Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
- Panax japonicus (T. Nees) C.A. Mey., strain 62, in cell culture passport and some earlier publications referenced to as P. japonicus var. repens [33];
- Dioscorea deltoidea Wall. ex Griseb., super-producer of furostanol-type steroidal glycosides, strain DM-05–03 [26];
- Tribulus terrestris L., strain Tter8 [27].
2.2. Bioreactor Cultivation of Cell Suspensions, Biomass Preparation and Phytochemical Analysis
- Laboratory-scale bioreactors: 20 L (15 L working volume) glass bubble-type bioreactors custom-designed at the Department of cell biology and biotechnology, Institute of Plant Physiology, Moscow, Russia;
- Industrial-scale bioreactors: 630 L (550 L working volume) bubble-type bioreactors of 1T series (CUC “EBEE”, Yoshkar-Ola, Russia) with air supply through ring-type gas distributor ∅ 750 mm (Supplementary Materials Figure S1).
2.3. Evaluation of the Hypoglycemic Activity of Cell Biomass Preparations
2.3.1. Laboratory Animal Husbandry
2.3.2. Collection and Evaluation of Biological Material
2.3.3. Adrenaline Model of Hyperglycemia
2.3.4. Experimental Type 2 Diabetes Model (T2DM)
2.4. Statistical Data Processing
3. Results
3.1. Cell Culture Growth in Bioreactors and Their Biochemical Composition
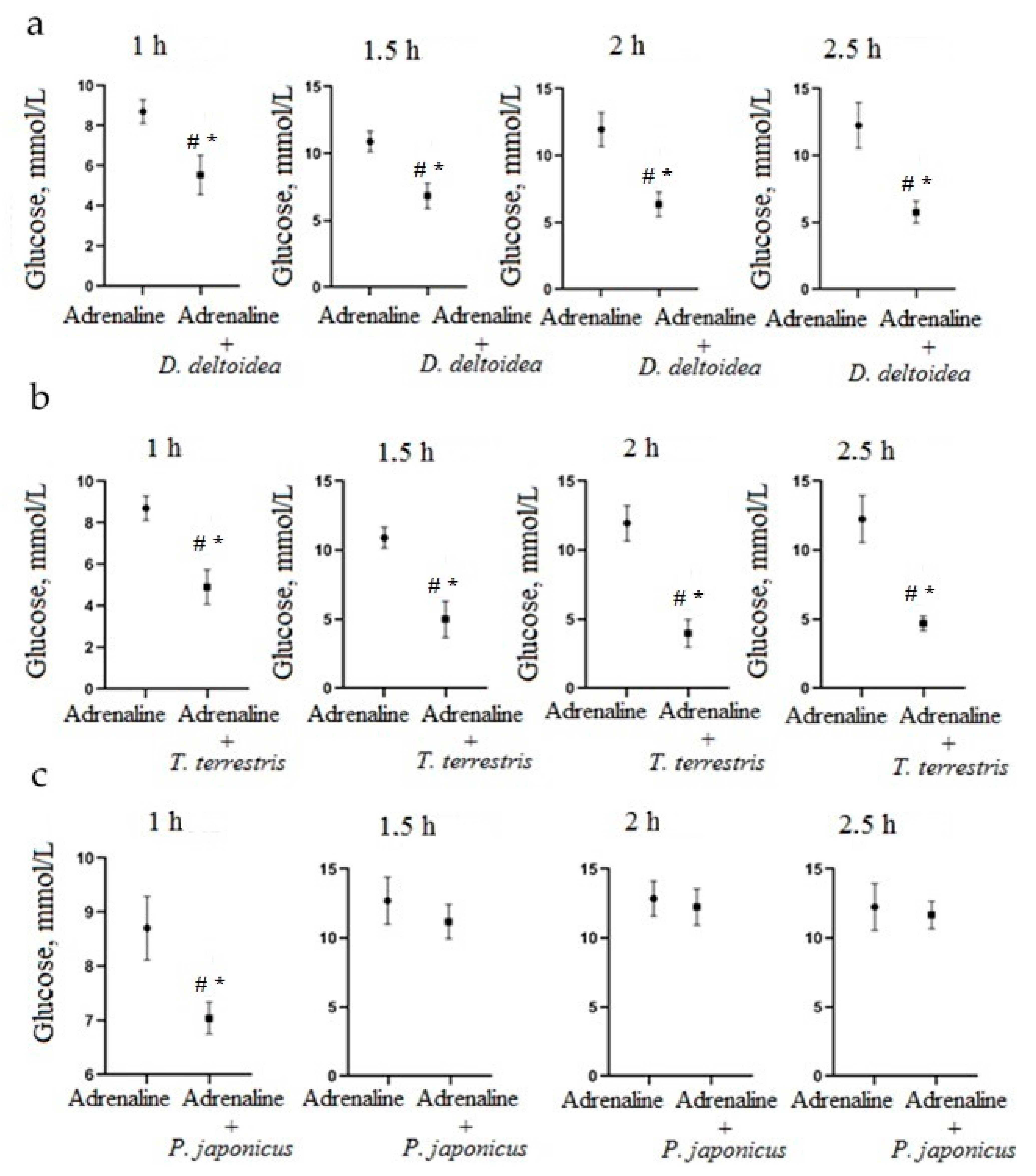
3.2. Hypoglycemic Activity of Cell Culture Biomass in an Adrenaline Hyperglycemia Model
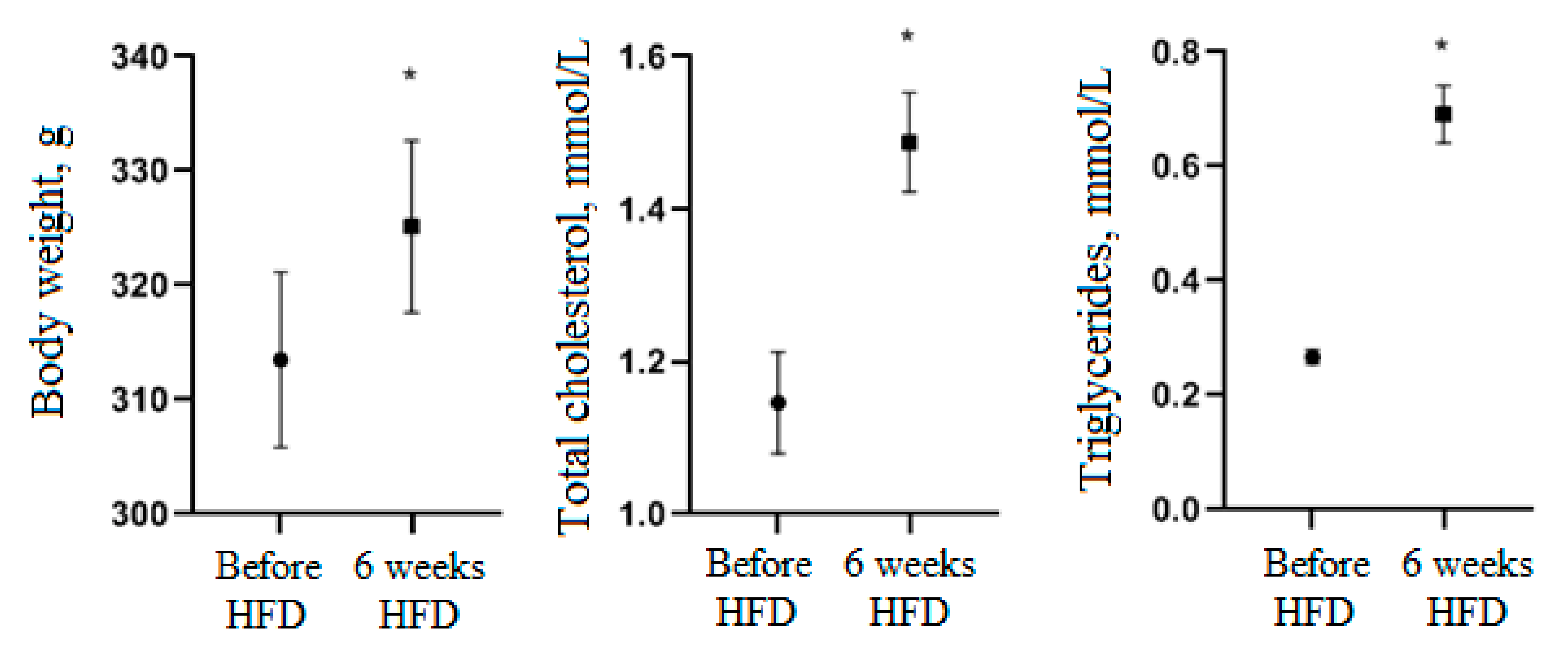
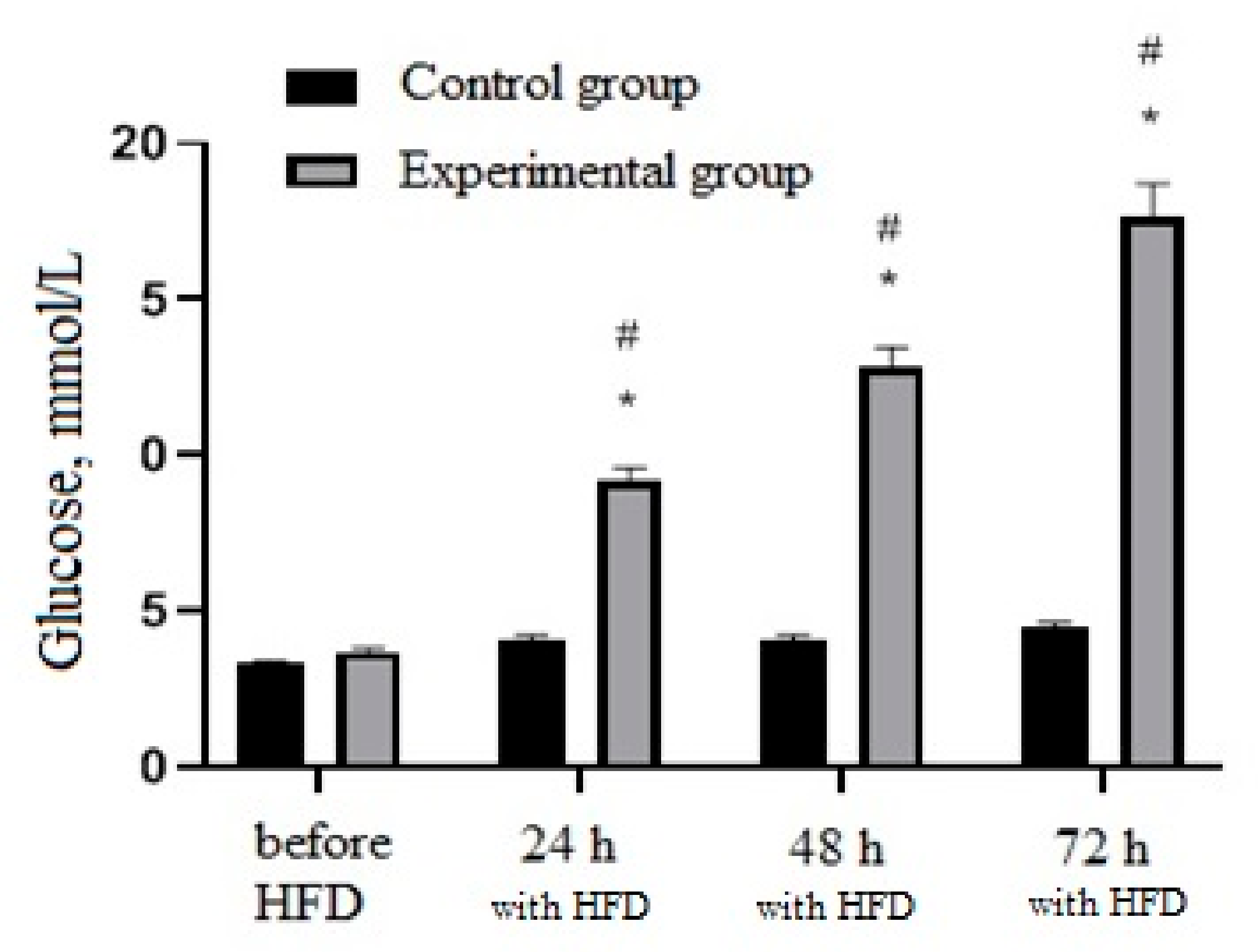
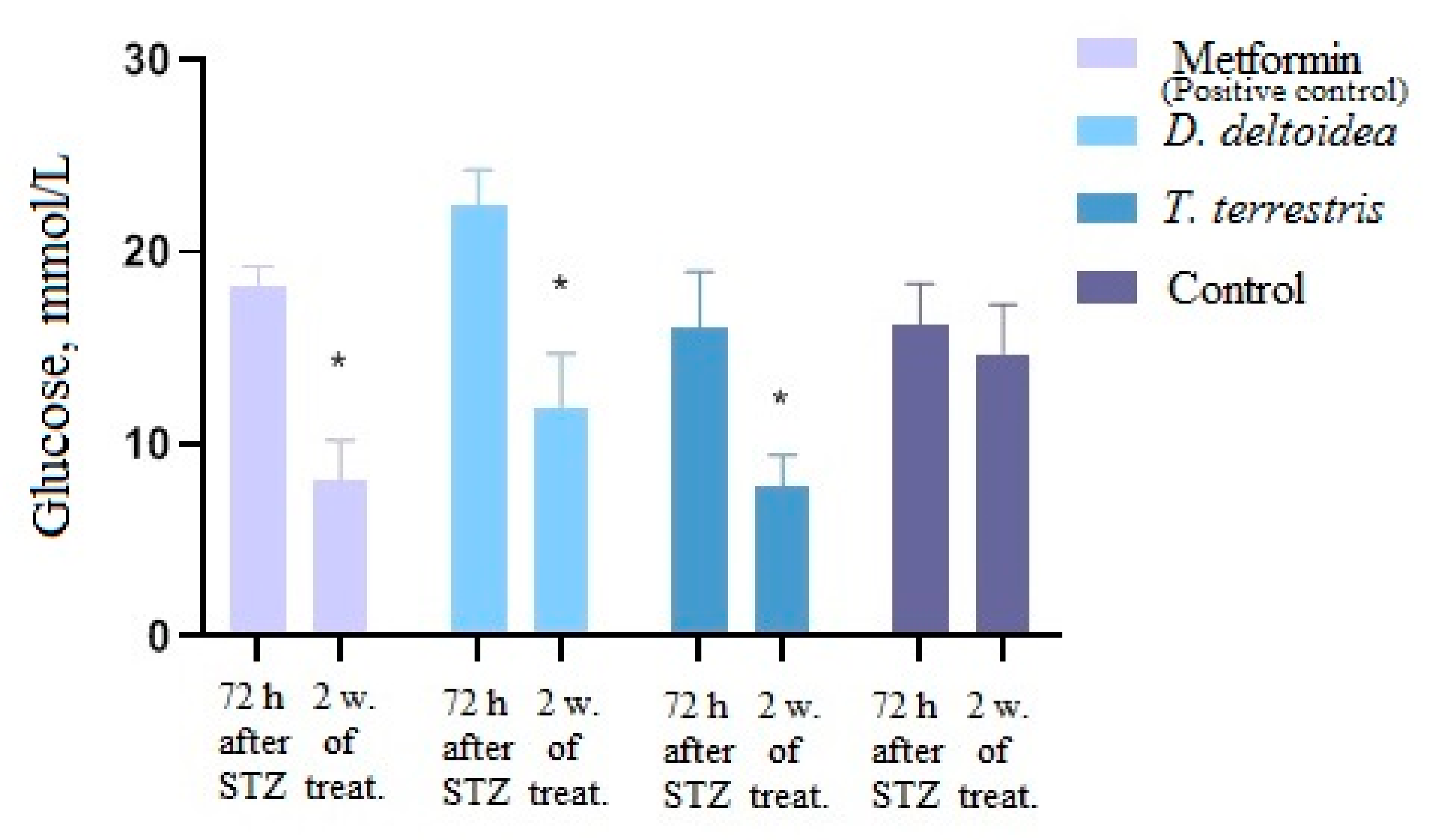
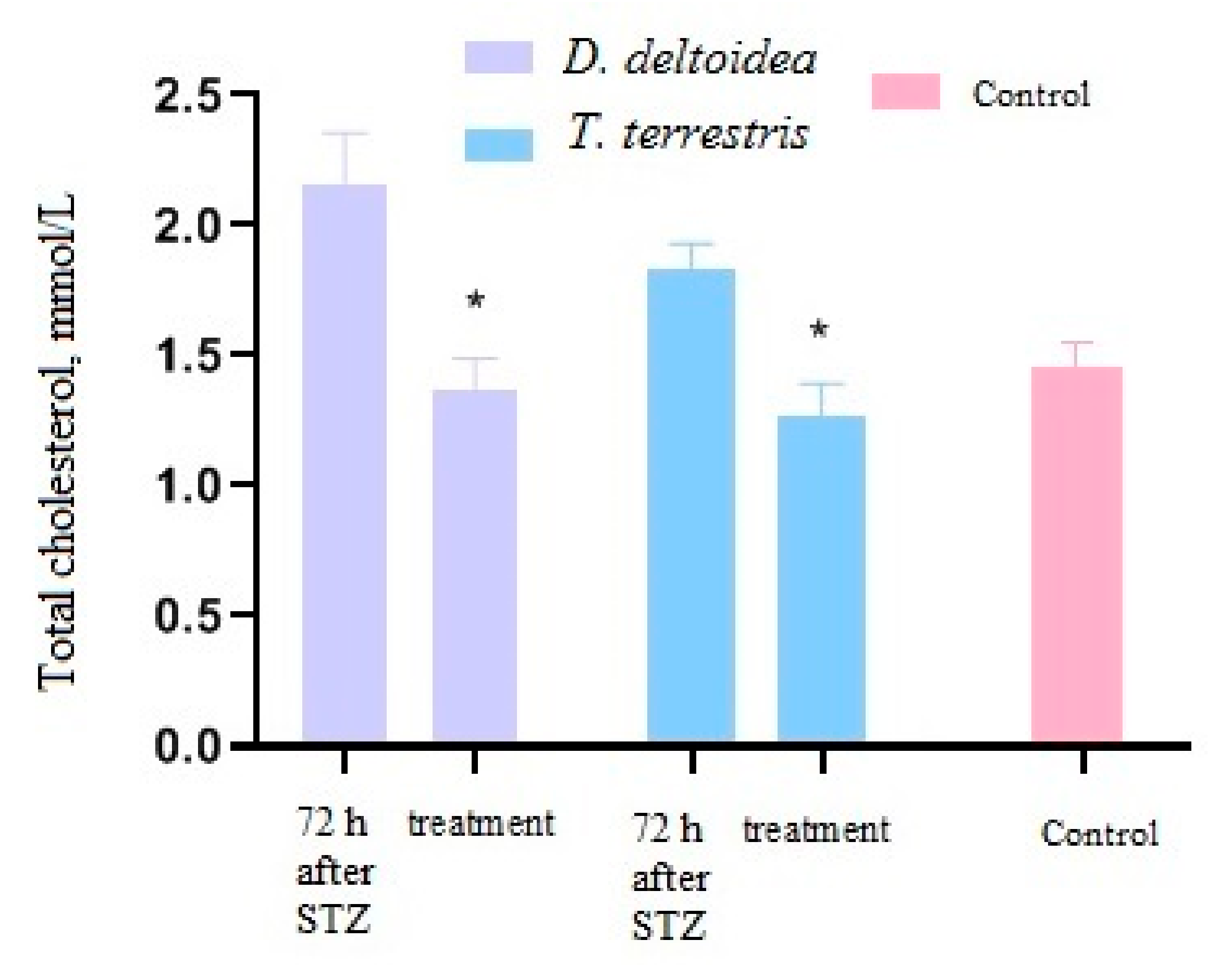
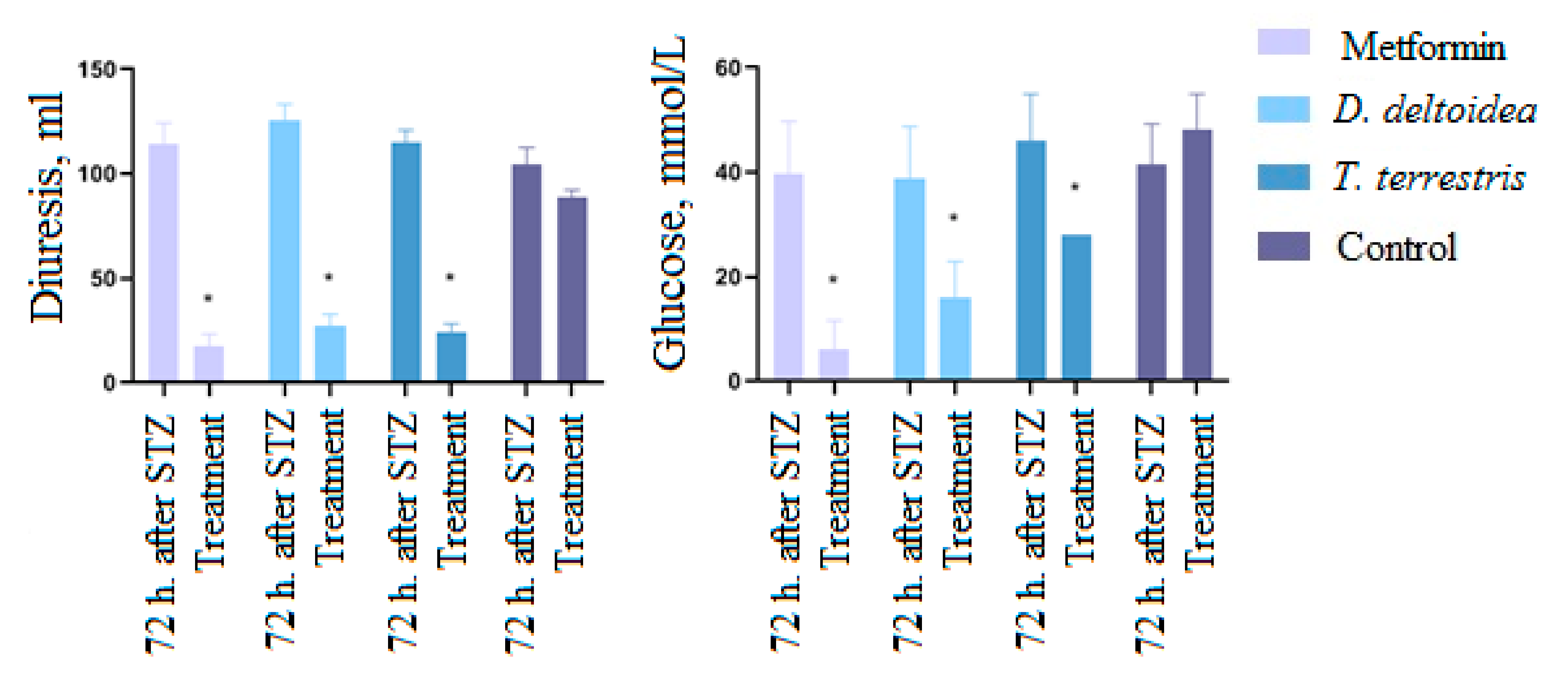
3.3. Activity of Cell Biomass Phytopreparations in Experimental STZ-Induced Model of Type 2 Diabetes Mellitus
3.4. Results of Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Non-Commun. Dis. 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Standl, E.; Khunti, K.; Hansen, T.-B.; Schnell, O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiol. 2019, 26, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Co, J.; Coupland, C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: Open cohort study in primary care. BMJ Clin. Res. Ed. 2016, 352, i1450. [Google Scholar]
- Dedov, I.I.; Shestakova, M.V.; Vikulova, O.K. Epidemiology of diabetes mellitus in Russian Federation: Clinical and statistical report according to the federal diabetes registry. Diabetes Mellit. 2017, 20, 13–41. [Google Scholar] [CrossRef] [Green Version]
