Effect of Geumgwe-Sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Geumgwe-Sinkihwan
2.2. Experimental Animals
2.3. Measurement of Plasma and Urine Biochemical Parameters
2.4. Morphological Measurement of Renal Tissues
2.5. Western Blot Analysis
2.6. Quantitative Real-Time Reverse Transcription-PCR (Real-Time RT-qPCR)
2.7. Immunohistochemical Analysis of Renal Tissues
2.8. Statistical Analysis
3. Results
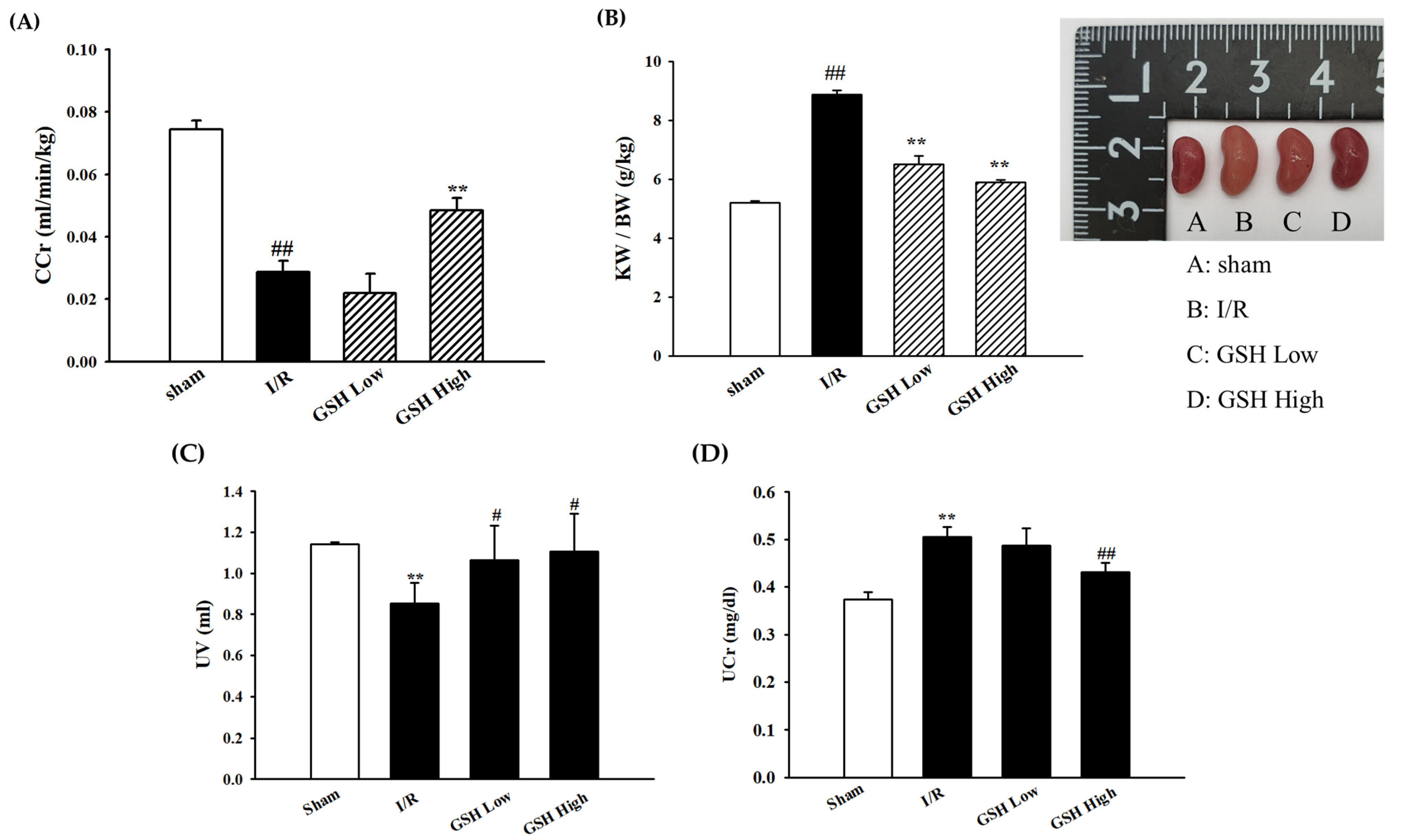
3.1. Effect of GSH on Renal Function Parameters
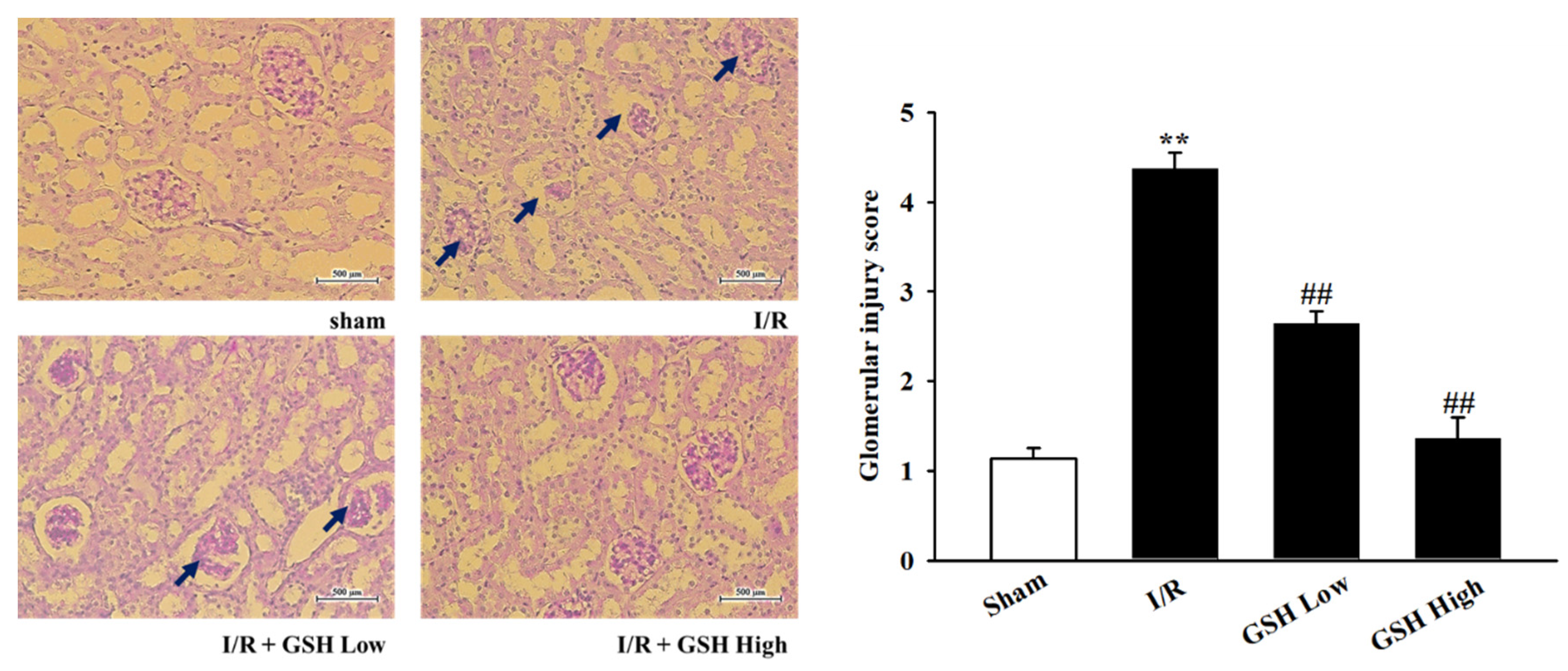
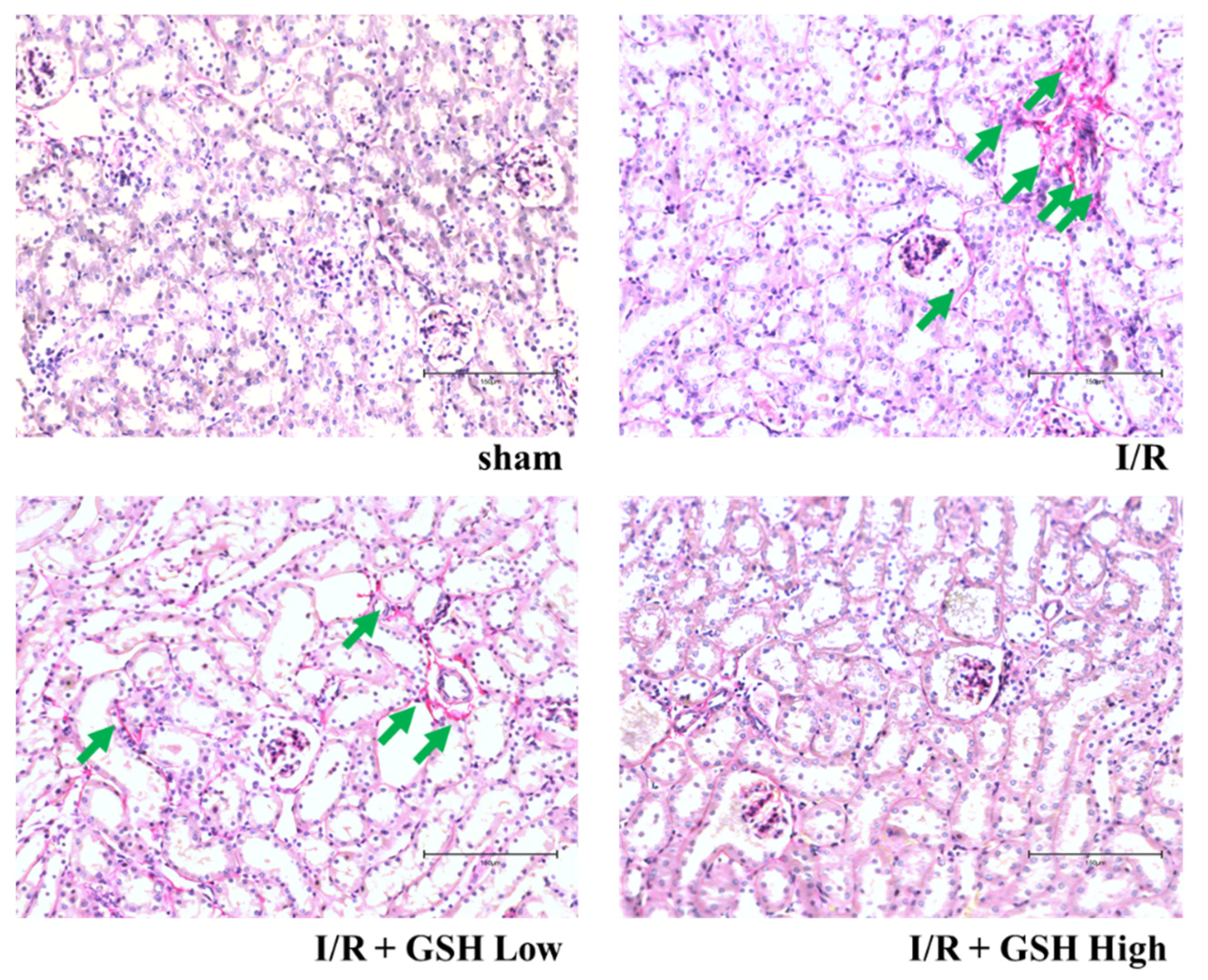
3.2. Effect of GSH on Renal Morphology and Renal Fibrosis
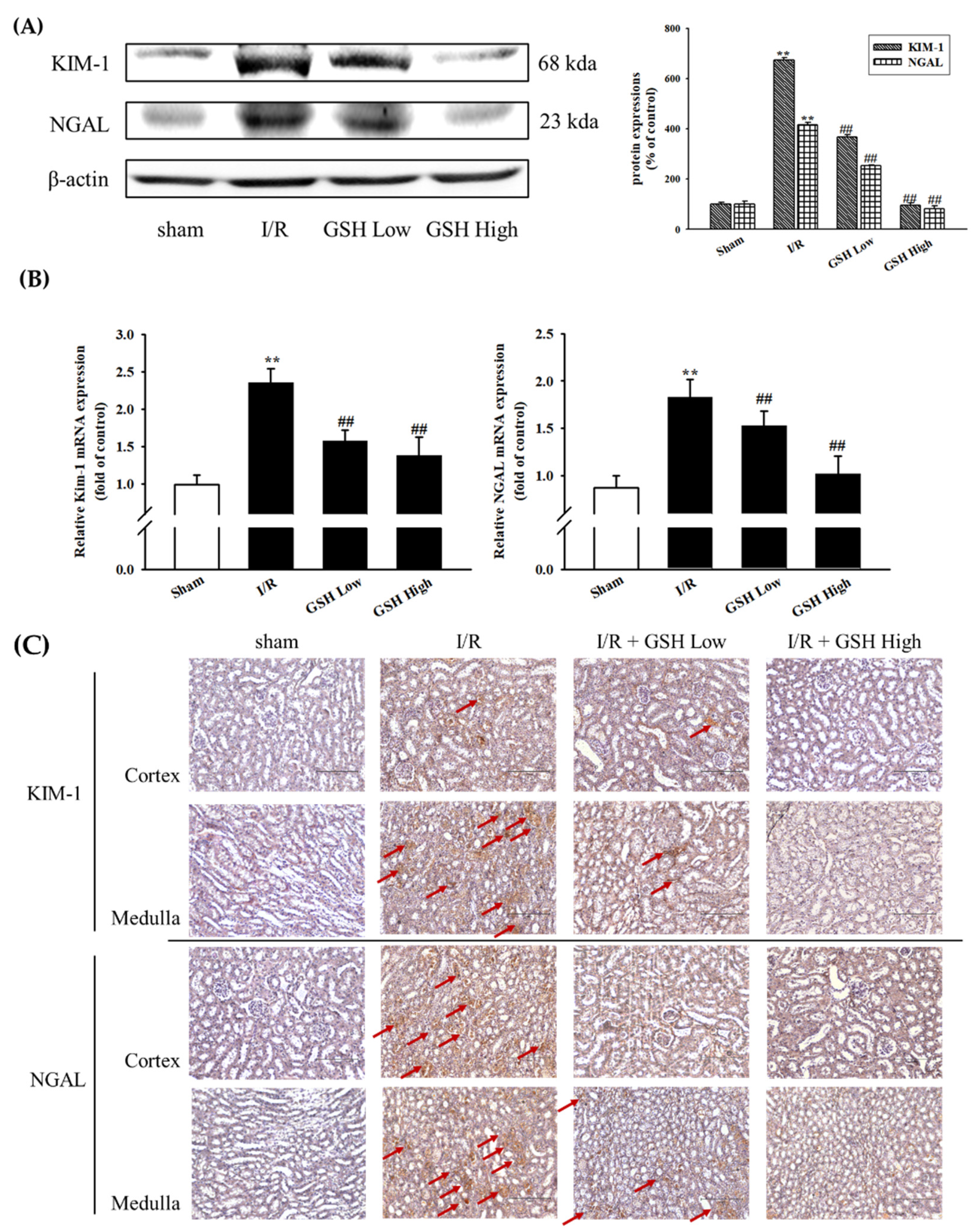
3.3. Effect of GSH on I/R-Induced Renal Nephropathy
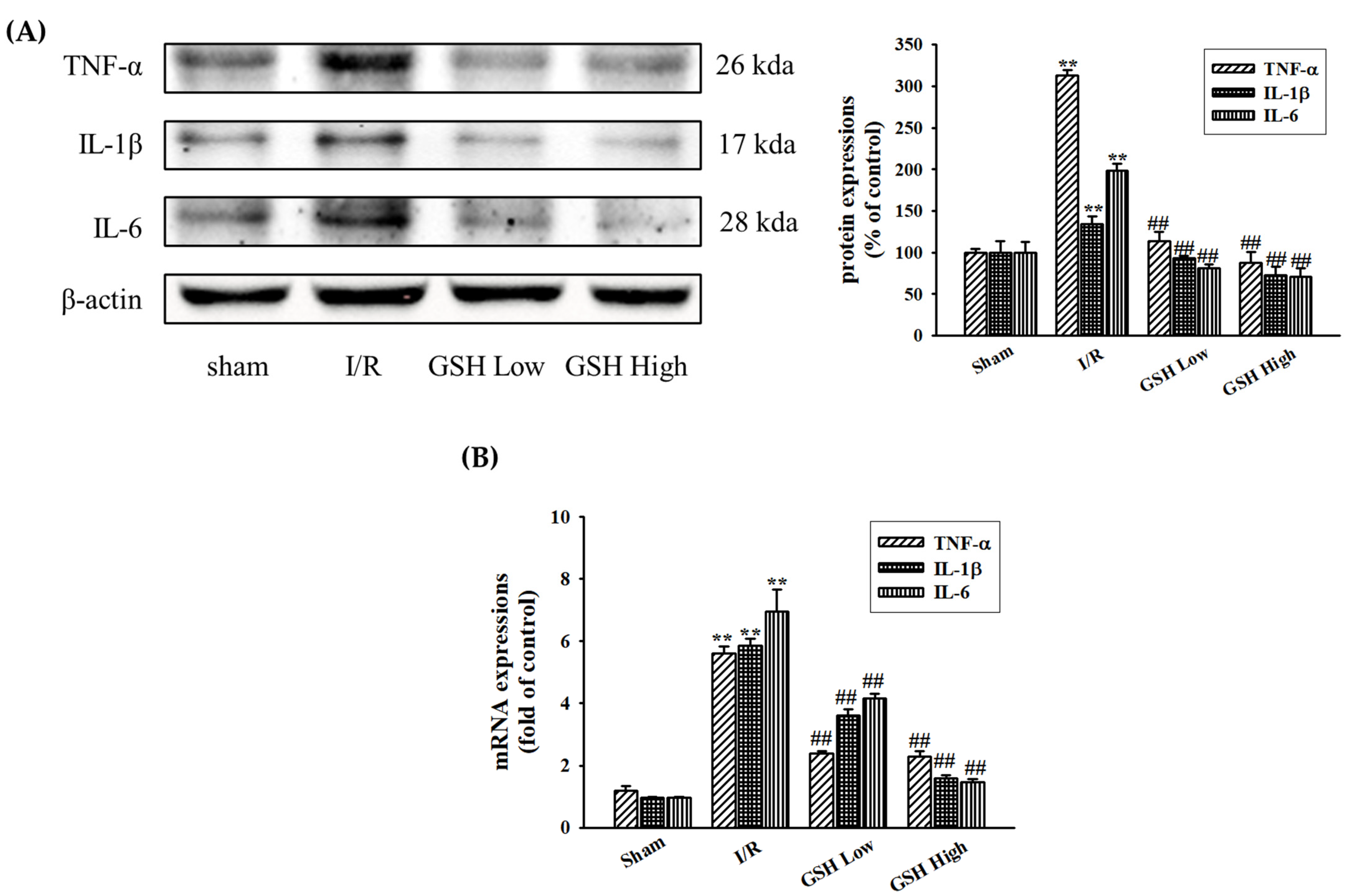
3.4. Effect of GSH on Renal Inflammatory Cytokines
3.5. Effect of GSH on Regulation of NLRP3 Inflammasome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez-Llama, P.; Andrews, P.; Nielsen, S.; Ecelbarger, C.A.; Knepper, M.A. Impaired aquaporin and urea transporter expression in rats with adriamicine-induced nephrotic syndrome. Kidney Int. 1998, 53, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Moses, A.M.; Miller, M.; Streeten, D.H. Pathophysiologic and pharmacologic alterations in the release and action of ADH. Metabolism 1976, 25, 697–721. [Google Scholar] [CrossRef]
