Maternal Diet, Infection, and Risk of Cord Blood Inflammation in the Bangladesh Projahnmo Pregnancy Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure Measures
2.1.1. Maternal Nutritional Status
2.1.2. Micronutrient Intake
2.1.3. Maternal Infections
2.2. Outcome Measures
2.2.1. Blood Spot Collection
2.2.2. Inflammatory Protein Analysis
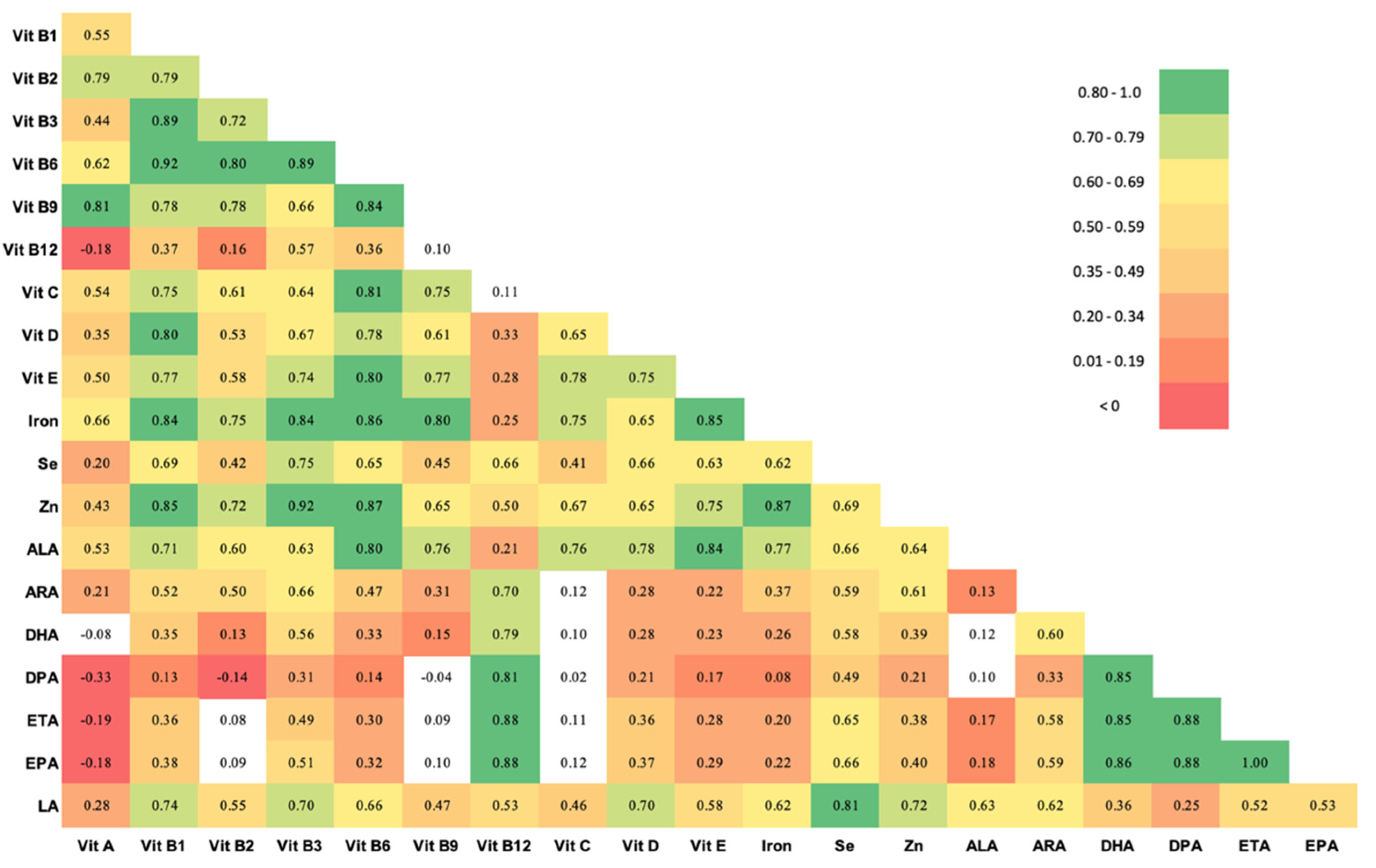
2.3. Statistical Analysis
2.4. Statistical Power
3. Results
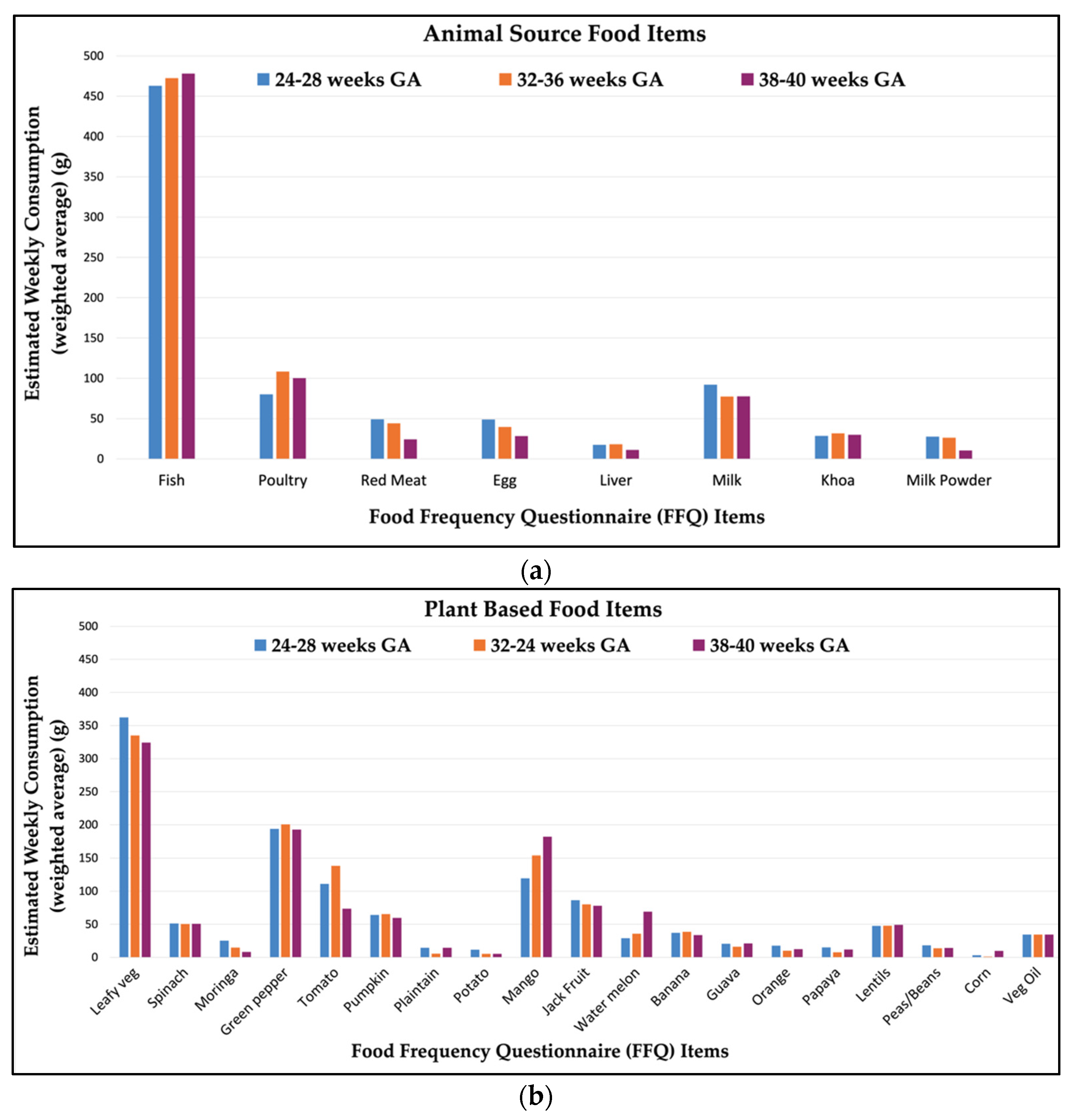
3.1. Maternal Dietary Intake
3.2. Infection Prevalence
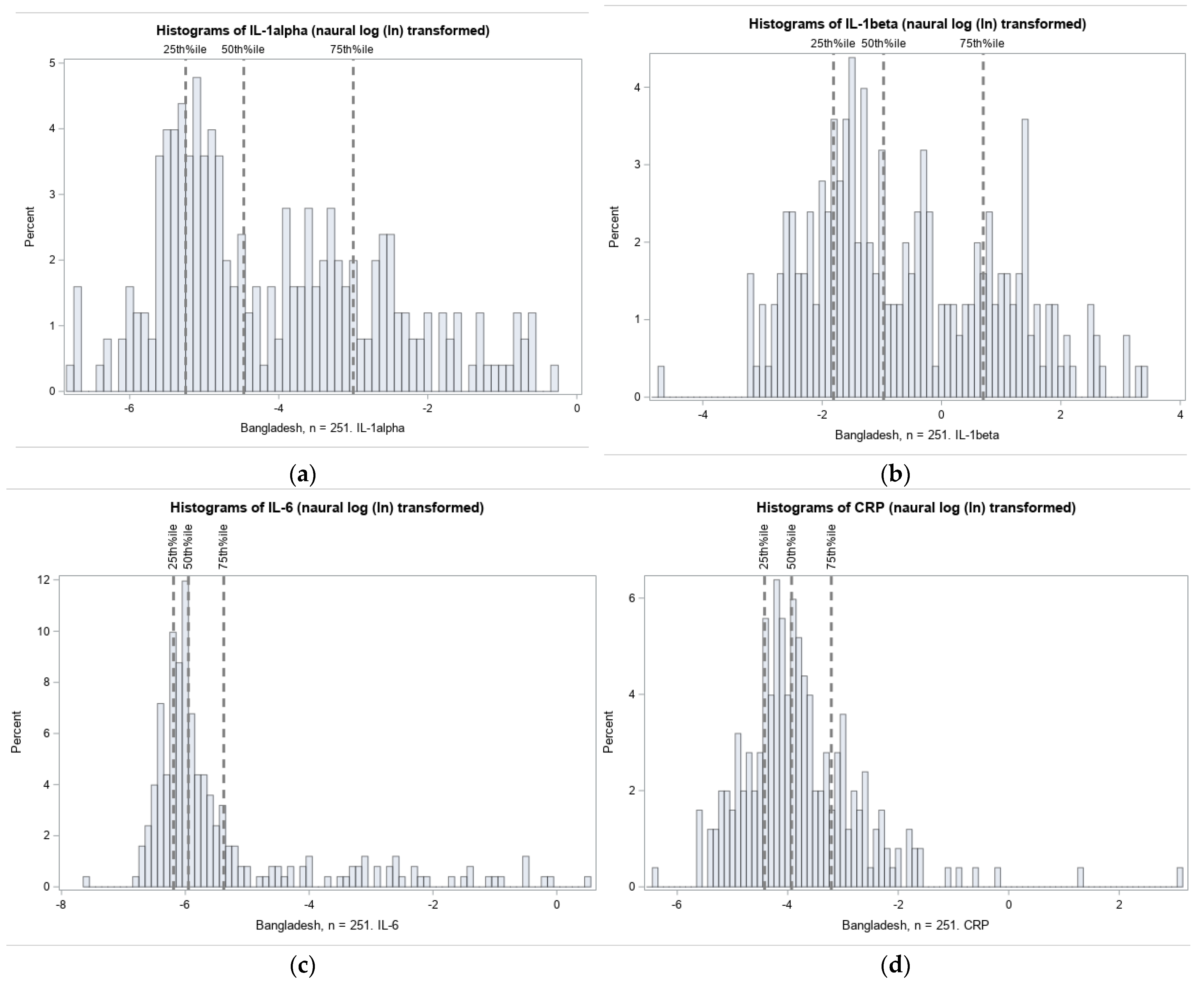
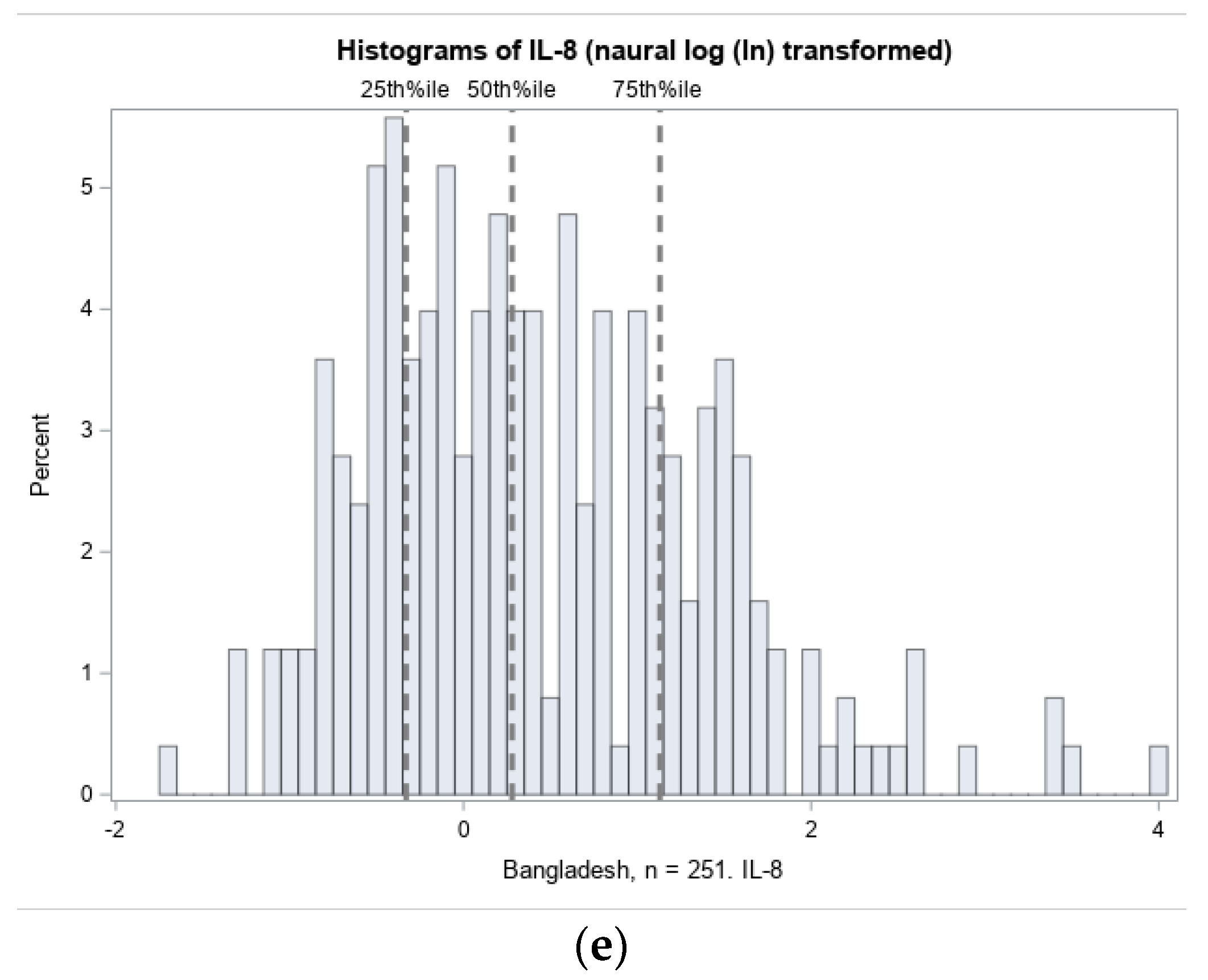
3.2.1. Distribution of Cord Blood Inflammation Biomarkers
3.2.2. Maternal Nutrition, Infection and Odds of Elevated Inflammatory Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B. International Child Development Steering Group Developmental Potential in the First 5 Years for Children in Developing Countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Raiten, D.J.; Sakr Ashour, F.A.; Ross, A.C.; Meydani, S.N.; Dawson, H.D.; Stephensen, C.B.; Brabin, B.J.; Suchdev, P.S.; van Ommen, B. INSPIRE Consultative Group Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J. Nutr. 2015, 145, 1039S–1108S. [Google Scholar] [CrossRef] [PubMed]
- Kutlesic, V.; Brewinski Isaacs, M.; Freund, L.S.; Hazra, R.; Raiten, D.J. Executive Summary: Research Gaps at the Intersection of Pediatric Neurodevelopment, Nutrition, and Inflammation in Low-Resource Settings. Pediatrics 2017, 139, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Darmstadt, G.L.; Saha, S.K. Population-Based Incidence and Etiology of Community-Acquired Neonatal Sepsis in Mirzapur, Bangladesh. J. Infect. Dis. 2009, in press. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Rasmussen, S.A.; Feldkamp, M.L.; Honein, M.A. National Birth Defects Prevention Study Prevalence of Self-Reported Infection during Pregnancy among Control Mothers in the National Birth Defects Prevention Study. Birth Defects Res. Part. A Clin. Mol. Teratol. 2009, 85, 193–201. [Google Scholar] [CrossRef]
- Chico, R.M.; Mayaud, P.; Ariti, C.; Mabey, D.; Ronsmans, C.; Chandramohan, D. Prevalence of Malaria and Sexually Transmitted and Reproductive Tract Infections in Pregnancy in Sub-Saharan Africa: A Systematic Review. JAMA 2012, 307, 2079–2086. [Google Scholar] [CrossRef]
- Strunk, T.; Inder, T.; Wang, X.; Burgner, D.; Mallard, C.; Levy, O. Infection-Induced Inflammation and Cerebral Injury in Preterm Infants. Lancet Infect. Dis. 2014, 14, 751–762. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Beatty, N.; Sassi, R.R.S.; Yamamoto, H.S.; Allred, E.N.; Leviton, A. ELGAN Investigators Systemic Inflammation in the Extremely Low Gestational Age Newborn Following Maternal Genitourinary Infections. Am. J. Reprod. Immunol. 2015, 73, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, I.; Mace, G.; Sediki, M.; Lirussi, F.; Riethmuller, D.; Lentz, N.; Ramanah, R.; Hoyek, T.; Spagnolo, G.; Laurent, N.; et al. Changes in Maternal Blood Inflammatory Markers as a Predictor of Chorioamnionitis: A Prospective Multicenter Study. Am. J. Reprod. Immunol. 2015, 73, 79–90. [Google Scholar] [CrossRef]
- Romero, R.; Chaemsaithong, P.; Docheva, N.; Korzeniewski, S.J.; Tarca, A.L.; Bhatti, G.; Xu, Z.; Kusanovic, J.P.; Chaiyasit, N.; Dong, Z.; et al. Clinical Chorioamnionitis at Term V: Umbilical Cord Plasma Cytokine Profile in the Context of a Systemic Maternal Inflammatory Response. J. Perinat. Med. 2016, 44, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Kaukola, T.; Herva, R.; Perhomaa, M.; Pääkkö, E.; Kingsmore, S.; Vainionpää, L.; Hallman, M. Population Cohort Associating Chorioamnionitis, Cord Inflammatory Cytokines and Neurologic Outcome in Very Preterm, Extremely Low Birth Weight Infants. Pediatr. Res. 2006, 59, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Haskell, M.J.; Raqib, R.; Stephensen, C.B. Vitamin A Status Is Associated with T-Cell Responses in Bangladeshi Men. Br. J. Nutr. 2009, 102, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.M.; Amadi, B.; Mwiya, M.; Nkamba, H.; Tomkins, A.; Goldblatt, D. Dendritic Cell Anergy Results from Endotoxemia in Severe Malnutrition. J. Immunol. 