- Bikbov, M.M.; Fayzrakhmanov, R.R.; Kazakbaeva, G.M.; Zainullin, R.M.; Arslangareeva, I.I.; Gilmanshin, T.R.; Salavatova, V.F.; Nikitin, N.A.; Mukhamadieva, S.R.; Yakupova, D.F.; et al. Prevalence, awareness and control of diabetes in Russia: The Ural Eye and Medical Study on adults aged 40+ years. PLoS ONE 2019, 14, e0215636. [Google Scholar] [CrossRef]
- Altobelli, E.; Angeletti, P.M.; Profeta, V.F.; Petrocelli, R. Lifestyle risk factors for type 2 diabetes mellitus and National Diabetes Care Systems in European countries. Nutrients 2020, 12, 2806. [Google Scholar] [CrossRef]
- Shao, J.W.; Jiang, J.L.; Zou, J.J.; Yang, M.Y.; Chen, F.M.; Zhang, Y.J.; Jia, L. Therapeutic potential of ginsenosides on diabetes: From hypoglycemic mechanism to clinical trials. J. Funct. Foods 2020, 64, 103630. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, J.; Hu, J.; Lou, G.; Xiong, H.; Peng, C.; Zheng, S.; Huang, Q. The role of diosgenin in diabetes and diabetic complications. J. Steroid Biochem. Mol. Biol. 2020, 198, 105575. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Yadav, S.; Srivastava, A.; Purohit, I.; Shrivastava, N. Plant derived bioactive molecules: Culture vessels to bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 47–60. [Google Scholar]
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, H.K.; Georgiev, M.I.; Park, S.-Y.; Dandin, V.S.; Paek, K.-Y. The safety assessment of food ingredients derived from plant cell, tissue and organ cultures: A review. Food Chem. 2015, 176, 426–432. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Luo, L.; Hu, H.; Meng, X.; Li, X.; Chen, S. Predicting the potential global distribution of diosgenin-contained Dioscorea species. Chin. Med. 2018, 13, 58. [Google Scholar] [CrossRef]
- Choi, H.-K.; Son, J.-S.; Na, G.-H.; Hong, S.-S.; Park, Y.-S.; Song, J.-Y. Mass production of paclitaxel by plant cell culture. J. Plant Biotechnol. 2002, 29, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, A.; Khoshvishkaie, E.; Azadbakht, M. Plant cells technology as an effective biotechnological approach for high scale production of pharmaceutical natural compounds; a meta-analysis study. Pharm. Biomed. Res. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent application of plant cell culture technology in cosmetics and food. Eng. Life Sci. 2020, 21, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [Green Version]
