Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review
Abstract
:1. Introduction
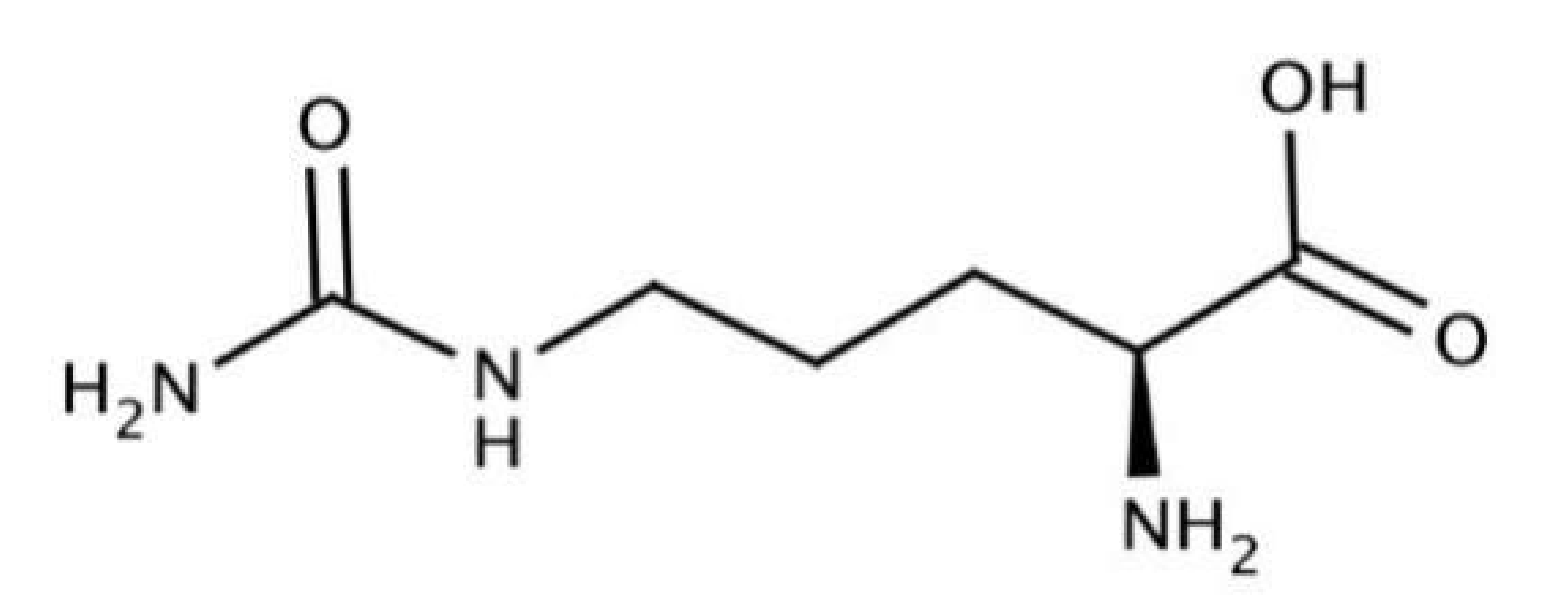
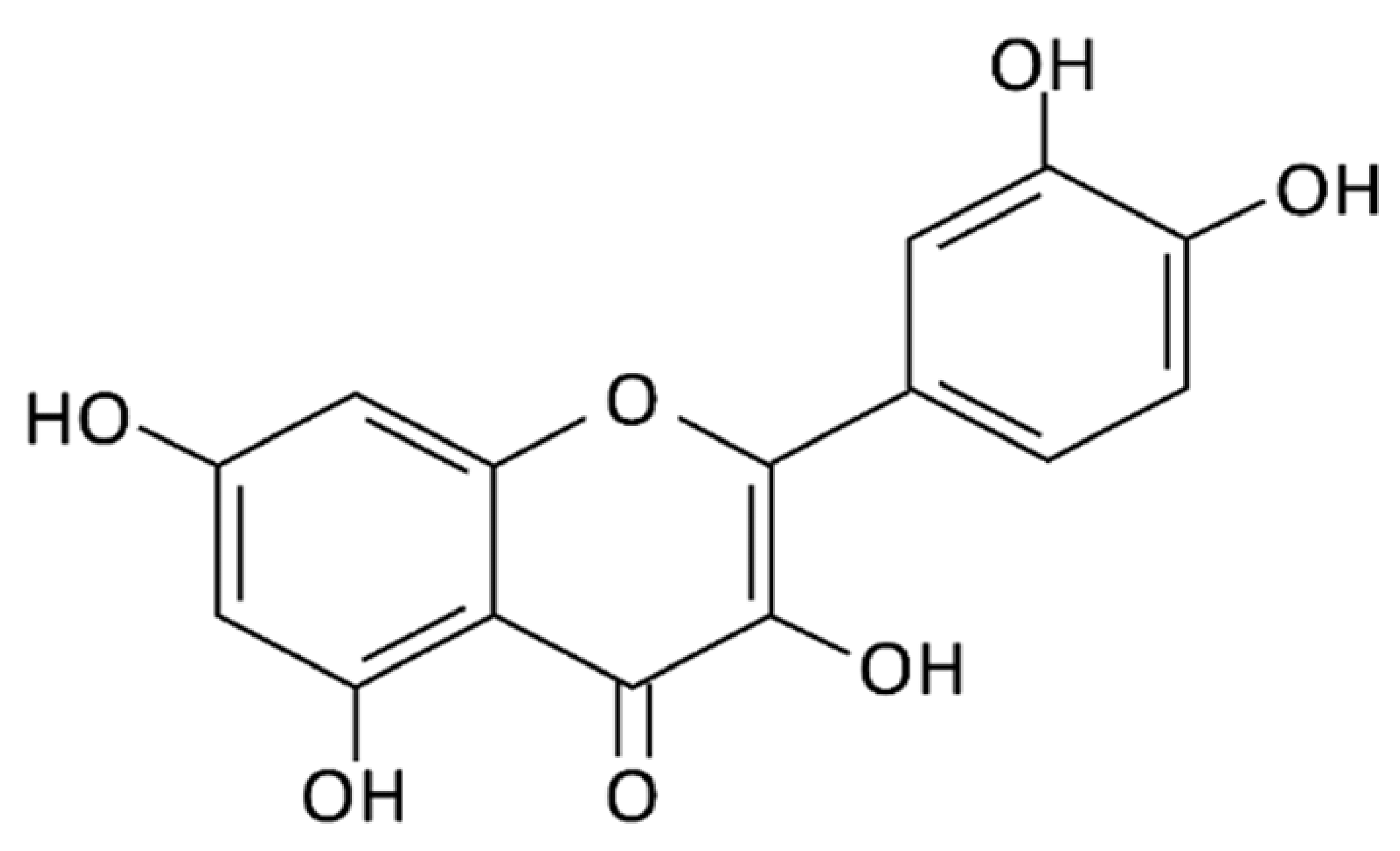
2. Overview on Citrulline and Quercetin
3. Metabolism of Citrulline and Quercetin
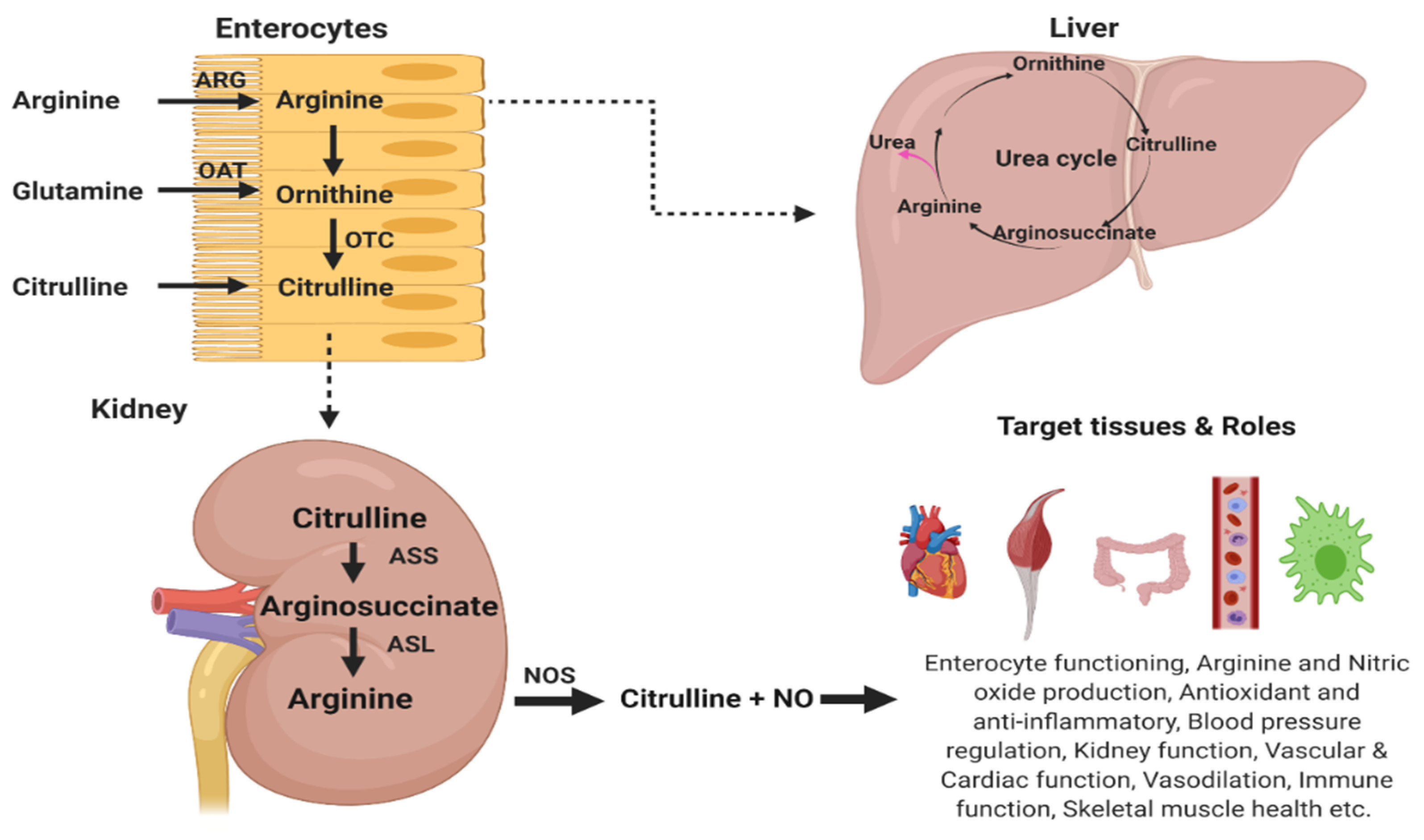
3.1. Metabolism of l-Citrulline
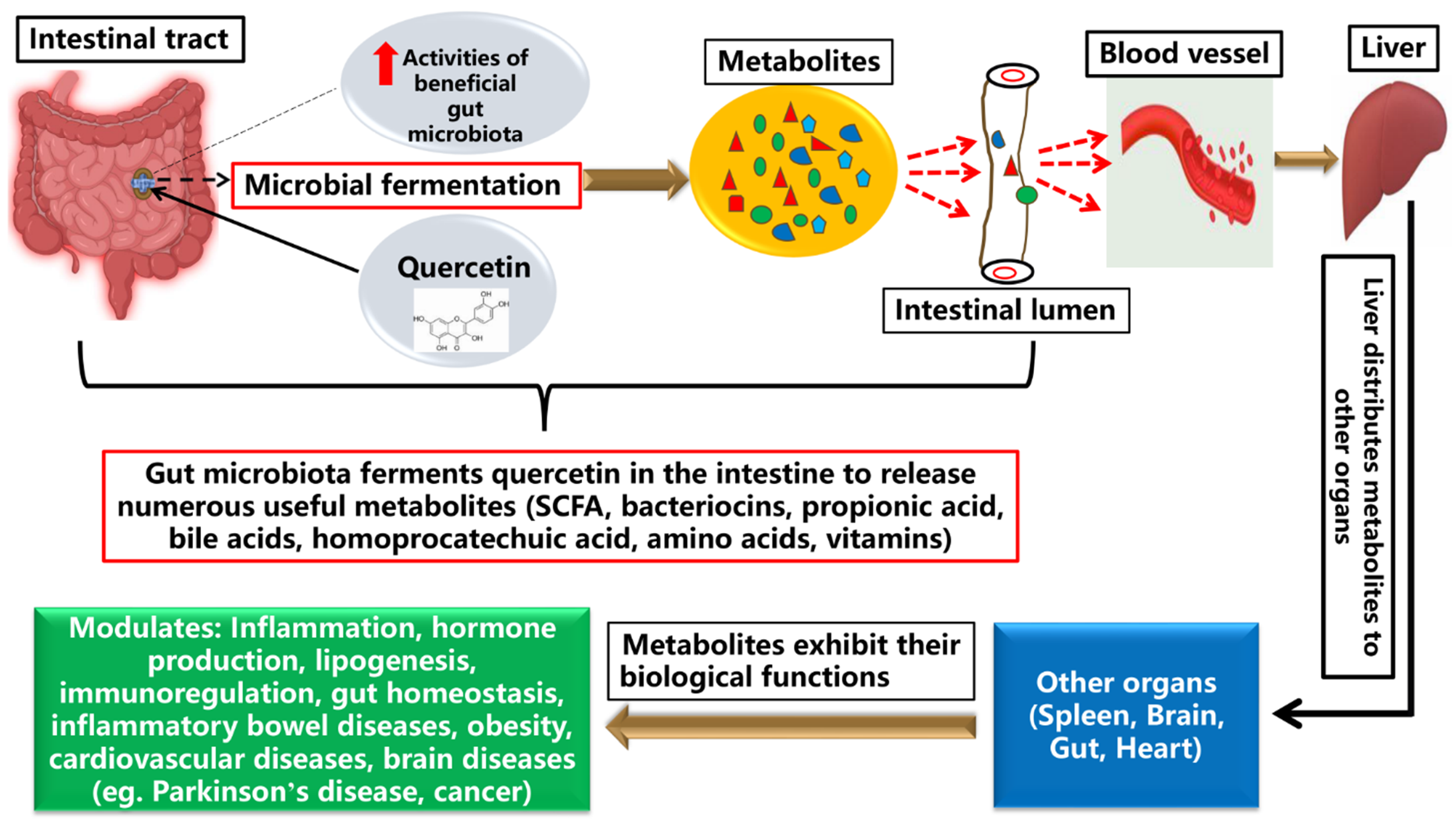
3.2. Metabolism of Quercetin
4. Role of Citrulline and Quercetin in Gut Functioning
4.1. Effects of Citrulline on Gut Functions of Animals
4.2. Effects of Quercetin on Gut Functions of Animals
4.3. Potential Combinatory Roles for Citrulline and Quercetin in Gastrointestinal Health of Animals
- (a)
- Anti-inflammatory and immunomodulatory functions
- (b)
- Nitric oxide regulation
- (c)
- Anti-oxidative functions
- (d)
- In vitro effects of citrulline and quercetin on intestinal cell integrity
5. Knowledge Gaps and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Briskey, D.; Tucker, P.S.; Johnson, D.W.; Coombes, J.S. Microbiota and the nitrogen cycle: Implications in the development and progression of CVD and CKD. Nitric Oxide 2016, 57, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Jala, V.R.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Bortoluzzi, C.; Rochell, S.J.; Applegate, T.J. Threonine, arginine, and glutamine: Influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 2018, 97, 937–945. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Boudry, G.; Le Huërou-Luron, I.; Michel, C. Dietary Protein and Colonic Microbiota—The Molecular Nutrition of Amino Acids and Proteins; Academic Press: Cambridge, MA, USA, 2016; pp. 207–220. [Google Scholar]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [Green Version]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Benazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Kaore, S.N.; Kaore, N.M. Citrulline: Pharmacological Perspectives and Role as a Biomarker in Diseases and Toxicities. In Biomarkers in Toxicology; Gupta, R., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 883–905. [Google Scholar]
- Lange, S.M.; McKell, M.C.; Schmidt, S.M.; Zhao, J.; Crowther, R.R.; Green, L.C.; Bricker, R.L.; Arnett, E.; Köhler, S.E.; Schlesinger, L.S.; et al. L-Arginine Synthesis from L-Citrulline in Myeloid Cells Drives Host Defense against Mycobacteria In Vivo. J. Immunol. 2019, 202, 1747. [Google Scholar] [CrossRef] [Green Version]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Dey, N.; Bhattacharjee, S. Accumulation of Polyphenolic Compounds and Osmolytes under Dehydration Stress and Their Implication in Redox Regulation in Four Indigenous Aromatic Rice Cultivars. Rice Sci. 2020, 27, 329–344. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gabe, S.M.; Seidner, D.L.; Lee, H.M.; Olivier, C. Citrulline correlations in short bowel syndrome-intestinal failure by patient stratification: Analysis of 24 weeks of teduglutide treatment from a randomized controlled study. Clin. Nutr. 2020, 39, 2479–2486. [Google Scholar] [CrossRef] [Green Version]
- Jirka, A.; Layec, S.; Picot, D.; Bernon-Ferreira, S.; Grasset, N.; Flet, L.; Thibault, R.; Darmaun, D. Effect of oral citrulline supplementation on whole body protein metabolism in adult patients with short bowel syndrome: A pilot, randomized, double-blind, cross-over study. Clin. Nutr. 2019, 38, 2599–2606. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; Gou, L.; Fu, X.; Li, S.; Lan, N.; Yin, X. Protective effect of l-citrulline against ethanol-induced gastric ulcer in rats. Environ. Toxicol. Pharmacol. 2012, 34, 280–287. [Google Scholar] [CrossRef]
- Ioannou, H.P.; Diamanti, E.; Piretzi, K.; Drossou-Agakidou, V.; Augoustides-Savvopoulou, P. Plasma citrulline levels in preterm neonates with necrotizing enterocolitis. Early Hum. Dev. 2012, 88, 563–566. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.P.; Rezzi, S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef]
