Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
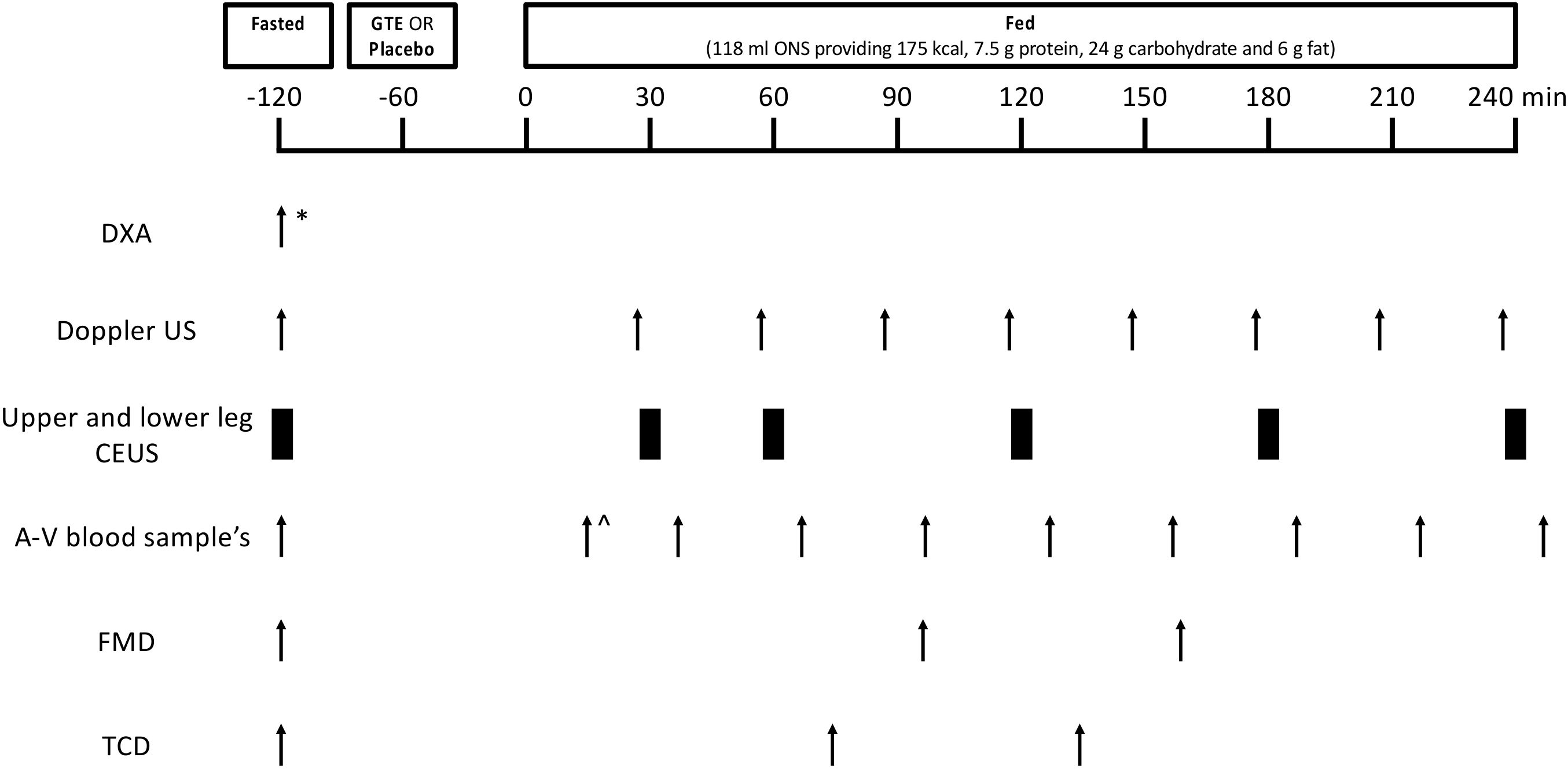
2.2. Volunteers and Study Design
2.3. Study Supplements and ONS Feeding
2.4. Measurement of LBF Using Doppler Ultrasound
2.5. Measurement of MBF Using CEUS
2.6. Measurement of Endothelial Function and Cerebral Blood Flow
2.7. Blood Glucose and Plasma Insulin
2.8. Statistical Analysis
3. Results
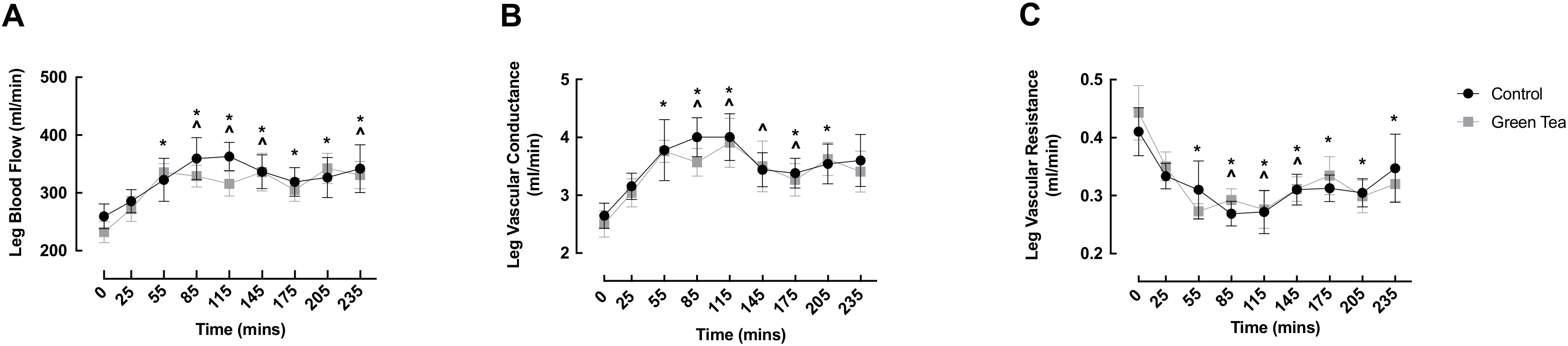
3.1. LBF, LVC and LVR
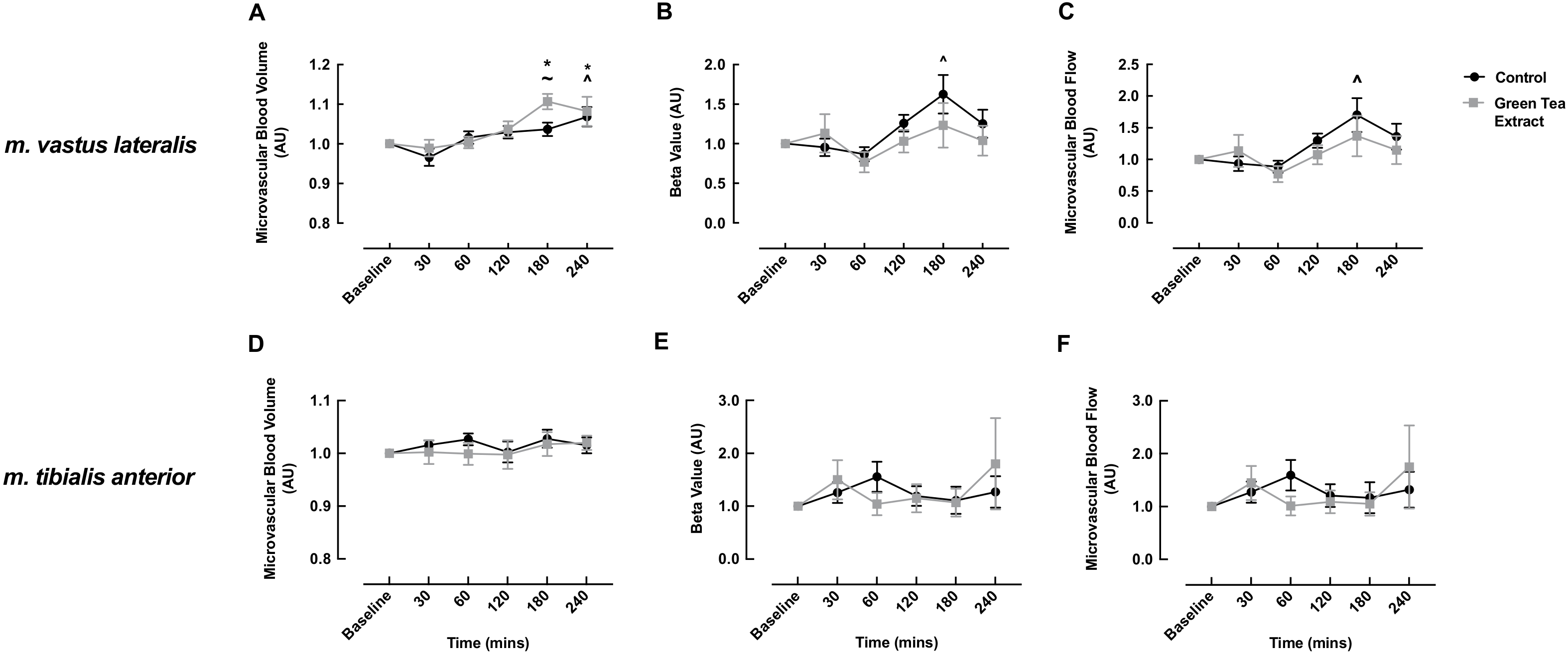
3.2. MBV, MFV and MBF
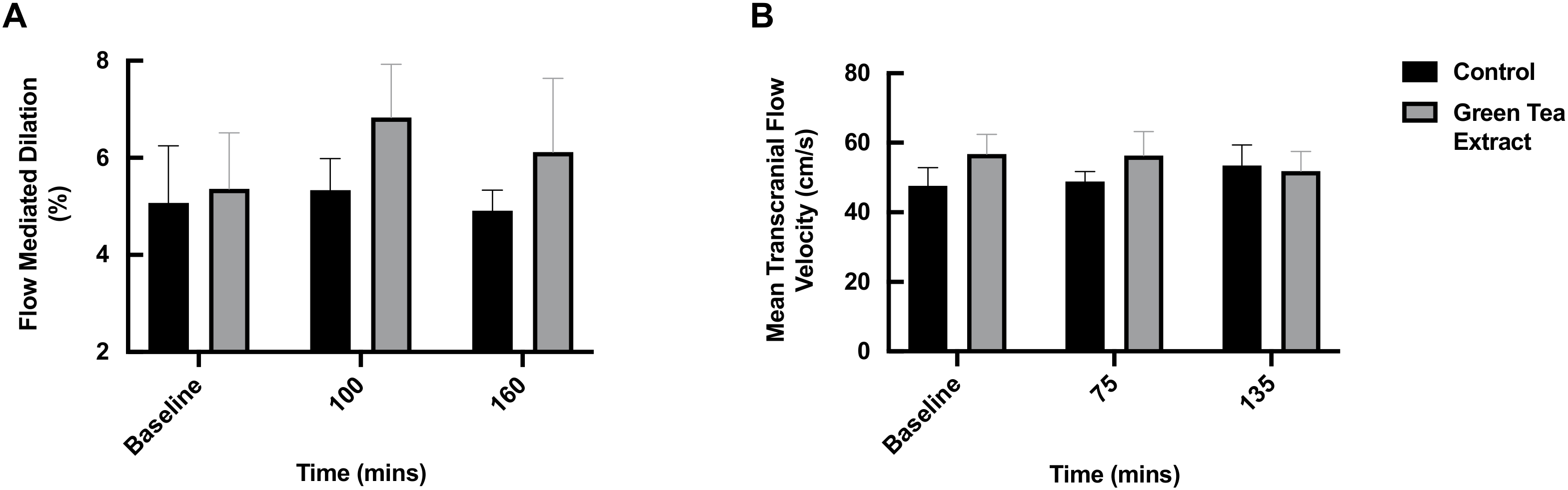
3.3. FMD and TCD
3.4. Blood Glucose and Plasma Insulin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrenson, L.; Poole, J.G.; Kim, J.; Brown, C.; Patel, P.; Richardson, R.S. Vascular and metabolic response to isolated small muscle mass exercise: Effect of age. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1023–H1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSouza, C.A.; Shapiro, L.F.; Clevenger, C.M.; Dinenno, F.A.; Monahan, K.D.; Tanaka, H.; Seals, D.R. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000, 102, 1351–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keske, M.A.; Ng, H.L.; Premilovac, D.; Rattigan, S.; Kim, J.A.; Munir, K.; Yang, P.; Quon, M.J. Vascular and metabolic actions of the green tea polyphenol epigallocatechin gallate. Curr. Med. Chem. 2015, 22, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, A.J.; Uberoi, A.; Wray, D.W.; Nishiyama, S.; Lawrenson, L.; Richardson, R.S. Differential effects of aging on limb blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H272–H278. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.R.; Lai, N.T.; Griffiths, K.A.; Molyneaux, L.M.; Yue, D.K.; Sullivan, D.R.; Celermajer, D.S. Meal-related increases in vascular reactivity are impaired in older and diabetic adults: Insights into roles of aging and insulin in vascular flow. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1404–H1410. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luukinen, H.; Koski, K.; Laippala, P.; Kivela, S.L. Factors predicting fractures during falling impacts among home-dwelling older adults. J. Am. Geriatr. Soc. 1997, 45, 1302–1309. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Laukkanen, P.; Heikkinen, E.; Kauppinen, M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing 1995, 24, 468–473. [Google Scholar] [CrossRef]
