Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of M. cochinchinensis Extracts
2.1.1. General Procedure
2.1.2. Preparation of Solvent Extract of Liquid Nitrogen-Treated Aril
2.1.3. Preparation of ASE (S)
2.1.4. Preparation of ASE (L)
2.1.5. Preparation of ASE-Nontreat
2.2. Total Polyphenol Content of M. cochinchinensis
2.3. Total Flavonoid Content of M. cochinchinensis
2.4. Melan-A Cell Culture
2.5. Cell Viability
2.6. Melanin Content Assay
2.7. Tyrosinase Activity Assay
2.8. Western Blotting
2.9. Real-Time Polymerase Chain Reaction (RT-qPCR)
2.10. Statistical Analysis
3. Results
3.1. Total Phenol and Flavonoid Contents in Parts of M. cochinchinensis
3.2. M. cochinchinensis Suppresses Tyrosinase Activity
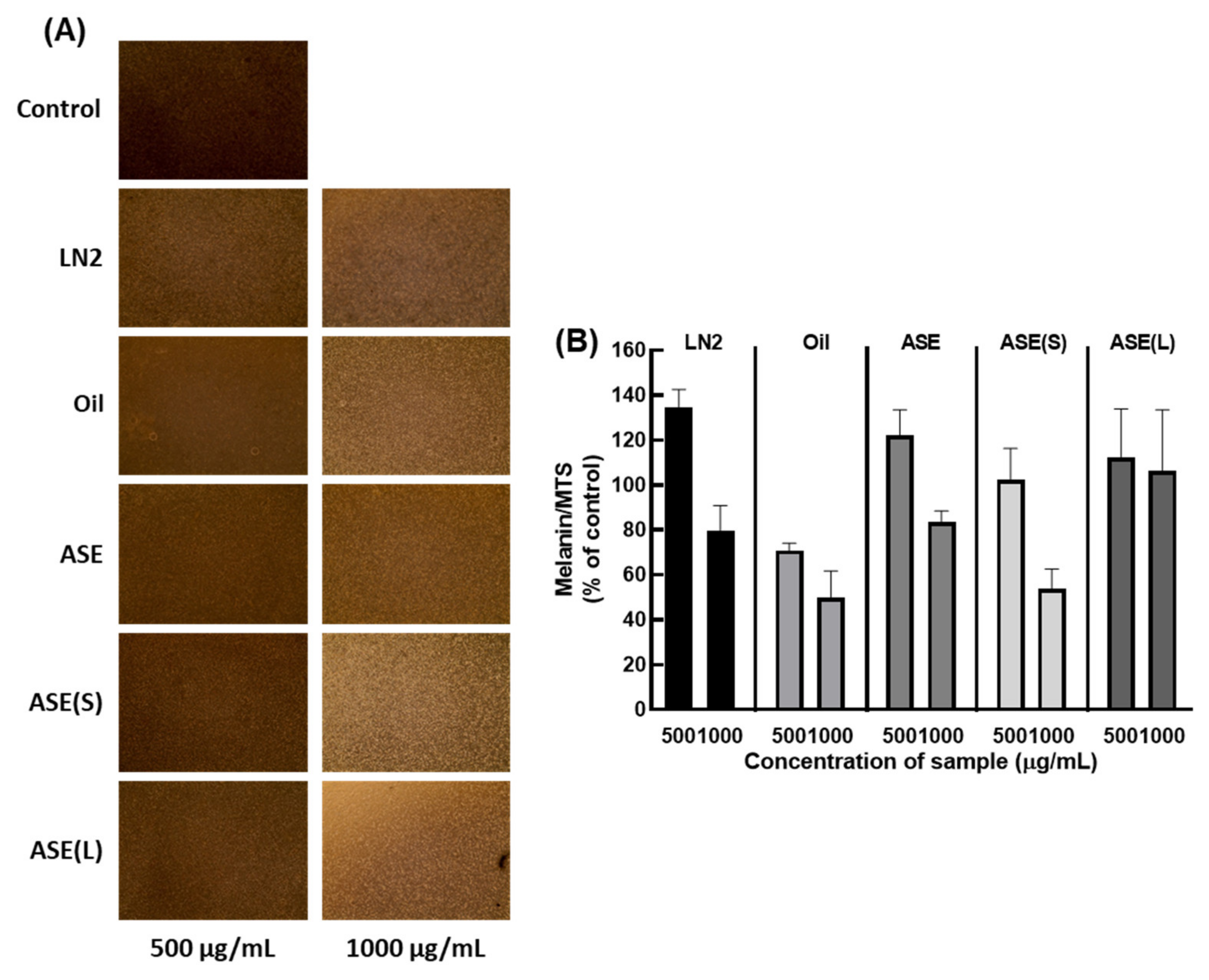
3.3. M. cochinchinensis Reduces Melanin Content in Live Cells
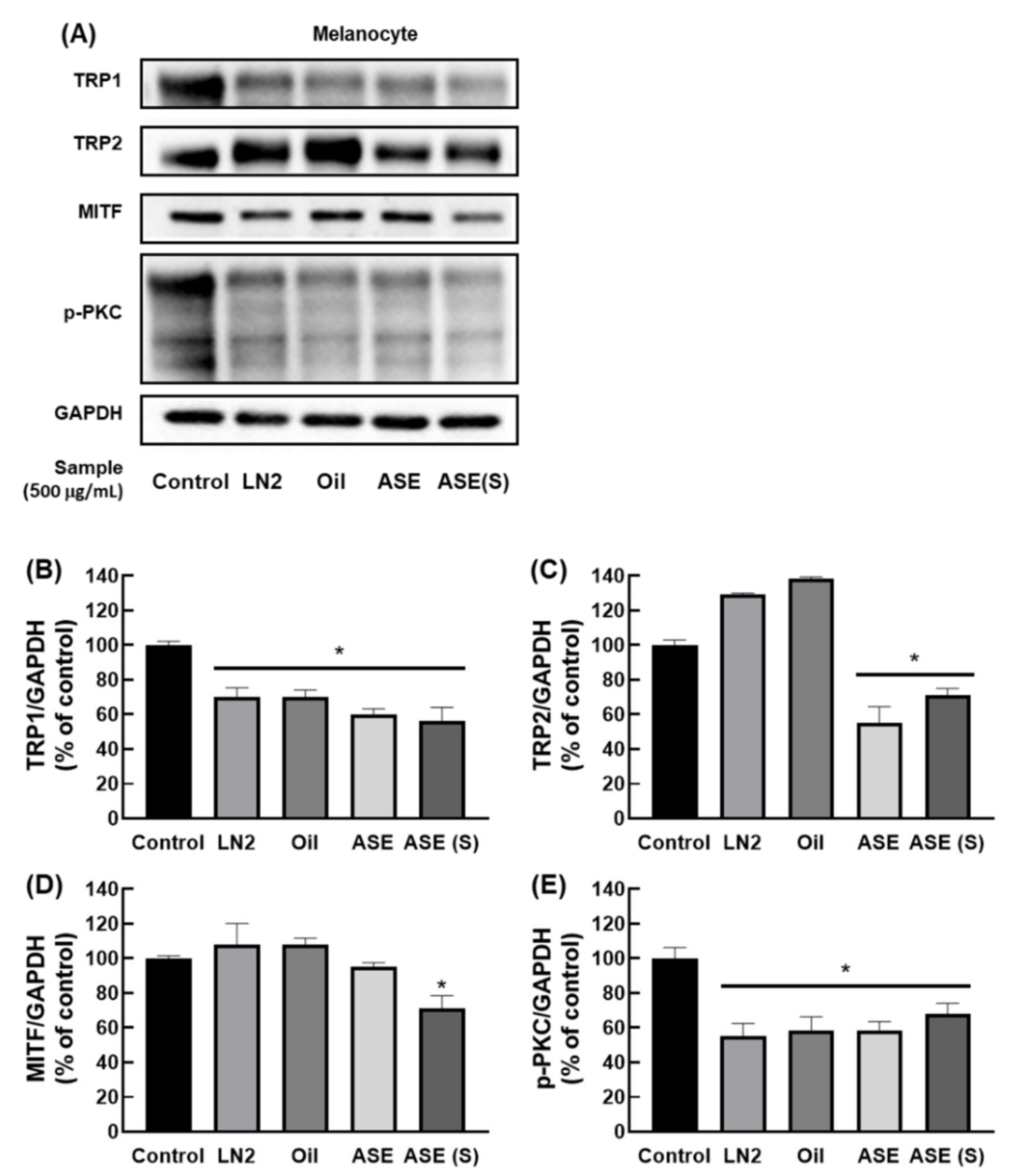
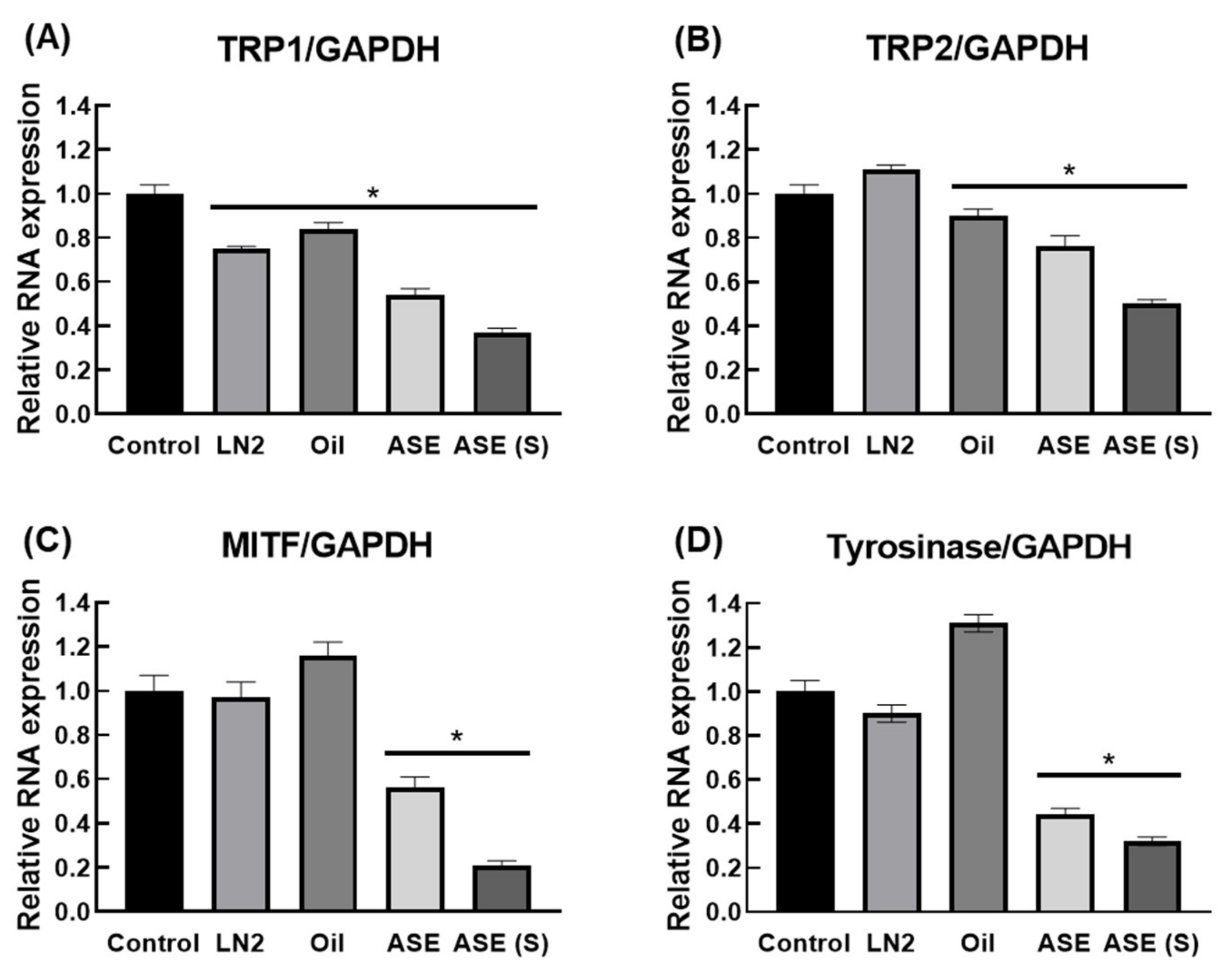
3.4. M. cochinchinensis Decreases Downstream Signal Transduction Protein Levels of the p-PKC Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Hu, X.; Zhang, B.; Wang, Z.; Hao, C.; Xin, J.; Guo, Q. Whitening Activity of Constituents Isolated from the Trichosanthes Pulp. Evid.-Based Complement. Altern. Med. 2020, 2020, 2582579. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, N.P.; Van Nieuwpoort, F.A.; Marrot, L.; Out, C.; Poorthuis, B.; Van Pelt, H.; Meunier, J.R.; Pavel, S. Increased melanogenesis is a risk factor for oxidative DNA damage—Study on cultured melanocytes and atypical nevus cells. Photochem. Photobiol. 2008, 84, 550–555. [Google Scholar] [CrossRef]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Investig. Dermatol. 2011, 131, E8–E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [Green Version]