- Lee, J.; Kang, D.G.; Kim, Y. Increased expression and shuttling of aquaporin-2 water channels in the kidney in DOCA-salt hypertensive rats. Clin. Exp. Hypertens. 2000, 22, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Kurtz, T.W.; Rosenzweig, J.; Weller, J.M. Renal hemodynamics in HgCl2-induced acute renal failure. Nephron 1977, 18, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Bagshaw, S.M. Normotensive ischemic acute renal failure. N. Engl. J. Med. 2007, 357, 2205–2206. [Google Scholar]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [Green Version]
- Thadhani, R.; Pascual, M.; Bonventre, J.V. Acute renal failure. N. Engl. J. Med. 1996, 334, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef]
- Fernandez-Llama, P.; Andrews, P.; Turner, R.; Saggi, S.; Dimari, J.; Kwon, T.H.; Nielsen, S.; Safirstein, R.; Knepper, M.A. Decreased abundance of collecting duct aquaporins in post-ischemic renal failure in rats. J. Am. Soc. Nephrol. 1999, 10, 1658–1668. [Google Scholar]
- Kwon, T.H.; Frokiaer, J.; Fernandez-Llama, P.; Knepper, M.A.; Nielsen, S. Reduced abundance of aquaporins in rats with bilateral ischemia/reperfusion-induced acute renal failure: Prevention by alpha-MSH. Am. J. Physiol. 1999, 277, 413–427. [Google Scholar]
