, G.; Nichetti, M.; Infantino, V.; Spadaccini, D.; Alalwan, T.A.; Faliva, M.A.; Perna, S. Rice Germ Macro- and Micronutrients: A New Opportunity for the Nutraceutics. Nat. Prod. Res. 2021, 35, 1532–1536. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Almeida, A.d.R.; Geraldo, M.R.F.; Ribeiro, L.F.; Silva, M.V.; Maciel, M.V.d.O.B.; Haminiuk, C.W.I. Compostos Bioativos Do Bagaço de Malte: Compostos Fenólicos, Ácidos Graxos e Capacidade Antioxidante in Vitro. Acta Sci. Technol. 2017, 39, 269–277. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Rodero, L.; Almajano, M.P. Brewing By-Products as a Source of Natural Antioxidants for Food Preservation. Antioxidants 2021, 10, 1512. [Google Scholar] [CrossRef]
- Stefanello, F.S.; dos Santos, C.O.; Bochi, V.C.; Fruet, A.P.B.; Soquetta, M.B.; Dörr, A.C.; Nörnberg, J.L. Analysis of Polyphenols in Brewer’s Spent Grain and Its Comparison with Corn Silage and Cereal Brans Commonly Used for Animal Nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the Extraction Solvent on Phenolic Content, Antioxidant, Antimicrobial and Antimutagenic Activities of Brewers’ Spent Grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of Its by-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef]
- Kao, T.-H. Health Potential for Beer Brewing Byproducts. In Current Topics on Superfoods; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Sun, Y.; Chen, Z.; Yang, Y.; Wang, Y.; Trabelsi, N. Functional Properties of Polyphenols in Grains and Effects of Physicochemical Processing on Polyphenols. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Luithui, Y.; Nisha, R.B.; Meera, M.S. Cereal By-Products as an Important Functional Ingredient: Effect of Processing. J. Food Sci. Technol. 2019, 56, 1. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current Extraction Techniques towards Bioactive Compounds from Brewer’s Spent Grain–A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Macias-Garbett, R.; Serna-Hernández, S.O.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Phenolic Compounds From Brewer’s Spent Grains: Toward Green Recovery Methods and Applications in the Cosmetic Industry. Front. Sustain. Food Syst. 2021, 5, 196. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Diez, M.T.S.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioprocess Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef]
- Fărcaş, A.; Tofană, M.; Socaci, S.; Scrob, S. Preliminary Study on Antioxidant Activity and Polyphenols Content in Discharged Waste from Beer Production. J. Agroaliment. Process. Technol. 2013, 19, 319–324. [Google Scholar]
- Bonifácio-Lopes, T.; Vilas Boas, A.A.; Coscueta, E.R.; Costa, E.M.; Silva, S.; Campos, D.; Teixeira, J.A.; Pintado, M. Bioactive Extracts from Brewer’s Spent Grain. Food Funct. 2020, 11, 8963–8977. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; Fitzgerald, R.J.; O’Brien, N.M. Phenolic Extracts of Brewers’ Spent Grain (BSG) as Functional Ingredients—Assessment of Their DNA Protective Effect against Oxidant-Induced DNA Single Strand Breaks in U937 Cells. Food Chem. 2012, 134, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Ismail, M. Isolation and Antioxidative Properties of Phenolics-Saponins Rich Fraction from Defatted Rice Bran. J. Cereal Sci. 2013, 57, 480–485. [Google Scholar] [CrossRef]