- Toricelli, M.; Melo, F.H.; Hunger, A.; Zanatta, D.; Strauss, B.E.; Jasiulionis, M.G. Timp1 promotes cell survival by activating the PDK1 signaling pathway in melanoma. Cancers 2017, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Alqarni, M.H.; Alam, P.; Shakeel, F.; Foudah, A.I.; Alshehri, S. Highly Sensitive and Ecologically Sustainable Reversed-Phase HPTLC Method for the Determination of Hydroquinone in Commercial Whitening Creams. Processes 2021, 9, 1631. [Google Scholar] [CrossRef]
- Whangsomnuek, N.; Mungmai, L.; Mengamphan, K.; Amornlerdpison, D. Bioactive compounds of aqueous extracts of flower and leaf of Etlingera elatior (Jack) RM Sm. for cosmetic application. Maejo Int. J. Sci. Technol. 2019, 13, 196–208. [Google Scholar]
- Kooyers, T.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Ishida, B.K.; Turner, C.; Chapman, M.H.; McKeon, T.A. Fatty acid and carotenoid composition of gac (Momordica cochinchinensis Spreng) fruit. J. Agric. Food Chem. 2004, 52, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.T. Overview of Vietnamese traditional medicine. Bull. Food Technol. 2005, 18, 91–97. [Google Scholar]
- Aoki, H.; Kieu, N.T.M.; Kuze, N.; Tomisaka, K.; Chuyen, N.V. Carotenoid Pigments in GAC fruit (Momordica cochinchinensis SPRENG). Biosci. Biotechnol. Biochem. 2002, 66, 2479–2482. [Google Scholar] [CrossRef] [Green Version]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Parks, S.E.; Stathopoulos, C. Gac fruit: Nutrient and phytochemical composition, and options for processing. Food Rev. Int. 2013, 29, 92–106. [Google Scholar] [CrossRef]