- Nosov, A.M. Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl. Biochem. Microbiol. 2012, 48, 609–624. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Nosov, A.M.; Popova, E.V.; Kochkin, D.V. Isoprenoid production via plant cell cultures: Biosynthesis, accumulation and scaling-up to bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 563–623. [Google Scholar]
- Popova, E.V.; Nosov, A.V.; Titova, M.V.; Kochkin, D.V.; Fomenkov, A.A.; Kulichenko, I.E.; Nosov, A.M. Advanced biotechnologies: Collections of higher plant cell cultures as a basis for development and production of medicinal preparations. Russ. J. Plant Physiol. 2021, 68, 385–400. [Google Scholar] [CrossRef]
- Amin, A.; Lotfy, M.; Shafiullah, M.; Adeghate, E. The protective effect of Tribulus terrestris in diabetes. Ann. N. Y. Acad. Sci. 2006, 1084, 391–401. [Google Scholar] [CrossRef]
- Han, L.-K.; Zheng, Y.-N.; Yoshikawa, M.; Okuda, H.; Kimura, Y. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement. Altern. Med. 2005, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Titova, M.V.; Popova, E.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Klyushin, A.G.; Titova, E.G.; Nebera, E.A.; Vasilevskaya, E.R.; Tolmacheva, G.S.; et al. Suspension cell culture of Dioscorea deltoidea—A renewable source of biomass and furostanol glycosides for food and pharmaceutical industry. Agronomy 2021, 11, 394. [Google Scholar] [CrossRef]
- Titova, M.V.; Shumilo, N.A.; Kulichenko, I.E.; Ivanov, I.M.; Sukhanova, E.S.; Nosov, A.M. Features of respiration and formation of steroidal glycosides in Dioscorea deltoidea cell suspension culture grown in flasks and bioreactors. Russ. J. Plant Physiol. 2015, 62, 557–563. [Google Scholar] [CrossRef]
- Khandy, M.T.; Kochkin, D.V.; Tomilova, S.V.; Galishev, B.A.; Sukhanova, E.S.; Klyushin, A.G.; Ivanov, I.M.; Nosov, A.M. Obtaining and study of callus and suspension plant cell cultures of Tribulus terrestris L., a producer of steroidal glycosides. Appl. Biochem. Microbiol. 2017, 53, 800–806. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Kachala, V.V.; Shashkov, A.S.; Chizhov, A.O.; Chirva, V.Y.; Nosov, A.M. Malonyl-ginsenoside content of a cell-suspension culture of Panax japonicus var. repens. Phytochemistry 2013, 93, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kochkin, D.V.; Galishev, B.A.; Glagoleva, E.S.; Titova, M.V.; Nosov, A.M. Rare triterpene glycoside of ginseng (ginsenoside malonyl-Rg1) detected in plant cell suspension culture of Panax japonicus var. repens. Russ. J. Plant Physiol. 2017, 64, 649–656. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Khandy, M.T.; Zaitsev, G.P.; Tolkacheva, N.V.; Shashkov, A.S.; Titova, M.V.; Chirva, V.Y.; Nosov, A.M. Protodioscin in Dioscorea deltoidea suspension cell culture. Chem. Nat. Comp. 2016, 52, 664–668. [Google Scholar] [CrossRef]
- Tomilova, S.V.; Khandy, M.T.; Kochkin, D.V.; Galishev, B.A.; Klyushin, A.G.; Nosov, A.M. Effect of synthetic auxin analogs (2.4-D and α-NAA) on growth and biosynthetic characteristics of suspension cell culture of Tribulus terrestris L. Russ. J. Plant Physiol. 2020, 67, 636–645. [Google Scholar] [CrossRef]
- Lagunin, A.; Povydysh, M.; Ivkin, D.; Luzhanin, V.; Krasnova, M.; Okovityi, S.; Nosov, A.; Titova, M.; Tomilova, S.; Filimonov, D.; et al. Antihypoxic action of Panax japonicus, Tribulus terrestris and Dioscorea deltoidea cell cultures: In silico and animal studies. Mol. Inf. 2020, 39, 2000093. [Google Scholar] [CrossRef]
- Demidova, E.V.; Reshetnyak, O.V.; Oreshnikov, A.V.; Nosov, A.M. Growth and biosynthetic characteristics of ginseng (Panax japonicus var. repens) deep-tank cell culture in bioreactors. Russ. J. Plant Physiol. 2006, 53, 134–140. [Google Scholar] [CrossRef]
- Titova, M.V.; Popova, E.V.; Shumilo, N.A.; Kulichenko, I.E.; Chernyak, N.D.; Ivanov, I.M.; Klushin, A.G.; Nosov, A.M. Stability of cryopreserved Polyscias filicifolia suspension cell culture during cultivation in laboratory and industrial bioreactors. Plant Cell Tiss. Organ Cult. 2021, 145, 591–600. [Google Scholar] [CrossRef]
- Vasil’eva, I.S.; Paseshnichenko, V.A. Steroid glycosides from suspension cultures of Dioscorea deltoidea cells and their biological activity. In Saponins Used in Traditional and Modern Medicine; Springer: Boston, MA, USA, 1996; pp. 15–22. [Google Scholar]
- Khandy, M.T.; Titova, M.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Nosov, A.M. Formation of protodioscin and deltoside isomers in suspension cultures of Nepal yam (Dioscorea deltoidea Wall.) cells. Appl. Biochem. Microbiol. 2016, 52, 657–662. [Google Scholar] [CrossRef]