- Dunford, E.C.; Au, J.S.; Devries, M.C.; Phillips, S.M.; MacDonald, M.J. Cardiovascular aging and the microcirculation of skeletal muscle: Using contrast-enhanced ultrasound. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1194–H1199. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Phillips, B.E.; Williams, J.P.; Rankin, D.; Smith, K.; Lund, J.N.; Atherton, P.J. Development of a new Sonovue contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol. Rep. 2013, 1, e00119. [Google Scholar] [CrossRef]
- Phillips, B.; Williams, J.; Atherton, P.; Smith, K.; Hildebrandt, W.; Rankin, D.; Greenhaff, P.; Macdonald, I.; Rennie, M.J. Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J. Appl. Physiol. 2012, 112, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.E.; Atherton, P.J.; Varadhan, K.; Wilkinson, D.J.; Limb, M.; Selby, A.L.; Rennie, M.J.; Smith, K.; Williams, J.P. Pharmacological enhancement of leg and muscle microvascular blood flow does not augment anabolic responses in skeletal muscle of young men under fed conditions. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E168–E176. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; Clayton, Z.S.; Zigler, M.C.; Richey, J.J.; Cuevas, L.M.; Rossman, M.J.; Battson, M.L.; Ziemba, B.P.; Hutton, D.A.; VanDongen, N.S.; et al. Lifelong voluntary aerobic exercise prevents age-and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J. Physiol. 2020, 599, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhang, Z.; Zheng, T.; Yang, Y.J.; Li, N.; Bai, M.; Peng, Y.; Zhang, J.; Li, Q.; Zhang, B. Association of green tea consumption with risk of coronary heart disease in Chinese population. Int. J. Cardiol. 2015, 179, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, P.V.; Liu, D. Green tea catechins and cardiovascular health: An update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef] [Green Version]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A.; Group, J.S. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef]
- Lorenz, M.; Urban, J.; Engelhardt, U.; Baumann, G.; Stangl, K.; Stangl, V. Green and black tea are equally potent stimuli of NO production and vasodilation: New insights into tea ingredients involved. Basic Res. Cardiol. 2009, 104, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.L.H.; Premilovac, D.; Rattigan, S.; Richards, S.M.; Muniyappa, R.; Quon, M.J.; Keske, M.A. Acute vascular and metabolic actions of the green tea polyphenol epigallocatechin 3-gallate in rat skeletal muscle. J. Nutr. Biochem. 2017, 40, 23–31. [Google Scholar] [CrossRef]