- Kim, S.W.; Jeon, Y.S.; Lee, J.U.; Kang, D.G.; Kook, H.; Ahn, K.Y.; Kim, S.Z.; Cho, K.W.; Kim, N.H.; Han, J.S.; et al. Diminished adenylate cyclase activity and aquaporin 2 expression in acute renal failure rats. Kidney Int. 2000, 57, 1643–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höcherl, K.; Schmidt, C.; Kurt, B.; Bucher, M. Inhibition of NF-κB ameliorates sepsis induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am. J. Physiol. 2010, 298, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, M.; Mik, E.G.; Johannes, T.; Payen, D.; Ince, C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol. Med. 2008, 14, 502–516. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin--an emerging troponin for kidney injury. Nephrol. Dial. Transplant. 2008, 23, 3737–3743. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Lee, H.T.; Rapoport, D.; Drexler, I.R.; Foster, K.; Yang, J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig. 2005, 115, 610–621. [Google Scholar] [CrossRef]
- Merika, L.; Rasić, S.; Serdarević, N. Importance of determination of urine neutrophile gelatinase associated lipocalin in early detection of acute kidney injury. Coll. Antropol. 2014, 38, 161–166. [Google Scholar]
- Kidher, E.; Harling, L.; Ashrafian, H.; Naase, H.; Chukwuemeka, A.; Anderson, J. Pulse wave velocity and neutrophil gelatinase-associated lipocalin as predictors of acute kidney injury following aortic valve replacement. J. Cardiothorac. Surg. 2014, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Solez, K.; Colvin, R.B.; Racusen, L.C.; Sis, B.; Halloran, P.F.; Birk, P.E. Banff ‘05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am. J. Transplant. 