2009, 183, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Shanks, N.; Lightman, S.L. The Maternal-Neonatal Neuro-Immune Interface: Are There Long-Term Implications for Inflammatory or Stress-Related Disease? J. Clin. Invest. 2001, 108, 1567–1573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shamim, A.A.; Kabir, A.; Merrill, R.D.; Ali, H.; Rashid, M.; Schulze, K.; Labrique, A.; West, K.P.; Christian, P. Plasma Zinc, Vitamin B (12) and α-Tocopherol Are Positively and Plasma γ-Tocopherol Is Negatively Associated with Hb Concentration in Early Pregnancy in North-West Bangladesh. Public Health Nutr. 2013, 16, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Claycombe, K.J.; Brissette, C.A.; Ghribi, O. Epigenetics of Inflammation, Maternal Infection, and Nutrition. J. Nutr. 2015, 145, 1109S–1115S. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. The Maturing Architecture of the Brain’s Default Network. Proc. Natl. Acad. Sci. USA 2008, 105, 4028–4032. [Google Scholar] [CrossRef]
- Monk, C.; Georgieff, M.K.; Xu, D.; Hao, X.; Bansal, R.; Gustafsson, H.; Spicer, J.; Peterson, B.S. Maternal Prenatal Iron Status and Tissue Organization in the Neonatal Brain. Pediatr. Res. 2016, 79, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Goines, P.E.; Croen, L.A.; Braunschweig, D.; Yoshida, C.K.; Grether, J.; Hansen, R.; Kharrazi, M.; Ashwood, P.; van de Water, J. Increased Midgestational IFN-γ, IL-4 and IL-5 in Women Bearing a Child with Autism: A Case-Control Study. Mol. Autism 2011, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.K.; O’Shea, T.M.; Allred, E.N.; Paneth, N.; Hirtz, D.; Fichorova, R.N.; Leviton, A. ELGAN Study Investigators Systemic Inflammation and Cerebral Palsy Risk in Extremely Preterm Infants. J. Child. Neurol. 2014, 29, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Kuban, K.; O’Shea, T.M.; Paneth, N.; Fichorova, R.; Allred, E.N.; Dammann, O. The Relationship between Early Concentrations of 25 Blood Proteins and Cerebral White Matter Injury in Preterm Newborns: The ELGAN Study. J. Pediatr. 2011, 158, 897–903. [Google Scholar] [CrossRef]
- Arifeen, S.E.; Mullany, L.C.; Shah, R.; Mannan, I.; Rahman, S.M.; Talukder, M.R.R.; Begum, N.; Al-Kabir, A.; Darmstadt, G.L.; Santosham, M.; et al. The Effect of Cord Cleansing with Chlorhexidine on Neonatal Mortality in Rural Bangladesh: A Community-Based, Cluster-Randomised Trial. Lancet 2012, 379, 1022–1028. [Google Scholar] [CrossRef]
- Wijnhoven, T.M.; de Onis, M.; Onyango, A.W.; Wang, T.; Bjoerneboe, G.-E.A.; Bhandari, N.; Lartey, A.; al Rashidi, B. Assessment of Gross Motor Development in the WHO Multicentre Growth Reference Study. Food Nutr. Bull. 2004, 25, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Bari, L.; Mannan, M.A. Food Composition Table for Bangladesh; Institute of Nutrition and Food Science, Centre for Advanced Research in Sciences, University of Dhaka: Dhaka, Bangladesh, 2013. [Google Scholar]
- Longvah, T.; Aṉantaṉ, I.; Bhaskarachary, K.; Venkaiah, K.; Longvah, T. Indian Food Composition Tables; National Institute of Nutrition, Indian Council of Medical Research: Hyderabad, India, 2017; pp. 2–58. [Google Scholar]
- Nahar, Q.; Choudhury, S.; Faruque, M.M.; Sultana, S.S.; Siddiquee, M.A. Dietary Guidelines for Bangladesh; BIRDEM: Dhaka, Bangladesh, 2014. [Google Scholar]
- Fichorova, R.N.; Onderdonk, A.B.; Yamamoto, H.; Delaney, M.L.; DuBois, A.M.; Allred, E.; Leviton, A. Extremely Low Gestation Age Newborns (ELGAN) Study Investigators Maternal Microbe-Specific Modulation of Inflammatory Response in Extremely Low-Gestational-Age Newborns. MBio 2011, 2, e00280-10. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Allred, E.N.; Kuban, K.C.K.; Dammann, O.; Paneth, N.; Fichorova, R.; Hirtz, D.; Leviton, A. Extremely Low Gestational Age Newborn (ELGAN) Study Investigators Elevated Concentrations of Inflammation-Related Proteins in Postnatal Blood Predict Severe Developmental Delay at 2 Years of Age in Extremely Preterm Infants. J. Pediatr. 2012, 160, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Allred, E.N.; Fichorova, R.N.; Kuban, K.C.K.; Michael O’Shea, T.; Dammann, O. ELGAN study investigators Systemic Inflammation on Postnatal Days 21 and 28 and Indicators of Brain Dysfunction 2years Later among Children Born before the 28th Week of Gestation. Early Hum. Dev. 2016, 93, 25–32. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Joseph, R.M.; Kuban, K.C.K.; Allred, E.N.; Ware, J.; Coster, T.; Fichorova, R.N.; Dammann, O.; Leviton, A. ELGAN Study Investigators Elevated Blood Levels of Inflammation-Related Proteins Are Associated with an Attention Problem at Age 24 Mo in Extremely Preterm Infants. Pediatr. Res. 2014, 75, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Richardson-Harman, N.; Alfano, M.; Belec, L.; Carbonneil, C.; Chen, S.; Cosentino, L.; Curtis, K.; Dezzutti, C.S.; Donoval, B.; et al. Biological and Technical Variables Affecting Immunoassay Recovery of Cytokines from Human Serum and Simulated Vaginal Fluid: A Multicenter Study. Anal. Chem. 2008, 80, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Fichorova, R.N.; Tang, V.F.; Allred, E.N.; McElrath, T.F.; Leviton, A. Elgan Study Investigators Relationship between Neonatal Blood Protein Concentrations and Placenta Histologic Characteristics in Extremely Low GA Newborns. Pediatr. Res. 2011, 69, 68–73. [Google Scholar] [CrossRef]
- Leviton, A.; Fichorova, R.N.; O’Shea, T.M.; Kuban, K.; Paneth, N.; Dammann, O.; Allred, E.N. ELGAN Study Investigators Two-Hit Model of Brain Damage in the Very Preterm Newborn: Small for Gestational Age and Postnatal Systemic Inflammation. Pediatr. Res. 2013, 73, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Bellomy, M.; Allred, E.N.; Fichorova, R.N.; Leviton, A. Systemic Inflammation Associated with Severe Intestinal Injury in Extremely Low Gestational Age Newborns. Fetal Pediatr. Pathol. 2013, 32, 222–234. [Google Scholar] [CrossRef] [PubMed]
- McElrath, T.F.; Allred, E.N.; van Marter, L.; Fichorova, R.N.; Leviton, A. ELGAN Study Investigators Perinatal Systemic Inflammatory Responses of Growth-Restricted Preterm Newborns. Acta Paediatr. 2013, 102, e439–e442. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.D.; Holzman, C.B.; Fichorova, R.N.; Tian, Y.; Jones, N.M.; Fu, W.; Senagore, P.K. Inflammation Biomarkers in Vaginal Fluid and Preterm Delivery. Hum. Reprod. 2013, 28, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Yanni, D.; Korzeniewski, S.J.; Allred, E.N.; Fichorova, R.N.; O’Shea, T.M.; Kuban, K.; Dammann, O.; Leviton, A. Both Antenatal and Postnatal Inflammation Contribute Information about the Risk of Brain Damage in Extremely Preterm Newborns. Pediatr. Res. 2017, 82, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Watt, K.; Brimbecombe, J.; Clough, A.; Judd, J.; Lindsay, D. The Role of Seasonality on the Diet and Household Food Security of Pregnant Women Living in Rural Bangladesh: A Cross-Sectional Study. Public Health Nutr. 2017, 20, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.Y.; Bloch, D.A.; Larsen, M.D. A Simple Method of Sample Size Calculation for Linear and Logistic Regression. Stat. Med. 1998, 17, 1623–1634. [Google Scholar] [CrossRef]
- World Health Organization UNICEF-WHO. Low Birthweight Estimates: Levels and Trends 2000–2015; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lee, A.C.; Kozuki, N.; Cousens, S.; Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.; Schmiegelow, C.; Adair, L.S.; et al. Estimates of Burden and Consequences of Infants Born Small for Gestational Age in Low and Middle Income Countries with INTERGROWTH-21st Standard: Analysis of CHERG Datasets. BMJ 2017, 358, j3677. [Google Scholar] [CrossRef] [PubMed]
- Filmer, D.; Pritchett, L.H. Estimating Wealth Effects without Expenditure Data-or Tears: An Application to Educational Enrollments in States of India. Demography 2001, 38, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Rutstein, S.; Johnson, K. The DHS Wealth Index. ORC Macro 2004, 6. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Yeh, K.-L.; Kautz, A.; Lohse, B.; Groth, S.W. Associations between Dietary Patterns and Inflammatory Markers during Pregnancy: A Systematic Review. Nutrients 2021, 13, 834. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Dietary Inflammatory Potential during Pregnancy Is Associated with Lower Fetal Growth and Breastfeeding Failure: Results from Project Viva. J. Nutr. 2016, 146, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Buss, C.; Wadhwa, P.D.; Entringer, S. Maternal Stress Potentiates the Effect of an Inflammatory Diet in Pregnancy on Maternal Concentrations of Tumor Necrosis Factor Alpha. Nutrients 2018, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Shivappa, N.; Hekmatdoost, A.; Hebert, J.R.; Davoodi, S.H.; Sadeghi, M. Association between Maternal Dietary Inflammatory Index (DII) and Abortion in Iranian Women and Validation of DII with Serum Concentration of Inflammatory Factors: Case-Control Study. Appl. Physiol. Nutr. Metab. 2017, 42, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Papazian, T.; Serhal, A.; Hout, H.; Younes, H.; Tayeh, G.A.; Azouri, J.; Moussa Lteif, F.H.; Kesrouani, A.; Khabbaz, L.R. Discrepancies among Different Tools Evaluating Mediterranean Diet Adherence during Pregnancy, Correlated to Maternal Anthropometric, Dietary and Biochemical Characteristics. Clin. Nutr. 2019, 38, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Hrolfsdottir, L.; Schalkwijk, C.G.; Birgisdottir, B.E.; Gunnarsdottir, I.; Maslova, E.; Granström, C.; Strøm, M.; Olsen, S.F.; Halldorsson, T.I. Maternal Diet, Gestational Weight Gain, and Inflammatory Markers during Pregnancy. Obesity 2016, 24, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Birch, C.S.; Brasch, N.E.; McCaddon, A.; Williams, J.H.H. A Novel Role for Vitamin B(12): Cobalamins Are Intracellular Antioxidants in Vitro. Free Radic. Biol. Med. 2009, 47, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.F.; Petrie, L. Folate Deficiency Enhances the Inflammatory Response of Macrophages. Mol. Immunol. 2013, 54, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, Z.; Jiang, X.; Hou, M.; Tang, Z.; Zhen, X.; Liang, Y.; Ma, J. Folic Acid Represses Hypoxia-Induced Inflammation in THP-1 Cells through Inhibition of the PI3K/Akt/HIF-1α Pathway. PLoS ONE 2016, 11, e0151553. [Google Scholar] [CrossRef]
- Kumar, K.A.; Lalitha, A.; Reddy, U.; Chandak, G.R.; Sengupta, S.; Raghunath, M. Chronic Maternal Vitamin B12 Restriction Induced Changes in Body Composition & Glucose Metabolism in the Wistar Rat Offspring Are Partly Correctable by Rehabilitation. PLoS ONE 2014, 9, e112991. [Google Scholar] [CrossRef]
- Guest, J.; Bilgin, A.; Hokin, B.; Mori, T.A.; Croft, K.D.; Grant, R. Novel Relationships between B12, Folate and Markers of Inflammation, Oxidative Stress and NAD(H) Levels, Systemically and in the CNS of a Healthy Human Cohort. Nutr. Neurosci. 2015, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sable, P.; Khaire, A.; Randhir, K.; Kale, A.; Joshi, S. Effect of Maternal Micronutrients (Folic Acid and Vitamin B12) and Omega 3 Fatty Acids on Indices of Brain Oxidative Stress in the Offspring. Brain Dev. 2014, 36, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, G.-S. Riboflavin, Vitamin B2, Attenuates NLRP3, NLRC4, AIM2, and Non-Canonical Inflammasomes by the Inhibition of Caspase-1 Activity. Sci. Rep. 2020, 10, 19091. [Google Scholar] [CrossRef]
- Seekamp, A.; Hultquist, D.E.; Till, G.O. Protection by Vitamin B2 against Oxidant-Mediated Acute Lung Injury. Inflammation 1999, 23, 449–460. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 Intake Is Inversely Related to, and the Requirement Is Affected by, Inflammation Status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef]
- Huang, S.C.; Wei, J.C.C.; Wu, D.J.; Huang, Y.C. Vitamin B(6) Supplementation Improves pro-Inflammatory Responses in Patients with Rheumatoid Arthritis. Eur. J. Clin. Nutr. 2010, 64, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Siddiqua, T.J.; Ahmad, S.M.; Ahsan, K.B.; Rashid, M.; Roy, A.; Rahman, S.M.; Shahab-Ferdows, S.; Hampel, D.; Ahmed, T.; Allen, L.H.; et al. Vitamin B12 Supplementation during Pregnancy and Postpartum Improves B12 Status of Both Mothers and Infants but Vaccine Response in Mothers Only: A Randomized Clinical Trial in Bangladesh. Eur. J. Nutr. 2016, 55, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Shaik-Dasthagirisaheb, Y.B.; Varvara, G.; Murmura, G.; Saggini, A.; Caraffa, A.; Antinolfi, P.; Tete’, S.; Tripodi, D.; Conti, F.; Cianchetti, E.; et al. Role of Vitamins D, E and C in Immunity and Inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 291–295. [Google Scholar] [PubMed]