- Sarvin, B.; Fedorova, E.; Shpigun, O.; Titova, M.; Nikitin, M.; Kochkin, D.; Rodin, I.; Stavrianidi, A. LC-MS determination of steroidal glycosides from Dioscorea deltoidea Wall cell suspension culture: Optimization of pre-LC-MS procedure parameters by Latin Square design. J. Chrom. B 2018, 1080, 64–70. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Kachala, V.V.; Nosov, A.M. Malonyl-ginsenoside Rb1 in cell suspension culture of Panax japonicus var. repens. Dokl. Biochem. Biophys. 2011, 441, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Kochkin, D.V.; Glagoleva, E.S.; Galishev, B.A.; Spiridovich, E.V.; Nosov, A.M.; Reshetnikov, V.N. Analysis of ginsenosides in roots of Panax ginseng introduced in Central botanic garden NAN Belarus. Dokl. Nat. Acad. Sci. Belarus 2018, 62, 447–454. (In Russian) [Google Scholar] [CrossRef]
- Salman, Z.K.; Refaat, R.; Selima, E.; El Sarha, A.; Ismail, M.A. The combined effect of metformin and L-cysteine on inflammation, oxidative stress and insulin resistance in streptozotocin-induced type 2 diabetes in rats. Eur. J. Pharmacol. 2013, 714, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Fan, S.; Jia, R.; Liu, Y. Ginsenoside Rg1 protects cardiomyocytes from hypoxia-induced injury through the PI3K/AKT/mTOR pathway. Pharmazie 2018, 73, 349–355. [Google Scholar]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Bayrasheva, V.K. Experimental modeling of diabetes and diabetic nephropathy. Sovrem. Probl. Nauk. Obraz. 2015, 4, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Domingo-Almenara, X.; Guijas, C.; Palermo, A.; Rinschen, M.M.; Isbell, J.; Benton, H.P.; Siuzdak, G. Enhanced in-source fragmentation annotation enables novel data independent acquisition and autonomous METLIN molecular identification. Anal. Chem. 2020, 92, 6051–6059. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Gouveia, S.; Castilho, P.C. Analysis of phenolic compounds in leaves from endemic trees from Madeira Island. A contribution to the chemotaxonomy of Laurisilva forest species. Ind. Crops Prod. 2015, 64, 135–151. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Liu, L.T.; Bian, J.J.; Yan, C.Q.; Ye, L.; Zhao, M.X.; Huang, Q.S.; Wang, W.; Liang, K.; Shi, Z.F.; et al. Identification of multiple constituents in shuganjieyu capsule and rat plasma after oral administration by ultra-performance liquid chromatography coupled with electrospray ionization and ion trap mass spectrometry. Acta Chromatograph. 2018, 30, 95–102. [Google Scholar] [CrossRef]
