The Relationships between Water Intake and Hydration Biomarkers and the Applications for Assessing Adequate Total Water Intake among Young Adults in Hebei, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Procedure
2.3. Anthropometric Measurements
2.4. Temperature and Humidity of the Environment
2.5. Assessment of Total Water Intake
2.5.1. Assessment of Total Drinking Fluids
2.5.2. Assessment of Water from Food
2.6. Urine Biomarkers
2.7. Plasma Biomarkers
2.8. Statistics
3. Results
3.1. Temperature and Humidity during the Study
3.2. Association between Fluids Intake and 24 h Urine and First Morning Urine Biomarkers
3.3. Association between Fluid Intake and Plasma Biomarkers
3.4. Partial Least Squares Model of the Relationship between TWI and 24 h Urinary Biomarkers
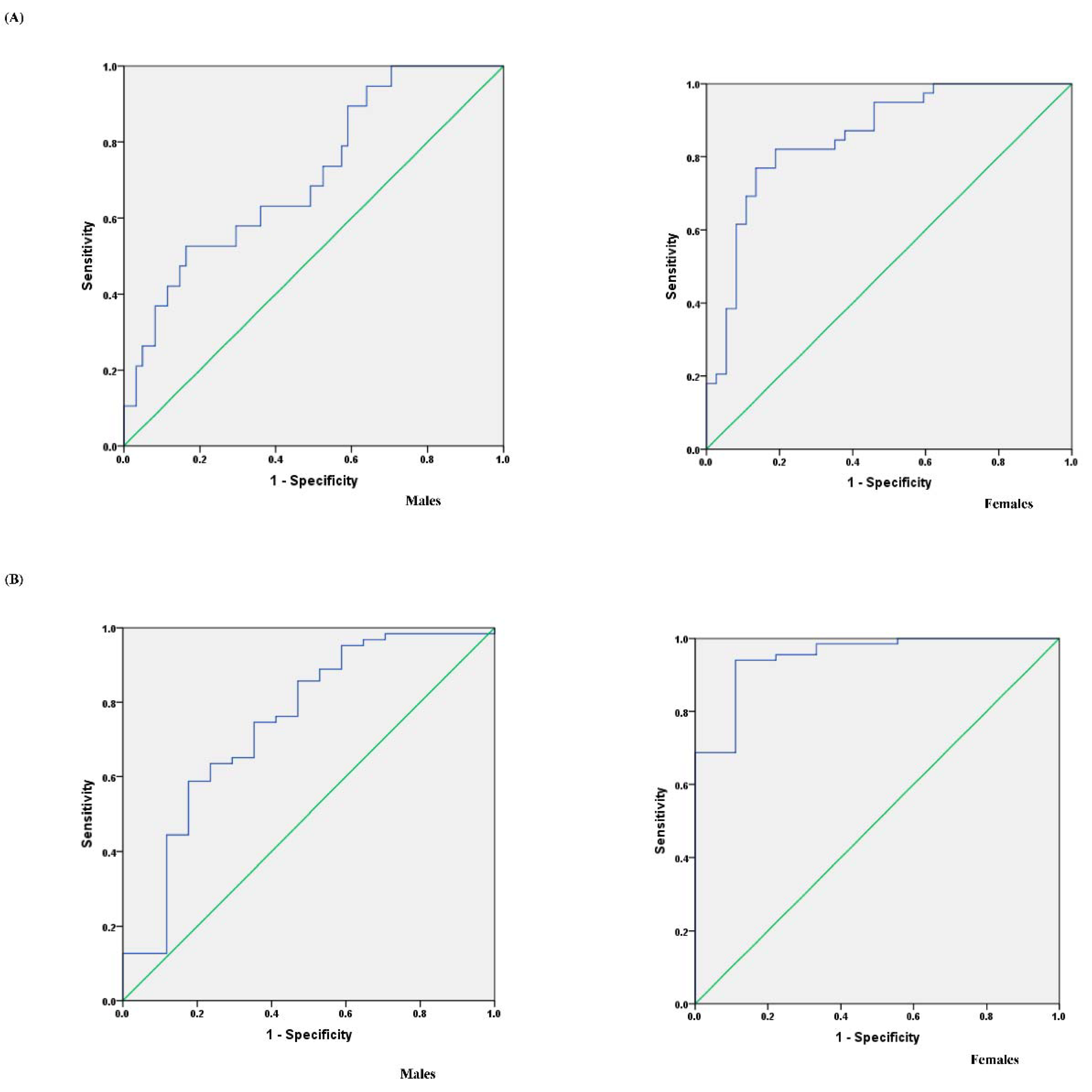
3.5. Determination of the TWI for Assessing Optimal Hydration and Assessing Dehydration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, A.C.; Reimers, K.J.; Buyckx, M.E. Hydration: Issues for the 21st century. Nutr. Rev. 2003, 61, 261–271. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Sahota, O.; Lobo, D.N. Acute and chronic effects of hydration status on health. Nutr. Rev. 2015, 732, 97–109. [Google Scholar] [CrossRef]
- Armstrong, L.E. Challenges of linking chronic dehydration and fluid consumption to health outcomes. Nutr. Rev. 2012, 70, S121–S127. [Google Scholar] [CrossRef]
- Zhang, N.; Du, S.M.; Zhang, J.F.; Ma, G.S. Effects of Dehydration and Rehydration on Cognitive Performance and Mood among Male College Students in Cangzhou, China: A Self-Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, L.A.; Lawrence, M.A.; Kenefick, R.W. Hydration Status and Thermoregulatory Responses in Drivers During Competitive Racing. J. Strength Cond. Res. 2018, 32, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Bar-David, Y.; Urkin, J.; Kozminsky, E. The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paediatr. 2005, 94, 1667–1673. [Google Scholar] [CrossRef]
- Baron, S.; Courbebaisse, M.; Lepicard, E.M.; Friedlander, G. Assessment of hydration status in a large population. Br. J. Nutr. 2015, 113, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Harvey, G.; Meir, R.; Brooks, L.; Holloway, K. The use of body mass changes as a practical measure of dehydration in team sports. J. Sci. Med. Sport 2008, 11, 600–603. [Google Scholar] [CrossRef]
- Ganio, M.S.; Armstrong, L.E.; Casa, D.J.; McDermott, B.P.; Lee, E.C.; Yamamoto, L.M.; Marzano, S.; Lopez, R.M.; Jimenez, L.; Le Bellego, L.; et al. Mild dehydration impairs cognitive performance and mood of men. Br. J. Nutr. 2011, 106, 1535–1543. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.A.; Gonzalez, M.A.; Adams, J.D.; Burchfield, J.M.; Moyen, N.E.; Robinson, F.B.; Schreiber, B.A.; Ganio, M.S. Reliability of 24-h void frequency as an index of hydration status when euhydrated and hypohydrated. Eur. J. Clin. Nutr. 2016, 70, 908–911. [Google Scholar] [CrossRef]