- Akoh, C.C.; Pressman, E.K.; Cooper, E.; Queenan, R.A.; Pillittere, J.; O’Brien, K.O. Low Vitamin D Is Associated with Infections and Proinflammatory Cytokines during Pregnancy. Reprod. Sci. 2018, 25, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; He, Y.; Mu, M.; Liu, K. Maternal Vitamin D Deficiency during Pregnancy and Low Birth Weight: A Systematic Review and Meta-Analysis. J. Matern. Fetal Neonatal Med. 2021, 34, 1167–1173. [Google Scholar] [CrossRef]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N. Engl. J. Med. 2018, 379, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zerofsky, M.S.; Jacoby, B.N.; Pedersen, T.L.; Stephensen, C.B. Daily Cholecalciferol Supplementation during Pregnancy Alters Markers of Regulatory Immunity, Inflammation, and Clinical Outcomes in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2388–2397. [Google Scholar] [CrossRef]
- Mikhail, M.S.; Anyaegbunam, A.; Garfinkel, D.; Palan, P.R.; Basu, J.; Romney, S.L. Preeclampsia and Antioxidant Nutrients: Decreased Plasma Levels of Reduced Ascorbic Acid, Alpha-Tocopherol, and Beta-Carotene in Women with Preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 150–157. [Google Scholar] [CrossRef]
- Rumbold, A.; Crowther, C.A. Vitamin E Supplementation in Pregnancy. Cochrane Database Syst. Rev. 2005, CD004069. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin D Supplementation during Pregnancy; E-Library of Evidence for Nutrition Actions (eLENA); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO guidelines approved by the guidelines review committee; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241549912. [Google Scholar]
- Kroot, J.J.C.; Tjalsma, H.; Fleming, R.E.; Swinkels, D.W. Hepcidin in Human Iron Disorders: Diagnostic Implications. Clin. Chem. 2011, 57, 1650–1669. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Iron Homeostasis and the Inflammatory Response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zou, Y.; Shen, Z.; Xiong, Y.; Zhang, W.; Liu, C.; Chen, S. Trace Elements, Ppars, and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2612. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.-F.; Hao, J.-H.; Chen, Y.-H.; Su, P.-Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.-Y.; Zhang, C.; et al. Maternal Zinc Deficiency during Pregnancy Elevates the Risks of Fetal Growth Restriction: A Population-Based Birth Cohort Study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef] [PubMed]
- Banupriya, N.; Vishnu Bhat, B.; Benet, B.D.; Sridhar, M.G.; Parija, S.C. Efficacy of Zinc Supplementation on Serum Calprotectin, Inflammatory Cytokines and Outcome in Neonatal Sepsis—A Randomized Controlled Trial. J. Matern. Fetal Neonatal Med. 2017, 30, 1627–1631. [Google Scholar] [CrossRef]
- Fritsche, K.L. Too Much Linoleic Acid Promotes Inflammation—Doesn’t It? Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 Fatty Acids and Risk for Cardiovascular Disease: A Science Advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Sankar, T.V.; Anandan, R.; Chakraborty, K.; Paul, B.N.; Sarma, D.; Syama Dayal, J.; Venkateshwarlu, G.; et al. DHA and EPA Content and Fatty Acid Profile of 39 Food Fishes from India. Biomed. Res. Int. 2016, 2016, 4027437. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The Endocrine Coupling of Skeletal Muscle and Bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Kutikhin, A.G. The rest of interleukins. In Interleukins in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–318. ISBN 9780128011218. [Google Scholar]
- Roebuck, K.A. Regulation of Interleukin-8 Gene Expression. J. Interferon Cytokine Res. 1999, 19, 429–438. [Google Scholar] [CrossRef] [PubMed]
- De Meeus, J.B.; Pourrat, O.; Gombert, J.; Magnin, G. C-Reactive Protein Levels at the Onset of Labour and at Day 3 Post-Partum in Normal Pregnancy. Clin. Exp. Obstet. Gynecol. 1998, 25, 9–11. [Google Scholar] [PubMed]