- Shu, G.; Kong, F.; Xu, D.; Yin, L.; He, C.; Lin, J.; Fu, H.; Wang, K.; Tian, Y.; Zhao, X. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci. Rep. 2020, 10, 12324. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Laurentius, A.; Wikanendra, G.B.; Cong, T.H.; Arozal, W. L-citrulline as Alternative Pharmacological Substance in Protecting Against Cardiovascular Disease. Pharm. Sci. Res. 2018, 5, 72–80. [Google Scholar]
- Joshi, V.; Joshi, M.; Silwal, D.; Noonan, K.; Rodriguez, S.; Penalosa, A. Systematized biosynthesis and catabolism regulate citrulline accumulation in watermelon. Phytochemistry 2019, 162, 129–140. [Google Scholar] [CrossRef]
- Yao, L.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Ann. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Marcolin, E.; San-Miguel, B.; Vallejo, D.; Tieppo, J.; Marroni, N.; Gonzalez-Gallego, J.; Tuñón, M.J. Quercetin Treatment Ameliorates Inflammation and Fibrosis in Mice with Nonalcoholic Steatohepatitis. J. Nutr. 2012, 142, 1821–1828. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the Dietary Flavonoid Quercetin Upon Performance and Health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food. Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Frank, K.; Patel, K.; Lopez, G. Citrulline Research Analysis. 2018. Available online: https://examine.com/supplements/citrulline/research/ (accessed on 24 April 2021).
- Cuzzocrea, S.; Salvemini, D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007, 71, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guslandi, M. Nitric oxide and inflammatory bowel diseases. Eur. J. Clin. Investig. 1998, 28, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin. Nutr. 2008, 27, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Crenn, P.; Cynober, L. Citrulline and the gut. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 620–626. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Jobgen, W.S.; Kim, S.W.; Lassala, A.; Li, P.; Matis, J.H.; Meininger, C.J.; Spencer, T.E. Pharmacokinetics and Safety of Arginine Supplementation in Animals. J. Nutr. 2007, 137, 1673S–1680S. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.H. Comparative nutrition and metabolism: Explication of open questions with emphasis on protein and amino acids. Proc. Natl. Acad. Sci. USA 2005, 102, 17897–17902. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, W.; Takada, S.; Hirao, J.; Shirai, M.; Iguchi, T.; Tsuji, M.; Nishiya, T.; Mori, K. Plasma citrulline is a sensitive safety biomarker for small intestinal injury in rats. Toxicol. Lett. 2018, 295, 416–423. [Google Scholar] [CrossRef]
- Agarwal, U.; Didelija, I.C.; Yuan, Y.; Wang, X.; Marini, J.C. Supplemental citrulline is more efficient than arginine in increasing systemic arginine availability in mice. J. Nutr. 2017, 147, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Uyanga, V.A.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. Dietary L-citrulline supplementation modulates nitric oxide synthesis and anti-oxidant status of laying hens during summer season. J. Anim. Sci. Biotechnol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Meesters, D.M.; van Barneveld, K.W.; Visschers, R.G.; Briede, J.J.; Vandendriessche, B.; van Eijk, H.M.; Bessems, B.A.; van den Hoven, N.; von Wintersdorff, C.J.; et al. Citrulline Supplementation Improves Organ Perfusion and Arginine Availability under Conditions with Enhanced Arginase Activity. Nutrients 2015, 7, 5217–5238. [Google Scholar] [CrossRef] [Green Version]
- Wijnands, K.A.; Vink, H.; Briede, J.J.; van Faassen, E.E.; Lamers, W.H.; Buurman, W.A.; Poeze, M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS ONE 2012, 7, e37439. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J. Agric. Food Chem. 2002, 50, 618–621. [Google Scholar] [CrossRef]