2007, 7, 518–526. [Google Scholar] [CrossRef]
- Yang, X.J.; Wu, Y.; Li, Q.Q.; Zhang, G.F.; Wang, M.; Yang, H.P.; Li, Q. CD36 promotes podocyte apoptosis by activating the pyrin domain-containing-3 (NLRP3) inflammasome in primary nephrotic syndrome. Med. Sci. Monit. 2018, 24, 6832–6839. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Ogura, Y.; Flavell, R.A. The inflammasome in pathogen recognition and inflammation. J. Leukoc. Biol. 2007, 82, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Yang, H.; Zhang, L.; Yang, B.; Wang, M.; Li, Q. Interleukin-17A participates in podocyte injury by inducing IL-1β secretion through ROS-NLRP3 inflammasome-caspase-1 pathway. Scand. J. Immunol. 2018, 87, e12645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.H.; Lee, H.K.; Jang, S.H.; Tai, A.L.; Yoon, J.J.; Kim, H.Y.; Lee, Y.J.; Lee, H.S.; Kang, D.G. Effect of Jesaeng-sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mouse. Korean J. Orient Med. Prescr. 2021, 29, 33–44. [Google Scholar]
- Liu, S.S.; Chen, Y.Y.; Wang, S.X.; Yu, Q. Protective effect of dabrafenib on renal ischemia-reperfusion injury in vivo and in vitro. Biochem. Biophys. Res. Commun. 2020, 522, 395–401. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jeon, Y.S.; Kim, J.H.; Kim, S.H. Study on antitumor activity and antimetastatic effect of Kamigumguesingihwan (KGSH). J. Korean Orient. Oncol. 1999, 5, 19–32. [Google Scholar]
- Edelstein, C.L. Biomarkers of acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Han, W.K.; Bonventre, J.V. Biologic markers for the early detection of acute kidney injury. Curr. Opin. Crit. Care 2004, 10, 476–482. [Google Scholar] [CrossRef]
- Jai, P.; Martin, H.B.; Marie, L.; Frank, O.; Pieter, A.K.; Harry, V.G. Inhibition of renal rho kinase attenuates ischemia/reperfusion-induced injury. J. Am. Soc. Nephrol 2008, 191, 368–375. [Google Scholar]
- Fioretto, P.; Mauer, M. Histopathology of diabetic nephropathy. Semin. Nephrol. 2007, 27, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Border, W.A.; Noble, N.A. TGF-beta in kidney fibrosis: A target for gene therapy. Kidney Int. 1997, 51, 1388–1396. [Google Scholar] [CrossRef] [Green Version]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Das, M.; Das, D.K. Molecular mechanism of preconditioning. IUBMB Life 2008, 60, 199–203. [Google Scholar] [CrossRef]
- Fu, W.J.; Li, B.L.; Wang, S.B.; Chen, M.L.; Deng, R.T.; Ye, C.Q. Changes of the tubular markers in type 2 diabetes mellitus with glomerular hyperfiltration. Diabetes Res. Clin. Pract. 