- Gotsch, F.; Romero, R.; Chaiworapongsa, T.; Erez, O.; Vaisbuch, E.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; Mazaki-Tovi, S.; Kim, C.J.; et al. Evidence of the Involvement of Caspase-1 under Physiologic and Pathologic Cellular Stress during Human Pregnancy: A Link between the Inflammasome and Parturition. J. Matern. Fetal Neonatal Med. 2008, 21, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Nutrition and the Developing Brain: Nutrient Priorities and Measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Neurobiology of Periventricular Leukomalacia in the Premature Infant. Pediatr. Res. 2001, 50, 553–562. [Google Scholar] [CrossRef]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The Developing Oligodendrocyte: Key Cellular Target in Brain Injury in the Premature Infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Inder, T.E.; Anderson, N.J.; Spencer, C.; Wells, S.; Volpe, J.J. White Matter Injury in the Premature Infant: A Comparison between Serial Cranial Sonographic and MR Findings at Term. AJNR Am. J. Neuroradiol. 2003, 24, 805–809. [Google Scholar] [PubMed]
- Volpe, J.J. Brain Injury in Premature Infants: A Complex Amalgam of Destructive and Developmental Disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Carlo, W.A.; McDonald, S.A.; Tyson, J.E.; Stoll, B.J.; Ehrenkranz, R.A.; Shankaran, S.; Goldberg, R.N.; Das, A.; Schendel, D.; Thorsen, P.; et al. Cytokines and Neurodevelopmental Outcomes in Extremely Low Birth Weight Infants. J. Pediatr. 2011, 159, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.K.; Joseph, R.M.; O’Shea, T.M.; Heeren, T.; Fichorova, R.N.; Douglass, L.; Jara, H.; Frazier, J.A.; Hirtz, D.; Rollins, J.V.; et al. Circulating Inflammatory-Associated Proteins in the First Month of Life and Cognitive Impairment at Age 10 Years in Children Born Extremely Preterm. J. Pediatr. 2017, 180, 116–123. [Google Scholar] [CrossRef]
- Ellman, L.M.; Deicken, R.F.; Vinogradov, S.; Kremen, W.S.; Poole, J.H.; Kern, D.M.; Tsai, W.Y.; Schaefer, C.A.; Brown, A.S. Structural Brain Alterations in Schizophrenia Following Fetal Exposure to the Inflammatory Cytokine Interleukin-8. Schizophr. Res. 2010, 121, 46–54. [Google Scholar] [CrossRef]
- Gilbert, N.M.; O’Brien, V.P.; Hultgren, S.; Macones, G.; Lewis, W.G.; Lewis, A.L. Urinary Tract Infection as a Preventable Cause of Pregnancy Complications: Opportunities, Challenges, and a Global Call to Action. Glob. Adv. Health Med. 2013, 2, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 1998; ISBN 9780195122978. [Google Scholar]
- Qamar, H.; Perumal, N.; Papp, E.; Gernand, A.D.; Al Mahmud, A.; Roth, D.E. Higher Maternal Parathyroid Hormone Concentration at Delivery Is Not Associated with Smaller Newborn Size. Endocr. Connect. 2021, 10, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Mumu, S.J.; Merom, D.; Ali, L.; Fahey, P.P.; Hossain, I.; Rahman, A.K.M.F.; Allman-Farinelli, M. Validation of a Food Frequency Questionnaire as a Tool for Assessing Dietary Intake in Cardiovascular Disease Research and Surveillance in Bangladesh. Nutr. J. 2020, 19, 42. [Google Scholar] [CrossRef]
- Jiang, N.M.; Cowan, M.; Moonah, S.N.; Petri, W.A. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol. Med. 2018, 24, 794–804. [Google Scholar] [CrossRef] [PubMed]

| Maternal Characteristics | Mean ± SD or N (%) |
|---|---|
| Gestational weeks at enrollment (by ultrasound dating < 20 weeks) 2 | 12.1 ± 3.8 |
| Age (years) | 23.7 ± 4.6 |
| Education (years) 2 | 6.0 ± 2.9 |
| Wealth index 3 | −0.04 ± 1.74 |
| Parity | |
| Nulliparous | 79 (31.5) |
| 1–2 | 119 (47.4) |
| 3+ | 53 (21.1) |
| Season during 24–28 week antenatal visit | |
| Grisma (summer, mid-April to mid June) | 115 (45.8) |
| Barsa (rainy, mid-June to mid-August) | 43 (17.1) |
| Sarat (autumn, mid-August to mid-October) | 39 (15.5) |
| Hermanta (late autumn, mid October to mid-December) | 1 (0.4) |
| Shhit (winter, mid-December to mid-February) | 1 (0.4) |
| Basanta (spring, mid-February to mid-April) | 52 (20.7) |
| Stunting (height < 145 cm) at enrollment 2 (cm) | 50 (20.1) |
| BMI at enrollment 2 | |
| Underweight (BMI < 18.5 kg/m2) | 79 (31.7) |
| Normal (18.5 < BMI < 25 kg/m2) | 152 (61.0) |
| Overweight (25 < BMI < 30 kg/m2) | 18 (7.2) |
| Mid-upper arm circumference < 22 cm at enrollment 3 (cm) | 85 (34.5) |
| Weight change from enrollment to 38–40 weeks GA3 (kg) | 5.6 (4.0) |
| Weight change by week (kg/week) | 0.23 ± 0.17 |
| Maternal hemoglobin at enrollment 2 (g/dL) | 11 (1.2) |
| Anemic (Hg < 11 g/dL) | 117 (47.4) |
| Betel nut/tobacco use (chewing/sniffing during this pregnancy) 4 | 91 (36.3) |
| Delivery and Infant Characteristics | |