- Ader, P.; Wessmann, A.; Wolffram, S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic. Biol. Med. 2000, 28, 1056–1067. [Google Scholar] [CrossRef]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Graefe, E.U.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int. J. Clin. Pharmacol. Ther. 1999, 37, 219–233. [Google Scholar]
- Azuma, K.; Ippoushi, K.; Ito, H.; Higashio, H.; Terao, J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J. Agric. Food Chem. 2002, 50, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.; van Trijp, J.M.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997, 114, 139–140. [Google Scholar] [CrossRef]
- Hollman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- Arts, I.C.; Sesink, A.L.; Faassen-Peters, M.; Hollman, P.C. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Cermak, R.; Landgraf, S.; Wolffram, S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef] [Green Version]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Dragoni, S.; Gee, J.; Bennett, R.; Valoti, M.; Sgaragli, G. Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro. Br. J. Pharmacol. 2006, 147, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, N.; Matsumoto, M.; Shinoki, A.; Hagio, M.; Inoue, R.; Hara, H. Nondigestible saccharides suppress the bacterial degradation of quercetin aglycone in the large intestine and enhance the bioavailability of quercetin glucoside in rats. J. Agric. Food Chem. 2009, 57, 9462–9468. [Google Scholar] [CrossRef]
- Guo, Y.; Mahm, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef]
- Lesser, S.; Cermak, R.; Wolffram, S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J. Nutr. 2004, 134, 1508–1511. [Google Scholar] [CrossRef]
- Vacek, J.; Papoušková, B.; Kosina, P.; Vrba, J.; Křen, V.; Ulrichová, J. Biotransformation of flavonols and taxifolin in hepatocyte in vitro systems as determined by liquid chromatography with various stationary phases and electrospray ionization-quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2012, 899, 109–115. [Google Scholar] [CrossRef]
- Cermak, R.; Wolffram, S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr. Drug Metab. 2006, 7, 729–744. [Google Scholar] [CrossRef]
- Galindo, P.; Rodriguez-Gómez, I.; González-Manzano, S.; Dueñas, M.; Jiménez, R.; Menéndez, C.; Vargas, F.; Tamargo, J.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS ONE 2012, 7, e32673. [Google Scholar] [CrossRef] [Green Version]
- Menendez, C.; Dueñas, M.; Galindo, P.; González-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodríguez-Gómez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular deconjugation of quercetin glucuronide: The flavonoid paradox revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Shimoi, K.; Saka, N.; Nozawa, R.; Sato, M.; Amano, I.; Nakayama, T.; Kinae, N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001, 29, 1521–1524. [Google Scholar]
- Araújo, K.C.; Costa, E.M.D.M.; Pazini, F.; Valadares, M.C.; de Oliveira, V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. [Google Scholar] [CrossRef]
- Beekmann, K.; Actis-Goretta, L.; van Bladeren, P.J.; Dionisi, F.; Destaillats, F.; Rietjens, I.M. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012, 3, 1008–1018. [Google Scholar] [CrossRef]
- Lodi, F.; Jimenez, R.; Moreno, L.; Kroon, P.A.; Needs, P.W.; Hughes, D.A.; Santos-Buelga, C.; Gonzalez-Paramas, A.; Cogolludo, A.; Lopez-Sepulveda, R.; et al. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis 2009, 204, 34–39. [Google Scholar] [CrossRef]
- Tribolo, S.; Lodi, F.; Connor, C.; Suri, S.; Wilson, V.G.; Taylor, M.A.; Needs, P.W.; Kroon, P.A.; Hughes, D.A. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis 2008, 197, 50–56. [Google Scholar] [CrossRef]