- Burchfield, J.M.; Ganio, M.S.; Kavouras, S.A.; Adams, J.D.; Gonzalez, M.A.; Ridings, C.B.; Moyen, N.E.; Tucker, M.A. 24-h Void number as an indicator of hydration status. Eur. J. Clin. Nutr. 2015, 69, 638–641. [Google Scholar] [CrossRef]
- Perrier, E.T.; Johnson, E.C.; McKenzie, A.L.; Ellis, L.A.; Armstrong, L.E. Urine colour change as an indicator of change in daily water intake: A quantitative analysis. Eur. J. Nutr. 2016, 55, 1943–1949. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, A.L.; Munoz, C.X.; Ellis, L.A.; Perrier, E.T.; Guelinckx, I.; Klein, A.; Kavouras, S.A.; Armstrong, L.E. Urine color as an indicator of urine concentration in pregnant and lactating women. Eur. J. Nutr. 2017, 56, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Du, S.; Zheng, M.; Tang, Z.; Yan, R.; Zhu, Y.; Ma, G. Urine color for assessment of dehydration among college men students in Hebei, China—A cross-sectional study. Asia Pac. J. Clin. Nutr. 2017, 26, 788–793. [Google Scholar]
- Kavouras, S.A.; Johnson, E.C.; Bougatsas, D.; Arnaoutis, G.; Panagiotakos, D.B.; Perrier, E.; Klein, A. Validation of a urine color scale for assessment of urine osmolality in healthy children. Eur. J. Nutr. 2016, 55, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, N.; Wang, Y.; Liang, S.; Liu, S.; Du, S.; Xu, Y.; He, H.; Cai, H.; Ma, G. Drinking patterns and hydration biomarkers among young adults with different levels of habitual total drinking fluids intake in Baoding, Hebei Province, China: A cross-sectional study. BMC Public Health 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Bellego, L.L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Torres, V.E.; Grantham, J.J.; Chapman, A.B.; Mrug, M.; Bae, K.T.; King, B.F., Jr.; Wetzel, L.H.; Martin, D.; Lockhart, M.E.; Bennett, W.M.; et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP). Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Chang, T.I.; Lee, J.; Kim, Y.H.; Han, S.H. Urine Osmolality and Renal Outcome in Patients with Chronic Kidney Disease: Results from the KNOW-CKD. Kidney Blood Press R 2019, 44, 1–12. [Google Scholar] [CrossRef]
- Yeh, H.C.; Lin, Y.S.; Kuo, C.C.; Weidemann, D.; Weaver, V.; Fadrowski, J.; Neu, A.; Navas-Acien, A. Urine osmolality in the US population: Implications for environmental biomonitoring. Environ. Res. 2015, 136, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, Y.; Tsutsumi, Y.; Ohnishi, T.; Kimachi, M.; Yamamoto, Y.; Fukuhara, S. Low Predialysis Plasma Calculated Osmolality Is Associated with Higher All-Cause Mortality: The Japanese Dialysis Outcomes and Practice Patterns Study (J-DOPPS). Nephron 2020, 144, 138–146. [Google Scholar] [CrossRef]