- Kim, J.A.; Formoso, G.; Li, Y.; Potenza, M.A.; Marasciulo, F.L.; Montagnani, M.; Quon, M.J. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J. Biol. Chem. 2007, 282, 13736–13745. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.; Wessler, S.; Follmann, E.; Michaelis, W.; Dusterhoft, T.; Baumann, G.; Stangl, K.; Stangl, V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004, 279, 6190–6195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1378–E1387. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.D.; Garner, C.T.; Mumford, P.W.; Beck, D.T.; Roberts, M.D. Higher doses of a green tea-based supplement increase post-exercise blood flow following an acute resistance exercise bout in recreationally resistance-trained college-aged men. J. Int. Soc. Sports Nutr. 2020, 17, 27. [Google Scholar] [CrossRef]
- Nagaya, N.; Yamamoto, H.; Uematsu, M.; Itoh, T.; Nakagawa, K.; Miyazawa, T.; Kangawa, K.; Miyatake, K. Green tea reverses endothelial dysfunction in healthy smokers. Heart 2004, 90, 1485–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyama, J.; Maeda, T.; Kouzuma, K.; Ochiai, R.; Tokimitsu, I.; Higuchi, Y.; Sugano, M.; Makino, N. Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circ. J. 2010, 74, 578–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widlansky, M.E.; Hamburg, N.M.; Anter, E.; Holbrook, M.; Kahn, D.F.; Elliott, J.G.; Keaney, J.F., Jr.; Vita, J.A. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007, 26, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell, C.F.; Forster, J.S.; Veasey, R.C.; Kennedy, D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Hum. Psychopharmacol. 2012, 27, 177–186. [Google Scholar] [CrossRef]
- Jang, H.J.; Ridgeway, S.D.; Kim, J.A. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Kondo, Y.; Goto, A.; Noma, H.; Iso, H.; Hayashi, K.; Noda, M. Effects of Coffee and Tea Consumption on Glucose Metabolism: A Systematic Review and Network Meta-Analysis. Nutrients 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Josic, J.; Olsson, A.T.; Wickeberg, J.; Lindstedt, S.; Hlebowicz, J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial. Nutr. J. 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sian, T.S.; Din, U.S.U.; Deane, C.