| Delivery at a health facility | 230 (92.0) |
| Gestational age at birth 2 (by ultrasound dating < 20 weeks) | 39.48 ± 1.2 |
| Infant sex (female) | 134 (53.4) |
| Birth weight 2 (g) | 2749.9 ± 413.7 |
| Birth weight z-score | −1.09 ± 1.01 |
| Low birth weight 2 (<2500 g) | 51 (22.9%) |
| Infant Size for Gestational Age 2 | |
| Small for gestational age (SGA) (birthweight z-score < 10 percentile) | 97 (43.9) |
| Appropriate for gestational age (AGA) 10th–90th percentile) | 122 (55.2) |
| Large for gestational age (LGA) (>90th percentile) | 2 (0.9) |
| Nutrient | Mean (SD) | Median (IQR) | Range | IOM Recommended Dietary Allowances * and Adequate Intakes † |
|---|---|---|---|---|
| Vitamin | ||||
| Vitamin A (µg) | 458 (357) | 380 (241–551) | 32.05–2741 | 770 * |
| Vitamin B1 (mg) | 0.28 (0.1) | 0.27 (0.21–0.35) | 0.09–0.56 | 1.4 * |
| Vitamin B2 (mg) | 0.47 (0.2) | 0.45 (0.33–0.60) | 0.12–1.60 | 1.4 * |
| Vitamin B3 (mg) | 8.82 (2.6) | 8.29 (6.8–10.7) | 3.55–16.62 | 18 * |
| Vitamin B6 (mg) | 0.59 (0.2) | 0.56 (0.42–0.74) | 0.20–1.49 | 2.0 * |
| Vitamin B9 (Folate) (µg) | 104.80 (54) | 95.2 (67.0–133) | 9.36–321 | 600 * |
| Vitamin B12 (µg) | 1.67 (0.5) | 1.77 (1.42–1.97) | 0.20–2.75 | 2.6 * |
| Vitamin C (mg) | 84.70 (46) | 77.6 (48.8–115) | 1.50–261 | 80–85 * |
| Vitamin D (µg) | 10.08 (4.4) | 9.43 (6.60–12.6) | 2.26–27.1 | 15 * |
| Vitamin E (mg) | 3.16 (0.8) | 3.06 (2.50–3.69) | 1.76–6.01 | 15 * |
| Mineral | ||||
| Iron (mg) | 3.57 (1.3) | 3.47 (2.5–4.5) | 1.05–7.14 | 27 * |
| Selenium (mg) | 0.04 (0.01) | 0.04 (0.03–0.05) | 0.01–0.08 | 0.06 * |
| Zinc (mg) | 3.22 (1.2) | 3.02 (2.3–4.0) | 1.07–7.60 | 11–12 * |
| Omega-3 LCPUFA | ||||
| Alpha Linolenic acid (mg) | 201.77 (107.4) | 188.8 (111–263) | 20.0–575 | 1400 † |
| Docosahexaenoic acid (mg) | 75.54 (16.1) | 83.0 (68.6–85.8) | 17.65–118 | - |
| Docosapentaenoic acid (mg) | 32.35 (9.4) | 34.6 (30.4–38.6) | 2.77–38.7 | - |
| Eicosapentaenoic acid (mg) | 26.39 (7.64) | 28.22 (24.8–31.6) | 2.26–31.6 | - |
| Eicosatrienoic acid (mg) | 21.68 (6.4) | 23.23 (19.4–25.6) | 3.27–35.7 | - |
| Omega-6 LCPUFA | ||||
| Linoleic acid (g) | 588 (305) | 511 (390–712) | 180–2270 | 13 † |
| Arachidonic acid (mg) | 87.41 (34) | 79.1 (62.2–105) | 33.2–325 | - |
| Nutrient 1 | Tertiles of Intake (Range) | IL-1α | IL-1β | IL-6 | IL-8 | CRP | Any Marker 2 |
|---|---|---|---|---|---|---|---|
| aOR 1 (95%CI) | aOR 1 (95%CI) | aOR 1 (95%CI) | aOR 1 (95%CI) | aOR 1 (95%CI) | aOR 1 (95%CI) | ||
| Vit A (mcg) | <33% (32.1–286) | 1.50 (0.7–3.21) | 2.13 (0.93–4.87) | 1.18 (0.56–2.52) | 1.76 (0.81–3.79) | 1.16 (0.56–2.41) | 1.27 (0.64–2.51) |
| >67% (482–2741) | 0.96 (0.44–2.1) | 1.81 (0.8–4.1) | 0.78 (0.36–1.69) | 0.79 (0.35–1.78) | 0.62 (0.29–1.35) | 1.15 (0.58–2.28) | |
| Vit B1 (mg) | <33% 0.09–0.22) | 2.13 (0.98–4.65) | 1.76 (0.79–3.95) | 2.07 (0.95–4.48) | 2.27 (1.05–4.91) * | 1.11 (0.52–2.34) | 1.14 (0.57–2.27) |
| >67% (0.32–0.56) | 1.30 (0.59–2.85) | 0.75 (0.34–1.68) | 1.02 (0.46–2.26) | 0.74 (0.32–1.67) | 1.01 (0.48–2.12) | 0.91 (0.46–1.8) | |
| Vit B2 (mg) | <33% (0.12–0.37) | 3.17 (1.39–7.19) * | 2.11 (0.93–4.8) | 2.25 (1.03–4.94) * | 2.45 (1.12–5.37) * | 2.52 (1.17–5.45) * | 2.84 (1.37–5.88) * |
| >67% (0.55–1.60) | 1.86 (0.83–4.15) | 1.14 (0.52–2.51) | 1.10 (0.5–2.41) | 0.74 (0.33–1.68) | 1.10 (0.5–2.42) | 1.45 (0.74–2.86) | |
| Vit B3 (mg) | <33% (3.55–7.34) | 2.30 (1.03–5.12) * | 2.94 (1.25–6.9) * | 1.60 (0.74–3.46) | 3.23 (1.42–7.31) * | 0.96 (0.46–2.02) | 1.70 (0.84–3.44) |
| >67% (0.20–0.56) | 1.61 (0.72–3.58) | 1.70 (0.75–3.84) | 0.80 (0.36–1.78) | 1.32 (0.57–3.06) | 0.62 (0.29–1.34) | 1.04 (0.52–2.06) | |
| Vit B6 (mg) | <33% (0.20–0.47) | 1.78 (0.82–3.87) | 1.92 (0.85–4.34) | 2.47 (1.12–5.44) * | 2.35 (1.08–5.13) * | 1.56 (0.74–3.3) | 1.43 (0.71–2.9) |
| >67% (0.68–1.49) | 1.20 (0.55–2.61) | 0.75 (0.34–1.67) | 1.09 (0.49–2.44) | 0.71 (0.31–1.63) | 0.90 (0.41–1.95) | 0.80 (0.4–1.58) | |
| Vit B9 (Folate) (mcg) | <33% (9.36–77.3) | 1.60 (0.73–3.51) | 2.04 (0.88–4.71) | 1.16 (0.54–2.47) | 2.28 (1.04–5.02)* | 1.36 (0.64–2.89) | 1.14 (0.57–2.3) |
| >67% (121–321) | 1.28 (0.6–2.73) | 1.26 (0.57–2.76) | 0.55 (0.25–1.19) | 0.78 (0.35–1.76) | 0.86 (0.4–1.85) | 0.89 (0.45–1.76) | |