- Tinrat, S.; Akkarachaneeyakorn, S.; Singhapol, C. Evaluation of antioxidant and antimicrobial activities of Momordica Cochinchinensis Spreng (Gac fruit) ethanolic extract. Int. J. Pharm. Sci. Res. 2014, 5, 3163–3169. [Google Scholar]
- Do, T.V.T.; Fan, L.; Suhartini, W.; Girmatsion, M. Gac (Momordica cochinchinensis Spreng) fruit: A functional food and medicinal resource. J. Funct. Foods 2019, 62, 103512. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef]
- Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J. Inflamm. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-Y.; Russakovsky, V.; Ohno, S.; Gilchrest, B. The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. J. Biol. Chem. 1993, 268, 11742–11749. [Google Scholar] [CrossRef]
- Park, H.; Gonzalez, S.; Lee, J.; Middelkamp-Hup, M.; Kaposi, S.; Peterson, S.; Gilchrest, B. Topical application of a PKC inhibitor reduces skin pigmentation. J. Investig. Dermatol. 2002, 119, 337. [Google Scholar]
- Imokawa, G.; Ishida, K. Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [Green Version]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S. Polyphenols, Flavonoids, and Antioxidant Activity Involved in Salt Tolerance in Wheat, Aegilops cylindrica and Their Amphidiploids. Front. Plant Sci. 2021, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C.; Koplan, J.P.; Nugent, R.; Dusenbury, C.; Puska, P.; Gaziano, T.A. Prevention of chronic disease by means of diet and lifestyle changes. In Disease Control Priorities in Developing Countries, 2nd ed.; The Work Bank: New York, NY, USA, 2006; Chapter 44. [Google Scholar]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol compounds and PKC signaling. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-G.; Kim, J.-E.; Lee, S.-Y.; Park, J.S.; Yeom, M.H.; Chen, H.; Bode, A.M.; Dong, Z.; Lee, K.W. The daidzein metabolite, 6,7,4′-Trihydroxyisoflavone, is a novel inhibitor of PKCα in suppressing solar UV-induced matrix metalloproteinase 1. Int. J. Mol. Sci. 2014, 15, 21419–21432. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Leevutinun, P.; Krisadaphong, P.; Petsom, A.J.J.C.S. Clinical evaluation of Gac extract (Momordica cochinchinensis) in an antiwrinkle cream formulation. J. Cosmet. Sci. 2015, 66, 175–187. [Google Scholar] [PubMed]
- Reungpatthanaphong, S.; Chawananorasest, K.; Kirdin, T.; Bamrungchai, M.; Reungpatthanaphong, P. Development of Thai Gac Fruit Extraction as a Multifunctional Cosmeceutical Ingredient for Antioxidant, Melanogenesis and Collagen Stimulating Activities. Key Eng. Mater. 2019, 819, 104–110. [Google Scholar] [CrossRef]
- Iwata, M.; Corn, T.; Iwata, S.; Everett, M.A.; Fuller, B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990, 95, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darias, M.J.; Andree, K.B.; Boglino, A.; Fernández, I.; Estévez, A.; Gisbert, E. Coordinated regulation of chromatophore differentiation and melanogenesis during the ontogeny of skin pigmentation of Solea senegalensis (Kaup, 1858). PLoS ONE 2013, 8, e63005. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Transcript | Forward/ Reverse | Primer Sequence | Annealing Temp. (°C) |
|---|---|---|---|
| MITF | F | 5’-GGTGGATGGGATAAGGGAAAG-3’ | 54.9 |