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Phillips, B.E.; et al. Cocoa Flavanols Adjuvant to an Oral Nutritional Supplement Acutely Enhances Nutritive Flow in Skeletal Muscle without Altering Leg Glucose Uptake Kinetics in Older Adults. Nutrients 2021, 13, 1646. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.E.; Atherton, P.J.; Varadhan, K.; Limb, M.C.; Williams, J.P.; Smith, K. Acute cocoa flavanol supplementation improves muscle macro- and microvascular but not anabolic responses to amino acids in older men. Appl. Physiol. Nutr. Metab. 2016, 41, 548–556. [Google Scholar] [CrossRef]
- Ullmann, U.; Haller, J.; Decourt, J.D.; Girault, J.; Spitzer, V.; Weber, P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int. J. Vitam. Nutr. Res. 2004, 74, 269–278. [Google Scholar] [CrossRef]
- Hadi, S.; Alipour, M.; Aghamohammadi, V.; Shahemi, S.; Ghafouri-Taleghani, F.; Pourjavidi, N.; Foroughi, M.; Chraqipoor, M. Improvement in fasting blood sugar, anthropometric measurement and hs-CRP after consumption of epigallocatechin-3-gallate (EGCG) in patients with type 2 diabetes mellitus. Nutr. Food Sci. 2019, 50, 348–359. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Bukhari, S.S.I.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P.; et al. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018, 37, 2011–2021. [Google Scholar] [CrossRef] [Green Version]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999, 100, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoberg, K.A.; Rattigan, S.; Hiscock, N.; Richter, E.A.; Kiens, B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: Real-time imaging in humans and rat. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H450–H458. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Purkayastha, S.; Sorond, F. Transcranial Doppler ultrasound: Technique and application. Semin. Neurol. 2012, 32, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Sorond, F.A.; Schnyer, D.M.; Serrador, J.M.; Milberg, W.P.; Lipsitz, L.A. Cerebral blood flow regulation during cognitive tasks: Effects of healthy aging. Cortex 2008, 44, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Englund, E.K.; Rodgers, Z.B.; Langham, M.C.; Mohler, E.R., 3rd; Floyd, T.F.; Wehrli, F.W. Simultaneous measurement of macro- and microvascular blood flow and oxygen saturation for quantification of muscle oxygen consumption. Magn. Reson. Med. 2018, 79, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.N.; Rabin, D.; Diamond, M.P.; Lacy, W.W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981, 30, 936–940. [Google Scholar] [CrossRef]