- Perrier, E.; Rondeau, P.; Poupin, M.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I.; Vergne, S.; Klein, A. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur. J. Clin. Nutr. 2013, 67, 939–943. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, A.L.; Perrier, E.T.; Guelinckx, I.; Kavouras, S.A.; Aerni, G.; Lee, E.C.; Volek, J.S.; Maresh, C.M.; Armstrong, L.E. Relationships between hydration biomarkers and total fluid intake in pregnant and lactating women. Eur. J. Nutr. 2017, 56, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Malisova, O.; Athanasatou, A.; Pepa, A.; Husemann, M.; Domnik, K.; Braun, H.; Mora-Rodriguez, R.; Ortega, J.F.; Fernandez-Elias, V.E.; Kapsokefalou, M. Water Intake and Hydration Indices in Healthy European Adults: The European Hydration Research Study (EHRS). Nutrients 2016, 8, 204. [Google Scholar] [CrossRef]
- Kavouras, S.A.; Bougatsas, D.; Johnson, E.C.; Arnaoutis, G.; Tsipouridi, S.; Panagiotakos, D.B. Water intake and urinary hydration biomarkers in children. Eur. J. Clin. Nutr. 2017, 71, 530–535. [Google Scholar] [CrossRef]
- Zhang, N.; Du, S.; Tang, Z.; Zheng, M.; Yan, R.; Zhu, Y.; Ma, G. Hydration, Fluid Intake, and Related Urine Biomarkers among Male College Students in Cangzhou, China: A Cross-Sectional Study—Applications for Assessing Fluid Intake and Adequate Water Intake. Int. J. Environ. Res. Public Health 2017, 14, 513. [Google Scholar] [CrossRef] [Green Version]
- Bougatsas, D.; Arnaoutis, G.; Panagiotakos, D.B.; Seal, A.D.; Johnson, E.C.; Bottin, J.H.; Tsipouridi, S.; Kavouras, S.A. Fluid consumption pattern and hydration among 8-14 years-old children. Eur. J. Clin. Nutr. 2018, 72, 420. [Google Scholar] [CrossRef]
- Kenney, E.L.; Long, M.W.; Cradock, A.L.; Gortmaker, S.L. Prevalence of inadequate hydration among US children and disparities by gender and race/ethnicity: National Health and Nutrition Examination Survey, 2009–2012. Am. J. Public Health 2015, 105, e113–e118. [Google Scholar] [CrossRef]
- World Health Organization. Nutrients in Drinking Water; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for water. EFSA J. 2010, 8, 1459. [Google Scholar]
- Institute of Medicine (US). DRI, Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Ma, G.; Zhang, Q.; Liu, A.; Zuo, J.; Zhang, W.; Zou, S.; Li, X.; Lu, L.; Pan, H.; Hu, X. Fluid intake of adults in four Chinese cities. Nutr. Rev. 2012, 70 (Suppl 2), S105–S110. [Google Scholar] [CrossRef] [PubMed]
- Pross, N.; Demazieres, A.; Girard, N.; Barnouin, R.; Metzger, D.; Klein, A.; Perrier, E.; Guelinckx, I. Effects of changes in water intake on mood of high and low drinkers. PLoS ONE 2014, 9, e94754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, N.; Liang, S.; Wang, Y.; Liu, S.; Liu, S.; Du, S.; He, H.; Xu, Y.; Cai, H.; et al. The amounts and contributions of total drinking fluids and water from food to total water intake of young adults in Baoding, China. Eur. J. Nutr. 2018, 58, 2669–2677. [Google Scholar] [CrossRef]