- Choudhary, N.; Khatik, G.L.; Suttee, A. The possible role of saponin in type-II diabetes-a review. Curr. Diabetes Rev. 2021, 17, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Etuk, E.U. Animal models for studying diabetes mellitus. Agric. Biol. J. N. Am. 2010, 1, 130–134. [Google Scholar]
- Reddy, S.; Wu, D.; Elliott, R.B. Low dose streptozotocin causes diabetes in severe combined immunodeficient (SCID) mice without immune cell infiltration of the pancreatic islets. Autoimmunity 1995, 20, 83–92. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protocols Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Adewole, S.O.; Ojewole, J.A.O. Insulin-induced immunohistochemical and morphological changes in pancreatic β-cells of streptozotocin-treated diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 447–455. [Google Scholar] [CrossRef]
- Gandhi, S.; Srinivasan, B.P.; Akarte, A.S. Potential nephrotoxic effects produced by steroidal saponins from hydro alcoholic extract of Tribulus terrestris in STZ-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Chopra, D.; Kohli, S.K. Pharmacological effects of Tribulus terrestris: A review. Int. J. Contemp. Med. 2013, 1, 71–75. [Google Scholar] [CrossRef]
- AlKhaldi, K.; Daghestani, M.; Al-Haddad, T. In vitro anti-diabetic activity of Tribulus terrestris L. fruits extracts. Nutr. Food Sci. 2020, 50, 631–640. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Domingo, J.S.; Dioum, E.H.; Canto, C.; Barron, D.; et al. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar]
- Vuksan, V.; Sievenpiper, J.L. Herbal remedies in the management of diabetes: Lessons learned from the study of ginseng. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.; Ahmad, A.; Tantray, A.H.; Parry, P.A. Ethnopharmacological potential and medicinal uses of miracle herb Dioscorea spp. J. Ayurvedic Herb. Med. 2018, 4, 79–85. [Google Scholar] [CrossRef]
- Salehi, B.; Sener, B.; Kilic, M.; Sharifi-Rad, J.; Naz, R.; Yousaf, Z.; Mudau, F.N.; Fokou, P.V.T.; Ezzat, S.M.; El Bishbishy, M.H.; et al. Dioscorea plants: A genus rich in vital nutra-pharmaceuticals—A review. Iranian J. Pharm. Res. 2019, 18 (Suppl. 1), 68–89. [Google Scholar]