- Williamson, G.; Barron, D.; Shimoi, K.; Terao, J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic. Res. 2005, 39, 457–469. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Cermak, R.; Breves, G.M. In vitro degradation of the flavonol quercetin and of quercetin glycosides in the porcine hindgut. Arch. Anim. Nutr. 2006, 60, 180–189. [Google Scholar] [CrossRef]
- Sokolová, R.; Ramešová, Š.; Degano, I.; Hromadová, M.; Gál, M.; Žabka, J. The oxidation of natural flavonoid quercetin. Chem. Commun. 2012, 48, 3433–3435. [Google Scholar] [CrossRef]
- Ramešová, S.; Sokolová, R.; Degano, I.; Bulíčková, J.; Zabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef]
- Walle, T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef]
- Walle, T.; Walle, U.K.; Halushka, P.V. Carbon dioxide is the major metabolite of quercetin in humans. J. Nutr. 2001, 131, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachie, R.F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; Collado, M.C.; Ferreira, C.L.; Bressan, J.; Peluzio Mdo, C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Banerjee, A. Gastrointestinal Toxicity Biomarkers. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014; pp. 269–277. [Google Scholar]
- Grimaldi, D.; Guivarch, E.; Neveux, N.; Fichet, J.; Pene, F.; Marx, J.S.; Chiche, J.D.; Cynober, L.; Mira, J.P.; Cariou, A. Markers of intestinal injury are associated with endotoxemia in successfully resuscitated patients. Resuscitation 2013, 84, 60–65. [Google Scholar] [CrossRef]
- Derikx, J.P.M.; Luyer, M.D.P.; Heineman, E.; Buurman, W.A. Non-invasive markers of gut wall integrity in health and disease. World J. Gastroenterol. 2010, 16, 5272–5279. [Google Scholar] [CrossRef]
- Papadia, C.; Dhaliwal, W.; Kelly, P.; Corazza, G.R.; Franzè, A.; Di Sabatino, A. Plasma citrulline as a quantitative biomarker of HIV-associated duodenalmucosal damage in a tropical enteropathy population. Clin. Nutr. 2010, 29, 795–800. [Google Scholar] [CrossRef]
- Jäckel, S.; Emde, B.; Lai, V.; Weigt, S.; Hanschke, B.; Kasper, L. L-Citrulline as translational safety biomarker for the small intestine—An update. J. Pharmacol. Toxicol. Methods 2020, 105, 106863. [Google Scholar] [CrossRef]
- Shen, L.J.; Guan, Y.Y.; Wu, X.P.; Wang, Q.; Wang, L.; Xiao, T.; Wu, H.R.; Wang, J.G. Serum citrulline as a diagnostic marker of sepsis-induced intestinal dysfunction. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 230–236. [Google Scholar] [CrossRef]
- Sellmann, C.; Jin, C.J.; Engstler, A.J.; De Bandt, J.P.; Bergheim, I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur. J. Nutr. 2017, 56, 2519–2527. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Zayed, A.; Farag, M.A. Gut homeostasis and microbiota under attack: Impact of the different types of food contaminants on gut health. Crit. Rev. Food Sci. Nutr. 2020, 2020, 1–26. [Google Scholar] [CrossRef]
- Mouna, H.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Florence, L.; Cournède, N.; Doré, J.; Melchior, J.C. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin. Nutr. 2018, 38, 2304–2310. [Google Scholar] [CrossRef]
- Diling, C.; Yinrui, G.; Longkai, Q.; Xiaocui, T.; Yadi, L.; Jiaxin, F. Metabolic regulation of Ganoderma lucidum extracts in high sugar and fat diet-induced obese mice by regulating the gut-brain axis. J. Funct. Foods 2020, 65, 103639. [Google Scholar] [CrossRef]
- Ho, S.W.; El-Nezami, H.; Shah, N.P. Effects of supplementation of citrulline and Lactobacillus helveticus ASCC 511 on intestinal epithelial cell integrity. J. Funct. Foods 2020, 64, 103571. [Google Scholar] [CrossRef]
- Moinard, C.; Walrand, S.; Boirie, Y.; Cynober, L. Use of Citrulline for the Treatment of Conditions Linked to an Increase in Protein Carbonylation. 2007. Available online: http://europepmc.org/article/PAT/EP2136777 (accessed on 10 March 2021).