| Vit B12 (mcg) | <33% (0.20–1.60) | 0.60 (0.28–1.3) | 0.49 (0.21–1.13) | 0.42 (0.19–0.92) * | 0.49 (0.23–1.07) | 0.75 (0.35–1.6) | 0.83 (0.41–1.67) |
| >67% (1.89–2.75) | 0.63 (0.29–1.37) | 0.54 (0.24–1.24) | 0.38 (0.17–0.84) * | 0.42 (0.19–0.95) * | 0.71 (0.33–1.56) | 0.70 (0.35–1.4) | |
| Vit C (mg) | <33% (1.50–57.3) | 1.28 (0.59–2.8) | 1.28 (0.57–2.89) | 1.31 (0.61–2.81) | 1.23 (0.57–2.62) | 0.65 (0.31–1.38) | 0.89 (0.45–1.77) |
| >67% (101–261) | 1.18 (0.56–2.48) | 0.76 (0.35–1.65) | 0.82 (0.38–1.74) | 0.52 (0.24–1.15) | 0.52 (0.25–1.1) | 0.88 (0.45–1.73) | |
| Vit D (mcg) | <33% (2.26–7.71) | 0.66 (0.29–1.52) | 1.44 (0.59–3.52) | 1.33 (0.57–3.07) | 1.18 (0.51–2.73) | 0.90 (0.39–2.06) | 0.84 (0.39–1.79) |
| >67% (11.6–27.13) | 0.48 (0.23–0.99) * | 0.65 (0.31–1.37) | 0.80 (0.39–1.67) | 0.57 (0.27–1.2) | 0.86 (0.42–1.77) | 0.46 (0.23–0.91) * | |
| Vit E (mg) | <33% (1.76–2.74) | 0.91 (0.42–1.98) | 1.00 (0.43–2.34) | 0.82 (0.38–1.79) | 1.06 (0.49–2.3) | 0.90 (0.43–1.9) | 0.54 (0.27–1.09) |
| >67% (3.45–6.01) | 0.79 (0.38–1.65) | 0.76 (0.36–1.61) | 0.60 (0.28–1.26) | 0.42 (0.19–0.92) * | 0.56 (0.26–1.19) | 0.44 (0.22–0.89) * | |
| Iron (mg) | <33% (1.05–2.76) | 1.57 (0.73–3.38) | 2.37 (1.03–5.45) * | 1.52 (0.72–3.25) | 2.28 (1.05–4.97) * | 0.94 (0.45–1.94) | 1.06 (0.54–2.09) |
| >67% (4.20–7.14) | 1.30 (0.61–2.78) | 1.52 (0.68–3.37) | 0.96 (0.45–2.05) | 1.01 (0.45–2.24) | 0.70 (0.34–1.48) | 0.75 (0.38–1.46) | |
| Selenium (mg) | <33% (0.01–0.03) | 1.43 (0.67–3.06) | 1.62 (0.72–3.66) | 1.01 (0.48–2.16) | 1.79 (0.84–3.83) | 0.95 (0.46–1.97) | 0.95 (0.48–1.89) |
| >67% (0.04–0.08) | 1.23 (0.58–2.62) | 1.43 (0.66–3.1) | 1.01 (0.48–2.11) | 1.02 (0.47–2.23) | 0.65 (0.3–1.37) | 0.66 (0.33–1.3) | |
| Zinc (mg) | <33% (1.07–2.56) | 2.10 (0.95–4.65) | 2.52 (1.07–5.91) * | 2.08 (0.93–4.65) | 2.20 (1–4.86) | 1.60 (0.73–3.47) | 1.75 (0.86–3.58) |
| >67% (3.61–7.60) | 1.23 (0.56–2.7) | 1.26 (0.56–2.82) | 1.24 (0.57–2.73) | 0.68 (0.3–1.55) | 1.06 (0.49–2.28) | 0.97 (0.49–1.92) | |
| Linoleic acid (mg) 3 | <33% (1020–1994) | 1.72 (0.81–3.67) | 1.73 (0.76–3.9) | 1.35 (0.63–2.9) | 2.35 (1.07–5.15) * | 1.47 (0.69–3.12) | 1.89 (0.93–3.86) |
| >67% (2116–3561) | 0.78 (0.36–1.69) | 1.10 (0.51–2.38) | 0.85 (0.4–1.8) | 0.96 (0.43–2.12) | 0.87 (0.41–1.85) | 0.83 (0.42–1.64) | |
| Low Nutrient Intake Categories 4 | |||||||
| Water-soluble vitamins 5 | 1.08 (0.55–2.13) | 1.28 (0.64–2.57) | 1.19 (0.6–2.35) | 1.60 (0.79–3.24) | 1.40 (0.71–2.79) | 1.07 (0.58–1.97) | |
| Fat-soluble vitamins 5 | 1.33 (0.7–2.55) | 1.68 (0.86–3.27) | 1.18 (0.61–2.26) | 1.96 (1.01–3.82) * | 1.55 (0.81–2.95) | 1.34 (0.74–2.43) | |
| Minerals | 1.51 (0.8–2.85) | 2.12 (1.07–4.20) * | 1.23 (0.65–2.32) | 2.07 (1.07–3.99) * | 1.68 (0.9–3.15) | 1.49 (0.84–2.65) | |
| LCPUFAs | 1.03 (0.53–1.98) | 0.90 (0.45–1.80) | 1.99 (0.98–4.04) | 1.42 (0.72–2.83) | 1.38 (0.70–2.71) | 0.96 (0.53–1.75) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.C.; Cherkerzian, S.; Olson, I.E.; Ahmed, S.; Chowdhury, N.H.; Khanam, R.; Rahman, S.; Andrews, C.; Baqui, A.H.; Fawzi, W.; et al. Maternal Diet, Infection, and Risk of Cord Blood Inflammation in the Bangladesh Projahnmo Pregnancy Cohort. Nutrients 2021, 13, 3792. https://doi.org/10.3390/nu13113792
Lee AC, Cherkerzian S, Olson IE, Ahmed S, Chowdhury NH, Khanam R, Rahman S, Andrews C, Baqui AH, Fawzi W, et al. Maternal Diet, Infection, and Risk of Cord Blood Inflammation in the Bangladesh Projahnmo Pregnancy Cohort. Nutrients. 2021; 13(11):3792. https://doi.org/10.3390/nu13113792
Chicago/Turabian StyleLee, Anne CC, Sara Cherkerzian, Ingrid E Olson, Salahuddin Ahmed, Nabidul Haque Chowdhury, Rasheda Khanam, Sayedur Rahman, Chloe Andrews, Abdullah H Baqui, Wafaie Fawzi, and et al. 2021. "Maternal Diet, Infection, and Risk of Cord Blood Inflammation in the Bangladesh Projahnmo Pregnancy Cohort" Nutrients 13, no. 11: 3792. https://doi.org/10.3390/nu13113792
APA StyleLee, A. C., Cherkerzian, S., Olson, I. E., Ahmed, S., Chowdhury, N. H., Khanam, R., Rahman, S., Andrews, C., Baqui, A. H., Fawzi, W., Inder, T. E., Nartey, S., Nelson, C. A., Oken, E., Sen, S., & Fichorova, R. (2021). Maternal Diet, Infection, and Risk of Cord Blood Inflammation in the Bangladesh Projahnmo Pregnancy Cohort. Nutrients, 13(11), 3792. https://doi.org/10.3390/nu13113792