- McAnuff, M.A.; Harding, W.W.; Omoruyi, F.O.; Jacobs, H.; Morrison, E.Y.; Asemota, H.N. Hypoglycemic effects of steroidal sapogenins isolated from Jamaican bitter yam, Dioscorea polygonoides. Food Chem. Toxicol. 2005, 43, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; More, P.; Derle, A.; Patil, A.; Markad, P.; Asok, A.; Kumbhar, N.; Shaikh, M.L.; Ramanamurthy, B.; Shinde, V.; et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of Type II Diabetes Mellitus with inhibitory activity against a-Amylase and a-Glucosidase. PLoS ONE 2014, 9, e106039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roghani-Dehkordi, F.; Roghani, M.; Baluchnejadmojarad, T. Diosgenin mitigates streptozotocin diabetes-induced vascular dysfunction of the rat aorta: The involved mechanisms. J. Cardiovasc. Pharmacol. 2015, 66, 584–592. [Google Scholar] [CrossRef]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv. Nutr. 2015, 6, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wang, J.; Zhao, R.; Zhang, X.; Mei, Y. Ginsenoside-Rb1 improved diabetic cardiomyopathy through regulating calcium signaling by alleviating protein O-GlcNAcylation. J. Agric. Food. Chem. 2019, 67, 14074–14085. [Google Scholar] [CrossRef]


| Manipulation | Day of the Experiment 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 2 | 1–42 | 43 | 44 (24 h STZ) | 45 (48 h STZ) | 46 (72 h STZ) | 47–53 1st Week of Treatment | 54–60 2nd Week of Treatment | |
| High-fat diet | + | + | + | + | + | + | + | |
| STZ administration 35 mg/kg | + | |||||||
| Visual check for pathology development | + | + | + | + | + | |||
| Drug administration | + | + | ||||||
| Body weight measurement | + | + (21,42) | + (60) | + (60) | ||||
| Blood-glucose-concentration measurement | + (42) | + | + | + | + (53) | + (60) | ||
| Measuring the concentration of glucose in urine | + | + (42) | + | + | + (53) | + (60) | ||
| Daily urine volume measurement | + | + (42) | + | + | + (53) | + (60) | ||
| Blood cholesterol and triglyceride level measurement | + (42) | + | + (60) | |||||
| Euthanasia | + (60) | |||||||
| Histological examination | + (60) | |||||||
| Cell Culture | Bioreactor Volume | Cell Viability, V (%) | Maximum Accumulation of Dry Weight, Mmax_dw (g/L) | Productivity, Pi_Max (g/(l⋅Day) | Specific Growth Rate, µdw, (Day−1) |