- Becraft, A.R.; Sturm, M.L.; Mendez, R.L.; Park, S.H.; Lee, S.I.; Shay, N.F. Intake of Watermelon or Its Byproducts Alters Glucose Metabolism, the Microbiome, and Hepatic Proinflammatory Metabolites in High-Fat-Fed Male C57BL/6 J Mice. J. Nutr. 2020, 150, 434–442. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Chun, W.K.; Kim, A.; Kim, N.; Roh, H.J.; Lee, Y.; Yi, M.; Kim, S.; Park, C.; Kim, D.H. Dietary Probiotic Effect of Lactococcus lactis WFLU12 on Low-Molecular-Weight Metabolites and Growth of Olive Flounder (Paralichythys olivaceus). Front. Microbiol. 2018, 9, 2059. [Google Scholar] [CrossRef]
- Chen, R.Q.; Liao, C.B.; Guo, Q.; Wu, L.R.; Zhang, L.; Wang, X.F. Combined systems pharmacology and fecal metabonomics to study the biomarkers and therapeutic mechanism of type 2 diabetic nephropathy treated with Astragalus and Leech. RSC Adv. 2018, 8, 27448–27463. [Google Scholar] [CrossRef] [Green Version]
- Adamovsky, O.; Buerger, A.N.; Vespalcova, H.; Sohag, S.R.; Hanlon, A.T.; Ginn, P.E.; Craft, S.L.; Budinska, E.; Persico, M. et al. Evaluation of Microbiome-Host Relationships in the Zebrafish Gastrointestinal System Reveals Adaptive Immunity Is a Target of Bis(2-ethylhexyl) Phthalate (DEHP) Exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef]
- Jackel, S.; Pipp, F.C.; Emde, B.; Weigt, S.; Vigna, E.; Hanschke, B.; Kasper, L.; Siddharta, A.; Hellmann, J.; Czasch, S.; et al. L-citrulline: A preclinical safety biomarker for the small intestine in rats and dogs in repeat dose toxicity studies. J. Pharmacol. Toxicol. Methods 2021, 110, 107110. [Google Scholar] [CrossRef]
- Authier, S.; Tang, H.-M.; Abtout, S.; Ascah, A.; Pouliot, M.; Bujold, K.; Troncy, E.; Pugsley, M.K.; Forster, R. Gastroinstestinal motility: Motility and motor migrating complex (mmc) evaluations in rats, dogs and non-human primates. J. Pharmacol. Toxicol. Methods 2016, 81, 389–390. [Google Scholar] [CrossRef]
- Feenstra, F.A.; Kuik, S.J.; Derikx, J.P.M.; Heiner-Fokkema, M.R.; Kooi, E.M.W.; Bos, A.F.; Hulscher, J.B.F. Plasma citrulline during the first 48 h after onset of necrotizing enterocolitis in preterm infants. J. Pediatr. Surg. 2020, 56, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.; Rubio, A.; Lasserre, V.; Maccario, J.; Walrand, S.; Neveux, N.; Plenier, S.L.; Hebuterne, X.; Cynober, L.; Moinard, C. Dose-dependent beneficial effects of citrulline supplementation in short bowel syndrome in rats. Nutr. J. 2021, 85, 111118. [Google Scholar] [CrossRef] [PubMed]
- Rajcic, D.; Baumann, A.; Hernandez-Arriaga, A.; Brandt, A.; Nier, A.; Jin, C.J.; Sanchez, V.; Jung, F.; Camarinha-Silva, A.; Bergheim, I. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biol. 2021, 41, 101879. [Google Scholar] [CrossRef] [PubMed]
- Van Wijck, K.; Wijnands, K.A.P.; Meesters, D.M.; Boonen, B.; van Loon, L.J.C.; Buurman, W.A.; Dejong, C.H.C.; Lenaerts, K.; Poeze, M. L-citrulline improves splanchnic perfusion and reduces gut injury during exercise. Med. Sci. Sports Exerc. 2014, 46, 2039–2046. [Google Scholar] [CrossRef]
- Batista, M.A.; Nicoli, J.R.; dos Santos Martins, F.; Nogueira Machado, J.A.; Esteves Arantes, R.M.; Pacífico Quirino, I.E. Davisson Correia, M.I.T.; Cardoso, V.N. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. J. Parenter. Enter. Nutr. 2012, 36, 69–76. [Google Scholar] [CrossRef]
- Najmanová, I.; Pourová, J.; Vopršalová, M.; Pilařová, V.; Semecký, V.; Nováková, L.; Mladěnka, P. Flavonoid metabolite 3-(3-hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef]
- Osowska, S.; Moinard, C.; Loï, C.; Neveux, N.; Cynober, L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 2004, 53, 1781. [Google Scholar] [CrossRef] [Green Version]
- Gou, L.; Zhang, L.; Yin, C.; Jia, G.; Yin, X.; Zhuang, X.; Xu, X.; Liu, Y. Protective effect of l-citrulline against acute gastric mucosal lesions induced by ischemia-reperfusion in rats. Can. J. Physiol. Pharmacol. 2011, 89, 317–327. [Google Scholar] [CrossRef]
- Antunes, M.M.; Leocádio, P.C.L.; Teixeira, L.G.; Leonel, A.J.; Cara, D.C.; Menezes, G.B.; Generoso, S.d.V.; Cardoso, V.N.; Alvarez-Leite, J.I.; Correia, M.I.T.D. Pretreatment with L-citrulline positively affects the mucosal architecture and permeability of the small intestine in a murine mucositis model. J. Parenter. Enter. Nutr. 2016, 40, 279–286. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef]