- Standardization Administration of China. National Food Safety Standard GB5009.3–2016. In Determination of Moisture in Foods; China Standard Press: Beijing, China, 2016. [Google Scholar]
- Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention. China Food Composition; Peking University Medicine Press: Beijing, China, 2009. [Google Scholar]
- Perrier, E.T.; Buendia-Jimenez, I.; Vecchio, M.; Armstrong, L.E.; Tack, I.; Klein, A. Twenty-four-hour urine osmolality as a physiological index of adequate water intake. Dis. Markers 2015, 2015, 231063. [Google Scholar] [CrossRef] [Green Version]
- Alwis, U.S.; Haddad, R.; Monaghan, T.F.; Abrams, P.; Dmochowski, R.; Bower, W.; Wein, A.J.; Roggeman, S.; Weiss, J.P.; Mourad, S.; et al. Impact of food and drinks on urine production: A systematic review. Int. J. Clin. Prac. 2020, 74, e13539. [Google Scholar] [CrossRef]
- Szinnai, G.; Schachinger, H.; Arnaud, M.J.; Linder, L.; Keller, U. Effect of water deprivation on cognitive-motor performance in healthy men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R275–R280. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, D.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Johnson, E.C.; Munoz, C.X.; Swokla, B.; Le Bellego, L.; Jimenez, L.; Casa, D.J.; Maresh, C.M. Hydration biomarkers and dietary fluid consumption of women. J. Acad. Nutr. Diet. 2012, 112, 1056–1061. [Google Scholar] [CrossRef]
- Gandy, J.; Martinez, H.; Guelinckx, I.; Moreno, L.A.; Bardosono, S.; Salas-Salvado, J.; Kavouras, S.A. Relevance of Assessment Methods for Fluid Intake. Ann. Nutr. Metab. 2016, 68 (Suppl 2), 1–5. [Google Scholar] [CrossRef]


| Urine Biomarkers | Total Drinking Fluids | Water from Food | TWI | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| 24 h Volume (mL) | 0.784 | <0.001 | 0.371 | <0.001 | 0.747 | <0.001 |
| Osmolality (mOsm/kg) | −0.589 | <0.001 | −0.079 | 0.329 | −0.477 | <0.001 |
| USG | −0.397 | <0.001 | −0.098 | 0.225 | −0.370 | <0.001 |
| pH | −0.046 | 0.571 | 0.102 | 0.206 | 0.027 | 0.741 |
| Potassium (mmol/L) | −0.404 | <0.001 | −0.036 | 0.659 | −0.353 | <0.001 |
| Sodium (mmol/L) | −0.314 | <0.001 | 0.235 | 0.003 | −0.151 | 0.059 |
| Chloride (mmol/L) | −0.303 | <0.001 | 0.255 | 0.001 | −0.137 | 0.088 |
| Calcium (mmol/L) | −0.280 | <0.001 | −0.093 | 0.251 | −0.257 | 0.001 |
| Phosphorus (mmol/L) | −0.397 | <0.001 | −0.172 | 0.032 | −0.410 | <0.001 |
| Magnesium (mmol/L) | −0.405 | <0.001 | −0.173 | 0.031 | −0.409 | <0.001 |
| Urea (mmol/L) | −0.375 | <0.001 | −0.090 | 0.263 | −0.351 | <0.001 |
| Uric acid (mmol/L) | −0.482 | <0.001 | −0.143 | 0.075 | −0.458 | <0.001 |
| Creatinine (mmol/L) | −0.369 | <0.001 | −0.135 | 0.094 | −0.373 | <0.001 |
| FMU | ||||||
| Osmolality (mOsm/kg) | −0.428 | <0.001 | −0.009 | 0.916 | −0.349 | <0.001 |
| USG | −0.324 | <0.001 | −0.015 | 0.855 | −0.287 | <0.001 |