2012, 95, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Filho, L.T.; Grande, A.J.; Colonetti, T.; Della, E.S.P.; Rosa, M.I.D. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Pediatr. Nephrol. 2017, 10, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P.; Mishra, J.; Supavekin, S.; Patterson, L.T.; Steven, P.S. Gene expression in early ischemic renal injury: Clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol. Genet. Metab. 2003, 80, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.T.; Lv, L.L.; Pan, M.M.; Wen, Y.; Wang, B.; Li, Z.I.; Wu, M.; Wang, F.A.; Crowley, S.D.; Liu, B.C. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018, 9, 351. [Google Scholar] [CrossRef]
- Xiang, H.; Zhu, F.; Xu, Z.F.; Xoing, J. Role of inflammasomes in kidney diseases via both canonical and non-canonical pathways. Front. Cell Dev. Biol. 2020, 8, 106. [Google Scholar] [CrossRef] [Green Version]

| Herbal Name (Part) | Amount (g) | Origin |
|---|---|---|
| Rehmannia glutinosa (rhizome) 熟地黃 | 320 | Imsil, Korea |
| Discorea batatas (rhizome) 山藥 | 160 | Imsil, Korea |
| Cornus officinalis (fruit) 山茱萸 | 160 | Imsil, Korea |
| Poria cocos (Schw.) Wolf (Hoelen) 白茯笭 | 120 | Imsil, Korea |
| Alisma orientale (rhizome) 澤瀉 | 120 | Imsil, Korea |
| Paeomia suffructicosa (cortex) 牧丹皮 | 120 | Imsil, Korea |
| Achyranthes aspera (ro桔梗ot) 牛膝 | 120 | Imsil, Korea |
| Plantago ovata (root) 桔梗 | 120 | Imsil, Korea |