- Heiss, C.; Schroeter, H.; Balzer, J.; Kleinbongard, P.; Matern, S.; Sies, H.; Kelm, M. Endothelial function, nitric oxide, and cocoa flavanols. J. Cardiovasc. Pharm. 2006, 47 (Suppl. 2), S128–S135. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Phillips, B.E.; Wilkinson, D.J.; Williams, J.P.; Rankin, D.; Lund, J.N.; Smith, K.; Atherton, P.J. Supplementing essential amino acids with the nitric oxide precursor, l-arginine, enhances skeletal muscle perfusion without impacting anabolism in older men. Clin. Nutr. 2017, 36, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, W.; Machado, A.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Tokimitsu, I.; Hase, T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1550–R1556. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Liu, H.W.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. The green tea polyphenol epigallocatechin-3-gallate attenuates age-associated muscle loss via regulation of miR-486-5p and myostatin. Arch. Biochem. Biophys. 2020, 692, 108511. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Ishino, M.; Kitazawa, H.; Yoto, A.; Shimba, Y.; Mochizuki, Y.; Unno, K.; Meguro, S.; Tokimitsu, I.; Miura, S. Green tea extracts ameliorate high-fat diet-induced muscle atrophy in senescence-accelerated mouse prone-8 mice. PLoS ONE 2018, 13, e0195753. [Google Scholar] [CrossRef]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.M.; Stuart, S.; Boij, M.; Lexell, J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J. Appl. Physiol. 2002, 92, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, F.; Borg, K.; Edstrom, L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol. 1990, 80, 459–468. [Google Scholar] [CrossRef]
- Rattigan, S.; Clark, M.G.; Barrett, E.J. Hemodynamic actions of insulin in rat skeletal muscle: Evidence for capillary recruitment. Diabetes 1997, 46, 1381–1388. [Google Scholar] [CrossRef]
- Vincent, M.A.; Barrett, E.J.; Lindner, J.R.; Clark, M.G.; Rattigan, S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E123–E129. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Zock, P.L.; Draijer, R. Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS ONE 2011, 6, e16974. [Google Scholar] [CrossRef] [PubMed]