- Valenzano, M.C.; DiGuilio, K.; Mercado, J.; Teter, M.; To, J.; Ferraro, B.; Mixson, B.; Manley, I.; Baker, V.; Moore, B.A.; et al. Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients. PLoS ONE 2015, 10, e0133926. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Xie, W.; Jiang, Z.; Wang, M.; Wang, J.; Zhao, H.; Zhang, X. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, J.; Zhang, B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020, 99, 4892–4903. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in citrobacter rodentium-infected mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of nrf2 pathway. Molecules 2020, 25, 1053. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wei, H.K.; Xiang, Q.H.; Wang, J.; Zhou, Y.F.; Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016, 78, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.Y.; Zang, K.H.; Zuo, X.; Wu, X.A.; Bian, Z.X. Quercetin attenuates visceral hypersensitivity and 5-hydroxytryptamine availability in postinflammatory irritable bowel syndrome rats: Role of enterochromaffin cells in the colon. J. Med. Food. 2019, 22, 663–671. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Qian, D.; Peng, F.; Li, H.; Liang, C.; Du, M.; Gu, J.; Mai, J.; Bai, B.; et al. Quercetin modulates the gut microbiota as well as the metabolome in a rat model of osteoarthritis. Bioengineered 2021, 12, 6240–6250. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal mrna expression genes in broiler chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.-Y.; Zhu, Y.; Yang, X. Quercetin ameliorates gut microbiota dysbiosis that drives hypothalamic damage and hepatic lipogenesis in monosodium glutamate-induced abdominal obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Hong, Z.; Piao, M. Effect of quercetin monoglycosides on oxidative stress and gut microbiota diversity in mice with dextran sodium sulphate-induced colitis. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, M.-H.; Huang, H.-L.; Zhou, A.-C.; Chu, Z.-D.; Huang, X.-D.; Li, C.-W. Protective effects of citrulline supplementation in ulcerative colitis rats. PLoS ONE 2020, 15, e0240883. [Google Scholar] [CrossRef]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouaknine Krief, J.; Helly de Tauriers, P.; Dumenil, C.; Neveux, N.; Dumoulin, J.; Giraud, V.; Labrune, S.; Tisserand, J.; Julie, C.; Emile, J.F.; et al. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J. Immunother. Cancer 2019, 7, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vande Vyver, M.; Beelen, R.; De Keyser, J.; Nagels, G.; Van Binst, A.M.; Verborgh, C.; D’haeseleer, M. Plasma citrulline levels are increased in patients with multiple sclerosis. J. Neurol. Sci. 2018, 387, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Breuillard, C.; Curis, E.; Le Plenier, S.; Cynober, L.; Moinard, C. Nitric oxide production by peritoneal macrophages from aged rats: A short term and direct modulation by citrulline. Biochimie 2017, 133, 66–73. [Google Scholar] [CrossRef]
- Lange, S.M.; McKell, M.C.; Schmidt, S.M.; Hossfeld, A.P.; Chaturvedi, V.; Kinder, J.M.; McAlees, J.W.; Lewkowich, I.P.; Way, S.S.; Turner, J.; et al. L-Citrulline metabolism in mice augments cd4+ t cell proliferation and cytokine production in vitro, and accumulation in the mycobacteria-infected lung. Front. Immunol. 2017, 8, 1561. [Google Scholar] [CrossRef] [Green Version]
- Breuillard, C.; Bonhomme, S.; Couderc, R.; Cynober, L.; De Bandt, J.P. In vitro anti-inflammatory effects of citrulline on peritoneal macrophages in Zucker diabetic fatty rats. Br. J. Nutr. 2015, 113, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Su, Y.T.; Liu, T.Y.; Tsai, C.M.; Chang, C.H.; Yu, H.R. L-arginine and l-citrulline supplementation have different programming effect on regulatory T-cells function of infantile rats. Front. Immunol. 2018, 9, 2911. [Google Scholar] [CrossRef] [Green Version]
- Crenn, P.; Neveux, N.; Chevret, S.; Jaffray, P.; Cynober, L.; Melchior, J.C.; Annane, D.; for the COIITSS Study Group. Plasma L-citrulline concentrations and its relationship with inflammation at the onset of septic shock: A pilot study. J. Crit. Care 2014, 29, 315.e1–315.e6. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.; Mahdavi, R.; Vaghef-Mehrabany, E.; Maleki, V.; Karamzad, N.; Ebrahimi-Mameghani, M. Potential roles of Citrulline and watermelon extract on metabolic and inflammatory variables in diabetes mellitus, current evidence and future directions: A systematic review. Clin. Exp. Pharm. Physiol. 2020, 47, 187–198. [Google Scholar] [CrossRef]
- Romero, M.J.; Yao, L.; Sridhar, S.; Bhatta, A.; Dou, H.; Ramesh, G.; Brands, M.W.; Pollock, D.M.; Caldwell, R.B.; Cederbaum, S.D.; et al. L-Citrulline protects from kidney damage in type 1 diabetic mice. Front. Immunol. 2013, 4, 480. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, R.; Machado, M.; Fontinha, F.; Fernandez-Boo, S.; Conceição, L.E.; Dias, J.; Costas, B. Dietary arginine and citrulline supplementation modulates the immune condition and inflammatory response of European seabass. Fish. Shellfish Immunol. 2020, 106, 451–463. [Google Scholar] [CrossRef]
- Uzzan, M.; Soudan, D.; Peoc’h, K.; Weiss, E.; Corcos, O.; Treton, X. Patients with COVID-19 present with low plasma citrulline concentrations that associate with systemic inflammation and gastrointestinal symptoms. Dig. Liver Dis. 2020, 52, 1104–1105. [Google Scholar] [CrossRef]