- Ciulu, M.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Extraction and Purification of Ferulic Acid from Flax Shives, Wheat and Corn Bran by Alkaline Hydrolysis and Pressurised Solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D. Antioxidant Activity and Phenolic Content of Air-Classified Corn Bran. Cereal Chem. 2011, 88, 36–40. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Y.; Sun, X.; Raddatz, J.; Zhou, Z.; Chen, G. Effect of Microfluidisation on Antioxidant Properties of Corn Bran. Food Chem. 2013, 152, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rebolleda, S.; José, M.L.G.S.; Sanz, M.T.; Beltrán, S.; Solaesa, Á.G. Bioactive Compounds of a Wheat Bran Oily Extract Obtained with Supercritical Carbon Dioxide. Foods 2020, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of Ultrasound-Assisted Extraction of Phenolic Compounds from Wheat Bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, R.; Liu, C. Extraction and Antioxidant Activity of Phenolic Compounds from Wheat Bran Treated by Steam Explosion. Trop. J. Pharm. Res. 2015, 14, 1857–1863. [Google Scholar] [CrossRef][Green Version]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-Waste Technology through the Enzymatic Hydrolysis of Agro-Industrial by-Products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Guo, J.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Resistance Investigation of Wheat Bran Polyphenols Extracts on HEK293 Cells against Oxidative Damage. RSC Adv. 2015, 5, 16116–16124. [Google Scholar] [CrossRef]
- Vieira, E.F.; da Silva, D.D.; Carmo, H.; Ferreira, I.M. Protective Ability against Oxidative Stress of Brewers’ Spent Grain Protein Hydrolysates. Food Chem. 2017, 228, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In Vitro and in Vivo Antioxidant Activity of Polyphenols Extracted from Black Highland Barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive Properties of Dietary Rice Bran: Current Status and Future Prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Polonskiy, V.; Loskutov, I.; Sumina, A. Biological Role and Health Benefits of Antioxidant Compounds in Cereals. Biol. Commun. 2020, 65, 53–67. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol Nanoformulations for Cancer Therapy: Experimental Evidence and Clinical Perspective. Int. J. Nanomed. 2017, 12, 2689. [Google Scholar] [CrossRef]
- Makris, D.P.; Şahin, S. Polyphenolic Antioxidants from Agri-Food Waste Biomass. Antioxidants 2019, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Martín-Diana, A.B.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Peñas, E.; Rico, D. Wheat and Oat Brans as Sources of Polyphenol Compounds for Development of Antioxidant Nutraceutical Ingredients. Foods 2021, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; Fitzgerald, R.J.; O’Brien, N.M. Phenolic-Enriched Fractions from Brewers’ Spent Grain Possess Cellular Antioxidant and Immunomodulatory Effects in Cell Culture Model Systems. J. Sci. Food Agric. 2014, 94, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Burlini, I.; Huerta Lorenzo, B.; Grandini, A.; Vertuani, S.; Tacchini, M.; Sacchetti, G. Antioxidant and Antimicrobial Extracts Obtained from Agricultural By-Products: Strategies for a Sustainable Recovery and Future Perspectives. Food Bioprod. Process. 