| R | 5’-AGGACCTTGAAAACCGACAG-3’ | 55.1 | |
| TRP-1 | F | 5’-GGTCTCCCTACATTTCCAGC-3’ | 55 |
| R | 5’-AGCCCCAACTCTGTCTTTTC-3’ | 55.1 | |
| TRP-2 | F | 5’-GGAAGGAGTGAGCCAAGTTATG-3’ | 54.9 |
| R | 5’-TCCAGAAGTTTGACAGCCC-3’ | 55.4 | |
| Tyrosinase | F | 5’-GGGTTTTGGCTTTGTCATGG-3’ | 55.2 |
| R | 5’-CTAACTTACTCAGCCCAGCATC-3’ | 54.9 | |
| GAPDH | F | 5′-GCCAAGGTCATCCATGACAAC-3′ | 59.9 |
| R | 5′-GTCCACCACCCTGTTGCTGTA-3′ | 56.4 |
| LN2 | Oil | ASE | ASE(S) | ASE(L) | |
|---|---|---|---|---|---|
| Total polyphenol content (mg GAE/mL) | 1.16 ± 1.06 | - | 4.97 ± 0.96 | 5.54 ± 0.97 | 3.04 ± 1.12 |
| Total flavonoids content (mg catechin/mL) | 16.31 ± 6.38 | 11.19 ± 4.42 | - | - | 4.52 ± 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Hong, S.-C.; Lee, E.H.; Lee, J.W.; Yang, S.-H.; Kim, J.-C. Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell. Nutrients 2021, 13, 3894. https://doi.org/10.3390/nu13113894
Kim J, Hong S-C, Lee EH, Lee JW, Yang S-H, Kim J-C. Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell. Nutrients. 2021; 13(11):3894. https://doi.org/10.3390/nu13113894
Chicago/Turabian StyleKim, Juyong, Sung-Chul Hong, Eun Ha Lee, Jae Wook Lee, Seung-Hoon Yang, and Jin-Chul Kim. 2021. "Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell" Nutrients 13, no. 11: 3894. https://doi.org/10.3390/nu13113894
APA StyleKim, J., Hong, S.-C., Lee, E. H., Lee, J. W., Yang, S.-H., & Kim, J.-C. (2021). Preventive Effect of M. cochinchinensis on Melanogenesis via Tyrosinase Activity Inhibition and p-PKC Signaling in Melan-A Cell. Nutrients, 13(11), 3894. https://doi.org/10.3390/nu13113894