- Boonlaos, A.; Wechsirisan, W.; Chaibuth, P.; Chupia, V.; Chotinun, S.; Chuammitri, P. Quercetin enhances and modulates the fungal killing efficacy of chicken heterophils through immunological recognition, effector functions, and resolution. Comp. Immunol. Microbiol. Infect. Dis. 2020, 74, 101582. [Google Scholar] [CrossRef]
- Yang, J.X.; Maria, T.C.; Zhou, B.; Xiao, F.L.; Wang, M.; Mao, Y.J.; Li, Y. Quercetin improves immune function in Arbor Acre broilers through activation of NF-kappaB signaling pathway. Poult. Sci. 2020, 99, 906–913. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef] [Green Version]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Medina, F.S.D. Effects of Flavonoids and other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Habtemariam, S.; Belai, A. Natural Therapies of the Inflammatory Bowel Disease: The Case of Rutin and its Aglycone, Quercetin. Mini Rev. Med. Chem. 2018, 18, 234–243. [Google Scholar] [CrossRef]
- McCafferty, D.M. Peroxynitrite and inflammatory bowel disease. Gut 2000, 46, 436. [Google Scholar] [CrossRef] [Green Version]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef]
- Stettner, N.; Rosen, C.; Bernshtein, B.; Gur-Cohen, S.; Frug, J.; Silberman, A.; Sarver, A.; Carmel-Neidermann, N.N.; Eilam, R.; Biton, I.; et al. Induction of nitric-oxide metabolism in enterocytes alleviates colitis and inflammation-associated colon cancer. Cell Rep. 2018, 23, 1962–1976. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Uyanga, V.A.; Wang, M.; Tong, T.; Zhao, J.; Wang, X.; Jiao, H.; Onagbesan, O.M.; Lin, H. L-citrulline influences the body temperature, heat shock response and nitric oxide regeneration of broilers under thermoneutral and heat stress condition. Front. Physiol. 2021, 12, 671691. [Google Scholar] [CrossRef]
- Donald, J.A. Subchapter 103A: Nitric oxide in Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 603–605. [Google Scholar] [CrossRef]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaίno, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Zhang, Z.-Y.; Wang, R.-X. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 2020, 11, 956. [Google Scholar] [CrossRef]
- Ozu, O.Y.; Ertug, P.U.; Karabulut, E.; Kumcu, E.K.; Singirik, E.; Secilmis, M.A. Dose-dependent differential mechanism of quercetin-induced vasodilatations in isolated perfused rat mesenteric vascular bed. Int. J. Pharmacol. 2016, 12, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic potential of quercetin as an antiatherosclerotic agent in atherosclerotic cardiovascular disease: A review. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Martins-Perles, J.V.C.; Bossolani, G.D.P.; Zignani, I.; de Souza, S.R.G.; Frez, F.C.V.; de Souza Melo, C.G.; Barili, E.; de Souza Neto, F.P.; Guarnier, F.A.; Armani, A.L.C.; et al. Quercetin increases bioavailability of nitric oxide in the jejunum of euglycemic and diabetic rats and induces neuronal plasticity in the myenteric plexus. Auton. Neurosci. 2020, 227, 102675. [Google Scholar] [CrossRef] [PubMed]
- Faddah, L.M.; Baky, N.A.A.; Mohamed, A.M.; Al-Rasheed, N.M.; Al-Rasheed, N.M. Protective effect of quercetin and/or l-arginine against nano-zinc oxide-induced cardiotoxicity in rats. J. Nanopart. Res. 2013, 15, 1520. [Google Scholar] [CrossRef]
- Baky, N.A.A.; Faddah, L.M.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Shebali, W. Role of quercetin and l-arginine in alleviating zinc oxide nanoparticle hepatotoxicity in rats. Chiang Mai J. Sci. 2013, 40, 577–592. [Google Scholar]
- Abdelhalim, M.A.K.; Moussa, S.A.A.; Qaid, H.A.Y. The protective role of quercetin and arginine on gold nanoparticles induced hepatotoxicity in rats. Int. J. Nanomed. 2018, 13, 2821–2825. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Coles, K.E. Investigation into the Antioxidant Capacity of L-Arginine and L-Citrulline in Relation to Their Vascular Protective Properties; Cardiff University: Cardiff, UK, 2007. [Google Scholar]
- Salama, Y.A.; El-Karef, A.; El Gayyar, A.M.; Abdel-Rahman, N. Beyond its antioxidant properties: Quercetin targets multiple signalling pathways in hepatocellular carcinoma in rats. Life Sci. 2019, 236, 116933. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Sahiner, H.S.; Epikmen, E.T.; Fiaz, M.; Yousuf, M.R.; Khan, S.A.; Serin, I.; Ceylan, A.; Aksoy, M. Dietary quercetin maintains the semen quality in rabbits under summer heat stress. Theriogenology 2018, 122, 88–93. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the nrf2/nrf1 transcription pathway. Investig. Ophthalmol. Vis. Sci. 2010, 52, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-H.; Lin, M.-T.; Chang, C.-P. Ischemic and oxidative damage to the hypothalamus may be responsible for heat stroke. Curr. Neuropharmacol. 2013, 11, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.R.; Ferreira, A.J.; PalermoNeto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Chapman, J.C.; Liu, Y.; Zhu, L.; Rhoads, J.M. Arginine and citrulline protect intestinal cell monolayer tight junctions from hypoxia-induced injury in piglets. Pediatr. Res. 2012, 72, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, J.M.; Chen, W.; Gookin, J.; Wu, G.Y.; Fu, Q.; Blikslager, A.T.; Rippe, R.A.; Argenzio, R.A.; Cance, W.G.; Weaver, E.M.; et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut 2004, 53, 514–522. [Google Scholar] [CrossRef]