2020, 124, 397–407. [Google Scholar] [CrossRef]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant Properties of Diverse Cereal Grains: A Review on in Vitro and in Vivo Studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer Chemoprevention by Dietary Polyphenols: Promising Role for Epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. NeuroMolecular Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.; O’Callaghan, Y.; McCarthy, A.L.; Connolly, A.; Fitzgerald, R.J.; O’Brien, N.M. Aqueous and Enzyme-Extracted Phenolic Compounds from Brewers’ Spent Grain (BSG): Assessment of Their Antioxidant Potential. J. Food Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Vanniasinkam, T.; Luo, J.; Blanchard, C.L. Chemopreventive Potential of Cereal Polyphenols. Nutr. Cancer 2018, 70, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained Proliferation in Cancer: Mechanisms and Novel Therapeutic Targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.K.L.; Lam, W.S.; Chiu, L.C.M.; Ooi, V.E.C.; Sun, S.S.M.; Wong, Y.S. A Rice Bran Polyphenol, Cycloartenyl Ferulate, Elicits Apoptosis in Human Colorectal Adenocarcinoma SW480 and Sensitizes Metastatic SW620 Cells to TRAIL-Induced Apoptosis. Biochem. Pharmacol. 2009, 77, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Huynh, K.; Heshu, S.R.; Yeap, S.K.; Hazilawati, H.; Roselina, K. Water Extract of Brewers’ Rice Induces Apoptosis in Human Colorectal Cancer Cells via Activation of Caspase-3 and Caspase-8 and Downregulates the Wnt/β-Catenin Downstream Signaling Pathway in Brewers’ Rice-Treated Rats with Azoxymethane-Induced Colon Carc. BMC Complement. Altern. Med. 2015, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health; In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015; pp. 113–124. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoon, W.J.; Kim, S.S. Phytochemical Compositions of Immature Wheat Bran, and Its Antioxidant Capacity, Cell Growth Inhibition, and Apoptosis Induction through Tumor Suppressor Gene. Molecules 2016, 21, 1292. [Google Scholar] [CrossRef] [PubMed]
- Carusillo, A.; Mussolino, C. DNA Damage: From Threat to Treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Ahmad, A.; Khan, H.; Zubair, H.; Sarkar, F.; Hadi, S. The Prooxidant Action of Dietary Antioxidants Leading to Cellular DNA Breakage and Anticancer Effects: Implications for Chemotherapeutic Action against Cancer. Cell Biochem. Biophys. 2013, 67, 431–438. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-Oxidant Activity of Polyphenols and Its Implication on Cancer Chemoprevention and Chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ Spent Grain; Bioactivity of Phenolic Component, Its Role in Animal Nutrition and Potential for Incorporation in Functional Foods: A Review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nakamura, Y.; Koshimizu, K.; Takahashi, D.; Matsumoto, K.; Hagihara, K.; Taniguchi, H.; Nomura, E.; Hosoda, A.; Tsuno, T.; et al. FA15, a Hydrophobic Derivative of Ferulic Acid, Suppresses Inflammatory Responses and Skin Tumor Promotion: Comparison with Ferulic Acid. Cancer Lett. 2002, 180, 121–129. [Google Scholar] [CrossRef]
| Cereal By-Product | Extraction Method | Conditions | Polyphenols | References |
|---|---|---|---|---|
| Brewer’s spent grain | Supercritical carbon dioxide (SC-CO2) | - CO2 + Ethanol (0–60%, v/v) - p = 15–35 MPa - t° = 40–60 °C | Very low quantities of polyphenols | [2,40] |
| Ultrasound Assisted Extraction (UAE) | - Acetone/Water (60/40%, v/v) or NaOH/Water (0.75/99.25%, v/v) - Ultrasound frequency = 20 kHz - t° = 39 → 47 °C | p-Hydroxybenzoic acid, Ferulic acid, Sinapic acid | [40,41] | |
| Microwave Assisted Extraction (MAE) | - NaOH (0.75%)/Choline chloride:glycerol - t° = 100 °C | p-Coumaric acid, Ferulic acid, Syringic acid | [40,42] | |
| Methanol/Methanol-Water | - VMethanol = 10 mL - t° = 4 °C | Very low quantities of polyphenols | [40,43] | |