| pH | −0.072 | 0.374 | 0.046 | 0.571 | −0.020 | 0.806 |
| Potassium (mmol/L) | −0.380 | <0.001 | −0.011 | 0.892 | −0.327 | <0.001 |
| Sodium (mmol/L) | −0.339 | <0.001 | 0.154 | 0.055 | −0.207 | 0.009 |
| Chloride (mmol/L) | −0.328 | <0.001 | 0.194 | 0.015 | −0.186 | 0.020 |
| Calcium (mmol/L) | −0.155 | 0.054 | −0.064 | 0.425 | −0.152 | 0.059 |
| Phosphorus (mmol/L) | −0.352 | <0.001 | −0.220 | 0.006 | −0.387 | <0.001 |
| Magnesium (mmol/L) | −0.226 | 0.004 | −0.105 | 0.191 | −0.238 | 0.003 |
| Urea (mmol/L) | −0.358 | <0.001 | −0.068 | 0.401 | −0.325 | <0.001 |
| Uric acid (mmol/L) | −0.456 | <0.001 | −0.153 | 0.056 | −0.430 | <0.001 |
| Creatinine (mmol/L) | −0.271 | 0.001 | −0.060 | 0.460 | −0.259 | 0.001 |
| Plasma Biomarkers | Total Drinking Fluids | Water from Food | TWI | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Osmolality (mOsm/kg) | −0.030 | 0.714 | 0.011 | 0.889 | 0.018 | 0.827 |
| Potassium (mmol/L) | 0.045 | 0.577 | 0.024 | 0.769 | 0.055 | 0.497 |
| Sodium (mmol/L) | 0.124 | 0.122 | 0.101 | 0.173 | 0.130 | 0.106 |
| Chloride (mmol/L) | 0.014 | 0.859 | 0.110 | 0.173 | 0.042 | 0.606 |
| Calcium (mmol/L) | 0.158 | 0.049 | 0.037 | 0.646 | 0.141 | 0.079 |
| Phosphorus (mmol/L) | −0.049 | 0.543 | −0.328 | 0.000 | −0.170 | 0.034 |
| Magnesium (mmol/L) | 0.208 | 0.009 | −0.095 | 0.239 | 0.108 | 0.178 |
| VIP > 0.8 | VIP < 0.8 | ||
|---|---|---|---|
| Volume | 2.15 | pH | 0.68 |
| Osmolality | 0.86 | USG | 0.79 |
| Sodium (mmol/L) | 1.01 | Potassium (mmol/L) | 0.71 |
| Chloride (mmol/L) | 1.06 | Calcium (mmol/L) | 0.77 |
| Phosphorus (mmol/L) | 0.81 | Magnesium (mmol/L) | 0.72 |
| Creatinine (mmol/L) | 0.84 | Uric acid (mmol/L) | 0.77 |
| Urea (mmol/L) | 0.91 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ma, G.; Du, S.; Zhang, N. The Relationships between Water Intake and Hydration Biomarkers and the Applications for Assessing Adequate Total Water Intake among Young Adults in Hebei, China. Nutrients 2021, 13, 3805. https://doi.org/10.3390/nu13113805
Zhang J, Ma G, Du S, Zhang N. The Relationships between Water Intake and Hydration Biomarkers and the Applications for Assessing Adequate Total Water Intake among Young Adults in Hebei, China. Nutrients. 2021; 13(11):3805. https://doi.org/10.3390/nu13113805
Chicago/Turabian StyleZhang, Jianfen, Guansheng Ma, Songming Du, and Na Zhang. 2021. "The Relationships between Water Intake and Hydration Biomarkers and the Applications for Assessing Adequate Total Water Intake among Young Adults in Hebei, China" Nutrients 13, no. 11: 3805. https://doi.org/10.3390/nu13113805
APA StyleZhang, J., Ma, G., Du, S., & Zhang, N. (2021). The Relationships between Water Intake and Hydration Biomarkers and the Applications for Assessing Adequate Total Water Intake among Young Adults in Hebei, China. Nutrients, 13(11), 3805. https://doi.org/10.3390/nu13113805