- Jochmann, N.; Lorenz, M.; Krosigk, A.; Martus, P.; Bohm, V.; Baumann, G.; Stangl, K.; Stangl, V. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br. J. Nutr. 2008, 99, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Rauhut, F.; Hofer, C.; Gwosc, S.; Muller, E.; Praeger, D.; Zimmermann, B.F.; Wernecke, K.D.; Baumann, G.; Stangl, K.; et al. Tea-induced improvement of endothelial function in humans: No role for epigallocatechin gallate (EGCG). Sci. Rep. 2017, 7, 2279. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Vlachopoulos, C.; Aznaouridis, K.; Baou, K.; Vasiliadou, C.; Pietri, P.; Xaplanteris, P.; Stefanadi, E.; Stefanadis, C. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Jowko, E. Green Tea Catechins and Sport Performance. In Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2015. [Google Scholar]
- Birdsill, A.C.; Carlsson, C.M.; Willette, A.A.; Okonkwo, O.C.; Johnson, S.C.; Xu, G.; Oh, J.M.; Gallagher, C.L.; Koscik, R.L.; Jonaitis, E.M.; et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity 2013, 21, 1313–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Klibanov, A.L.; Clark, M.G.; Rattigan, S.; Barrett, E.J. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004, 53, 1418–1423. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.Y.; Juan, C.C.; Hwang, L.S.; Hsu, Y.P.; Ho, P.H.; Ho, L.T. Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur. J. Nutr. 2004, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Juan, C.C.; Ho, L.T.; Hsu, Y.P.; Hwang, L.S. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J. Agric. Food Chem. 2004, 52, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Choi, H.S.; Kim, D.H.; Han, M.Y.; Chang, U.J.; Yim, S.V.; Song, B.C.; Kim, C.H.; Kang, S.A. Epigallocatechin gallate stimulates glucose uptake through the phosphatidylinositol 3-kinase-mediated pathway in L6 rat skeletal muscle cells. J. Med. Food 2008, 11, 429–434. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Li, Q.; Liang, J.; Dai, X.Q.; Ding, Y.; Wang, J.B.; Li, Y. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine 2010, 17, 14–18. [Google Scholar] [CrossRef]