| Water | - VH2O = 50 mL - t° = 25 °C; 80 °C | 4-Hydroxybenzoic acid, p-Coumaric acid, Protocatechuic acid, Vanillin, Catechin, Vanillic acid | [44] | |
| Ethanol/ Ethanol-Water | - VEthanol (60–100%, v/v) = 50 mL - t° = 25 °C; 80 °C | 4-Hydroxybenzoic, p-Coumaric acid, Protocatechuic acid, Vanillin, Catechin, Syringic acid | [44] | |
| Acetone/ Acetone-Water | - Acetone (60%, v/v) - t° = 60 °C | p-Hydroxybenzoic acid, Protocatechuic acid, Chlorogenic acid, 8-8′-DiFA, 5-5′-DiFA, 5-5′,8′-O-4′-TriFA, p-Coumaric acid, Ferulic acid, Sinapic acid | [41] | |
| H2SO4 and NaOH | - H2SO4 + NaOH - t° = 120 °C | Ferulic acid | [40] | |
| Saponification | - CM = 1–4 M NaOH | Ferulic acid, p-Coumaric | [45] | |
| Rice bran | Supercritical carbon dioxide (SC-CO2) | - CO2 + Ethanol (0, 5 and 10%) - t° = 40 °C, 60 °C - p = 30, 40 MPa | (+)-Catechin, Chlorogenic acid, Caffeic acid, p-Coumaric acid, Protocatechuic acid, Cyanidin-3-glucoside | [2,46] |
| Ultrasound Assisted Extraction (UAE) for black and purple rice bran | - Solvent (20–60%) - t° = 30–60 °C - pH = 2–4 | [2,46] | ||
| Microwave Assisted Extraction (MAE) | - Methanol (100%) - t° = 185 °C - Microwave power = 1000 W | [46,47] | ||
| Green method | - Glycerol (10–70%, v/v) - t° = 40–70 °C - Liquid-to-solid ratio = 10–40 mL/g | [2,46] | ||
| Corn bran | Pressurized alkaline hydrolysis | - CM = 0.5 M NaOH—30% Ethanol - 15% Ammonia/Water - t° = 180 °C | Ferulic acid, p-Coumaric acid, Vanillin (derived) | [48] |
| Single alkaline and acid extraction | - VNaOH = 5 mL (2 N NaOH) - VHCl = 5 mL (2 N HCl) | Vanillic acid, Cis-ferulic acid, p-Coumaric acid, Caffeic acid, Syringic acid, Sinapic acid | [49] | |
| Single neutral extraction | - VEthanol (50%, v/v) = 10 mL | [49] | ||
| Acetone | - VAcetone (50%, v/v) = 20 mL - t° = 25 °C | p-Coumaric acid, Syringic acid, Ferulic acid | [50] | |
| Wheat bran | Supercritical carbon dioxide (SC-CO2) | - CO2 (8 ± 1 kg CO2/h) - t° = 40 ± 2 °C - p = 25.0 ± 1.0 MPa | p-Hydroxybenzaldehyde, Ferulic acid, Syringic acid, Syringic aldehyde, Vanillic acid, Vanillin | [51] |
| Ultrasound Assisted Extraction (UAE) | - Methanol/Ethanol/Acetone (70/70/70%, v/v) - Ultrasound frequency = 40 kHz - t° = 50 °C | [52] | ||
| Steam Explosion | -Ethanol -t° = 224 °C -p = 2.5 MPa | Ferulic acid predominantly | [53] | |
| Enzymatic Hydrolysis | - Multi-enzyme complex Viscozyme L/Xylanase/Feruloyl esterase | Ferulic acid | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fărcaș, A.; Drețcanu, G.; Pop, T.D.; Enaru, B.; Socaci, S.; Diaconeasa, Z. Cereal Processing By-Products as Rich Sources of Phenolic Compounds and Their Potential Bioactivities. Nutrients 2021, 13, 3934. https://doi.org/10.3390/nu13113934
Fărcaș A, Drețcanu G, Pop TD, Enaru B, Socaci S, Diaconeasa Z. Cereal Processing By-Products as Rich Sources of Phenolic Compounds and Their Potential Bioactivities. Nutrients. 2021; 13(11):3934. https://doi.org/10.3390/nu13113934
Chicago/Turabian StyleFărcaș, Anca, Georgiana Drețcanu, Teodora Daria Pop, Bianca Enaru, Sonia Socaci, and Zorița Diaconeasa. 2021. "Cereal Processing By-Products as Rich Sources of Phenolic Compounds and Their Potential Bioactivities" Nutrients 13, no. 11: 3934. https://doi.org/10.3390/nu13113934
APA StyleFărcaș, A., Drețcanu, G., Pop, T. D., Enaru, B., Socaci, S., & Diaconeasa, Z. (2021). Cereal Processing By-Products as Rich Sources of Phenolic Compounds and Their Potential Bioactivities. Nutrients, 13(11), 3934. https://doi.org/10.3390/nu13113934