|---|---|---|---|---|---|
| D. deltoidea | 630 L | 83.5 ± 4.8 | 9.10 ± 2.76 | 0.37 ± 0.07 | 0.11 ± 0.03 |
| T. terrestris | 20 L | 99.0 ± 1.0 | 14.0 ± 0.10 | 1.10 ± 0.20 | 0.3 ± 0.05 |
| P. japonicus | 630 L | 84.2 ± 3.6 | 8.16 ± 1.84 | 0.31 ± 0.04 | 0.09 ± 0.02 |
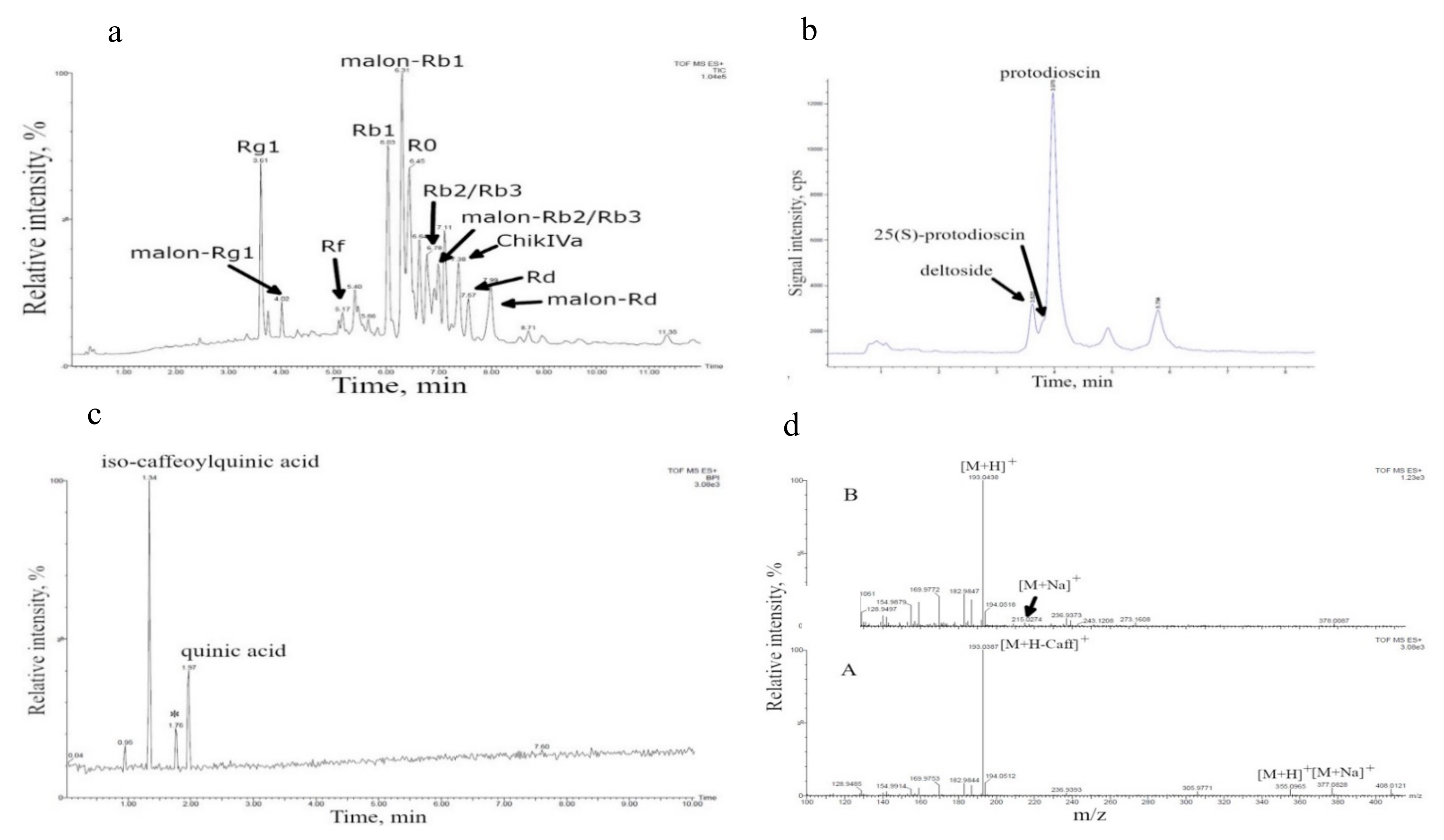
| Cell Culture | Bioreactor Volume | Bioactive Metabolites 1 | Total Content of Detected Bioactive Metabolites (% of Dry Cell Weight) |
|---|---|---|---|
| D. deltoidea | 630 L | Furostanol-type glycosides (deltoside, 25 (S)-protodioscin, protodioscin) [21,30] 2 | 4.62 ± 0.53 |
| T. terrestris | 20 L | Furostanol-type steroidal glycosides [27,31] Caffeoylquinic acid, quinic acid (this study) | 0.10 ± 0.03 not determined |
| P. japonicus | 630 L | Ginsenosides Rg1, malonyl-Rg1, Rb1, malonyl-Rb1, Rb2/Rb3, malonyl-Rb2/Rb3, Rd, malonyl-Rd, Rf, R0, chikusetsusaponin IVa) [28,29] | 3.46 ± 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povydysh, M.N.; Titova, M.V.; Ivanov, I.M.; Klushin, A.G.; Kochkin, D.V.; Galishev, B.A.; Popova, E.V.; Ivkin, D.Y.; Luzhanin, V.G.; Krasnova, M.V.; et al. Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus. Nutrients 2021, 13, 3811. https://doi.org/10.3390/nu13113811
Povydysh MN, Titova MV, Ivanov IM, Klushin AG, Kochkin DV, Galishev BA, Popova EV, Ivkin DY, Luzhanin VG, Krasnova MV, et al. Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus. Nutrients. 2021; 13(11):3811. https://doi.org/10.3390/nu13113811
Chicago/Turabian StylePovydysh, Maria N., Maria V. Titova, Igor M. Ivanov, Andrey G. Klushin, Dmitry V. Kochkin, Boris A. Galishev, Elena V. Popova, Dmitry Yu. Ivkin, Vladimir G. Luzhanin, Marina V. Krasnova, and et al. 2021. "Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus" Nutrients 13, no. 11: 3811. https://doi.org/10.3390/nu13113811
APA StylePovydysh, M. N., Titova, M. V., Ivanov, I. M., Klushin, A. G., Kochkin, D. V., Galishev, B. A., Popova, E. V., Ivkin, D. Y., Luzhanin, V. G., Krasnova, M. V., Demakova, N. V., & Nosov, A. M. (2021). Effect of Phytopreparations Based on Bioreactor-Grown Cell Biomass of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus on Carbohydrate and Lipid Metabolism in Type 2 Diabetes Mellitus. Nutrients, 13(11), 3811. https://doi.org/10.3390/nu13113811