- Ueda, M.; Nishiumi, S.; Nagayasu, H.; Fukuda, I.; Yoshida, K.; Ashida, H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 377, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Narumi, K.; Sonoda, J.; Shiotani, K.; Shigeru, M.; Shibata, M.; Kawachi, A.; Tomishige, E.; Sato, K.; Motoya, T. Simultaneous detection of green tea catechins and gallic acid in human serum after ingestion of green tea tablets using ion-pair high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2014, 945–946, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Okuda, S.; Miyazawa, T. Dose-dependent incorporation of tea catechins, (-)-epigallocatechin-3-gallate and (-)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 1997, 61, 1981–1985. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Johnell, K.; Laroche, M.L.; Fastbom, J.; Wastesson, J.W. The epidemiology of polypharmacy in older adults: Register-based prospective cohort study. Clin. Epidemiol. 2018, 10, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Volunteers (n = 12) |
|---|---|
| Gender (% males) | 42% |
| Age (year) | 74 ± 1 |
| Height (cm) | 168 ± 13 |
| Weight (kg) | 73.4 ± 13.1 |
| BMI (kg/m2) | 26.1 ± 2.5 |
| Lean mass (kg) | 45.7 ± 10.5 |
| Resting heart rate (bpm) | 62 ± 6 |
| Resting systolic blood pressure (mmHg) | 136 ± 11 |
| Resting diastolic blood pressure (mmHg) | 77 ± 8 |
| Grip strength (kg) | 27 ± 8 |
| SPPB | 9 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Din, U.S.U.; Sian, T.S.; Deane, C.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Atherton, P.J.; et al. Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults. Nutrients 2021, 13, 3895. https://doi.org/10.3390/nu13113895
Din USU, Sian TS, Deane CS, Smith K, Gates A, Lund JN, Williams JP, Rueda R, Pereira SL, Atherton PJ, et al. Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults. Nutrients. 2021; 13(11):3895. https://doi.org/10.3390/nu13113895
Chicago/Turabian StyleDin, Ushnah S. U., Tanvir S. Sian, Colleen S. Deane, Ken Smith, Amanda Gates, Jonathan N. Lund, John P. Williams, Ricardo Rueda, Suzette L. Pereira, Philip J. Atherton, and et al. 2021. "Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults" Nutrients 13, no. 11: 3895. https://doi.org/10.3390/nu13113895
APA StyleDin, U. S. U., Sian, T. S., Deane, C. S., Smith, K., Gates, A., Lund, J. N., Williams, J. P., Rueda, R., Pereira, S. L., Atherton, P. J., & Phillips, B. E. (2021). Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults. Nutrients, 13(11), 3895. https://doi.org/10.3390/nu13113895