| Total | 1240 |
| Group | BUN (mg/dL) | Cr (mg/dL) |
|---|---|---|
| sham | 29.2 ± 0.55 | 0.22 ± 0.01 |
| I/R | 51.96 ± 7.43 # | 0.40 ± 0.04 ## |
| GSH Low | 36.46 ± 3.62 ** | 0.32 ± 0.02 ** |
| GSH High | 28.56 ± 0.61 ** | 0.34 ± 0.01 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.H.; Lee, H.K.; Jang, S.H.; Tai, A.L.; Jang, Y.J.; Yoon, J.J.; Kim, H.Y.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Effect of Geumgwe-Sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mice. Nutrients 2021, 13, 3859. https://doi.org/10.3390/nu13113859
Han BH, Lee HK, Jang SH, Tai AL, Jang YJ, Yoon JJ, Kim HY, Lee HS, Lee YJ, Kang DG. Effect of Geumgwe-Sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mice. Nutrients. 2021; 13(11):3859. https://doi.org/10.3390/nu13113859
Chicago/Turabian StyleHan, Byung Hyuk, Hyeon Kyoung Lee, Se Hoon Jang, Ai Lin Tai, Youn Jae Jang, Jung Joo Yoon, Hye Yoom Kim, Ho Sub Lee, Yun Jung Lee, and Dae Gill Kang. 2021. "Effect of Geumgwe-Sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mice" Nutrients 13, no. 11: 3859. https://doi.org/10.3390/nu13113859
APA StyleHan, B. H., Lee, H. K., Jang, S. H., Tai, A. L., Jang, Y. J., Yoon, J. J., Kim, H. Y., Lee, H. S., Lee, Y. J., & Kang, D. G. (2021). Effect of Geumgwe-Sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mice. Nutrients, 13(11), 3859. https://doi.org/10.3390/nu13113859