- Noiri, E.; Peresleni, T.; Srivastava, N.; Weber, P.; Bahou, W.F.; Peunova, N.; Goligorsky, M.S. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. Am. J. Physiol. 1996, 270, C794–C802. [Google Scholar] [CrossRef]
- Gookin, J.L.; Rhoads, J.M.; Argenzio, R.A. Inducible nitric oxide synthase mediates early epithelial repair of porcine ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G157–G168. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Cai, X.; Chen, X.; Wang, X.; Xu, C.; Guo, Q.; Zhu, L.; Zhu, S.; Xu, J. Pre-protective effect of lipoic acid on injury induced by H2O2 in IPEC-J2 cells. Mol. Cell Biochem. 2013, 378, 73–81. [Google Scholar] [CrossRef]
- Cai, X.; Wang, J.; Chen, X.; Guo, Q.; Zhu, L.; Xu, J. The Research Progress in Intestinal Epithelial Oxidative Stress Cell Model. Acta Vet. Zoo Sin. 2014, 45, 337–346. [Google Scholar]
- Farkas, O.; Mátis, G.; Pászti-Gere, E.; Palócz, O.; Kulcsár, A.; Petrilla, J.; Csikó, G.; Neogrády, Z.; Gálfi, P. Effects of Lactobacillus plantarum 2142 and sodium n-butyrate in lipopolysaccharide-triggered inflammation: Comparison of a porcine intestinal epithelial cell line and primary hepatocyte monocultures with a porcine enterohepatic co-culture system. J. Anim. Sci 2014, 92, 3835–3845. [Google Scholar] [CrossRef] [Green Version]
- Manjeet, K.R.; Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int. J. Immunopharmocol. 1999, 21, 435–443. [Google Scholar]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of quercetin on proliferation and h₂o₂-induced apoptosis of intestinal porcine enterocyte cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef] [Green Version]
- Penissi, A.B.; Rudolph, M.I.; Piezzi, R.S. Role of mast cells in gastrointestinal mucosal defense. Biocell 2003, 27, 163–172. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zheng, M.; Gao, F.; Zhang, J.; Liu, F. Metabolomics analysis of l-arginine induced gastrointestinal motility disorder in rats using uplc-ms after magnolol treatment. Front. Pharmacol. 2019, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Ghimire, S.; Wongkuna, S.; Sankaranarayanan, R.; Ryan, E.P.; Bhat, G.J.; Scaria, J. Rice bran and quercetin produce a positive synergistic effect on human gut microbiota, elevate the level of propionate, and reduce the population of enterobacteriaceae family when determined using a bioreactor model. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Vazquez Prieto, M.A.; Bettaieb, A.; Rodriguez Lanzi, C.; Soto, V.C.; Perdicaro, D.J.; Galmarini, C.R.; Haj, F.G.; Miatello, R.M.; Oteiza, P.I. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2015, 59, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Amevor, F.K.; Cui, Z.; Du, X.; Ning, Z.; Shu, G.; Jin, N.; Deng, X.; Tian, Y.; Zhang, Z.; Kang, X.; et al. Combination of quercetin and vitamin E supplementation promotes yolk precursor synthesis and follicle development in aging breeder hens via liver-blood-ovary signal axis. Animals 2021, 11, 1915. [Google Scholar] [CrossRef] [PubMed]
- Amevor, F.K.; Cui, Z.; Ning, Z.; Du, X.; Jin, N.; Shu, G.; Deng, X.; Zhu, Q.; Tian, Y.; Li, D.; et al. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens. Poult. Sci. 2021, 101481. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential implications of quercetin in autoimmune diseases. Front. Immunol. 2012, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
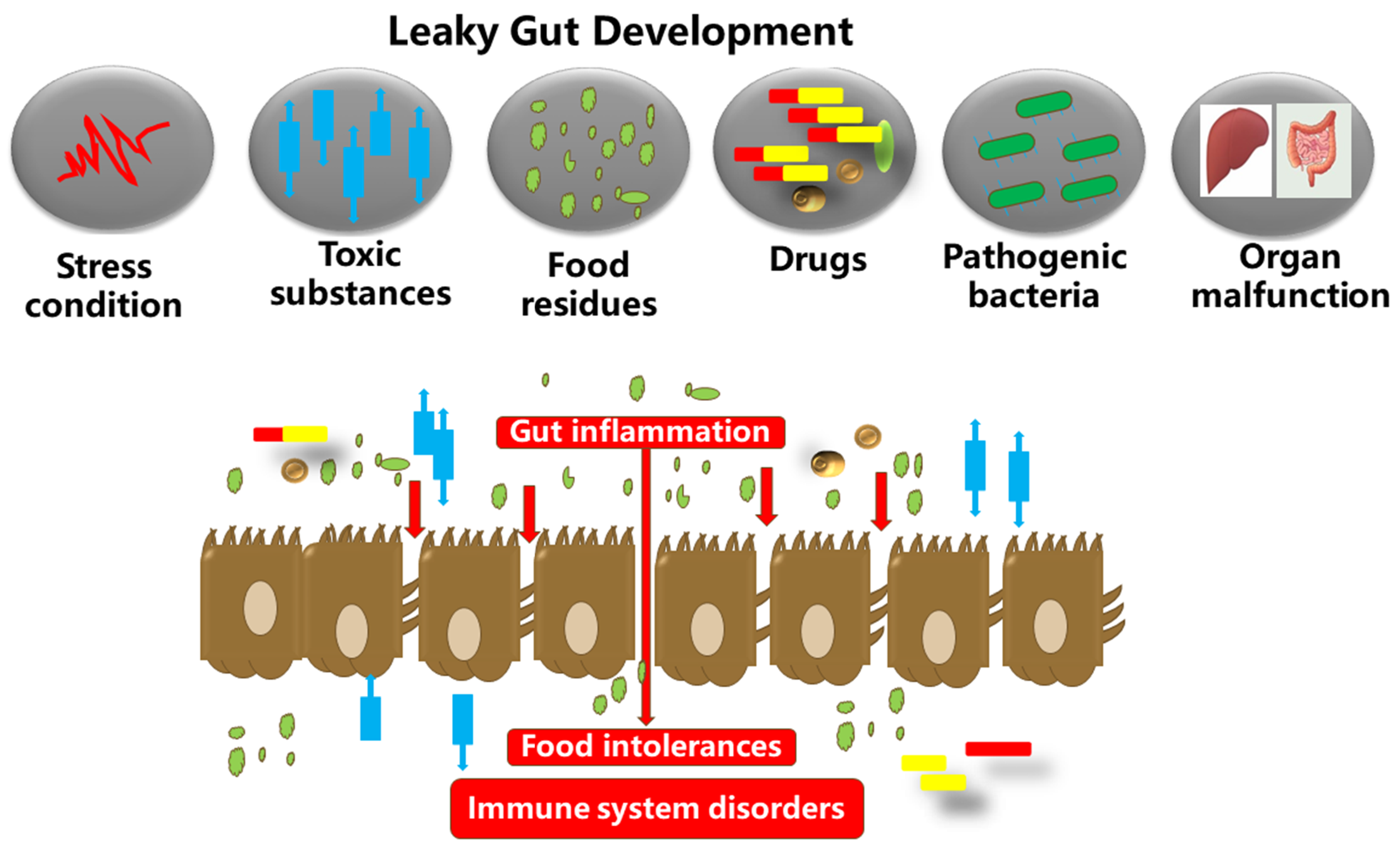
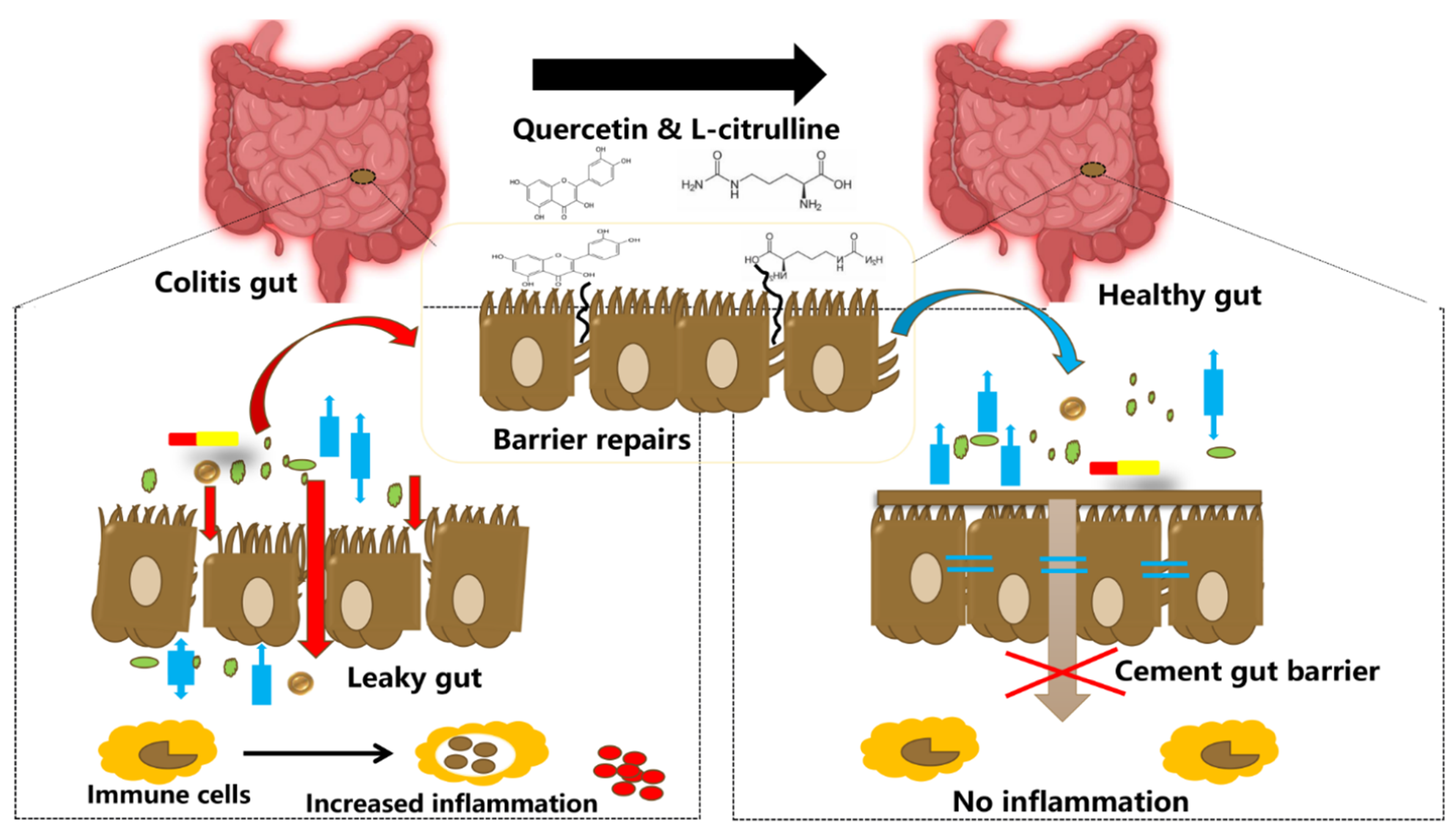
| Subject | Design | Main Findings | References |
|---|---|---|---|
| Wistar rats; Beagle dogs; Cynomolgus monkeys | Intestinal toxicity was induced using oncological drug candidates. | In treated animals, a > 50% decrease in plasma l-citrulline levels strongly correlated with histopathological findings in the small intestine such as single-cell necrosis and mucosa atrophy, intestinal crypt necrosis, villus atrophy, enterocyte loss, and clinical signs (bloody feces and diarrhea), indicating l-citrulline as a small intestine biomarker. | [104] |
| Dogs (5 males/5 females per group) | Oral doses of 0.75, 1.5, and 3 mg/kg/d of MS-229 over 4 weeks to induce small intestinal toxicity. | A dose- and exposure-dependent decrease in plasma citrulline was correlated with pathological findings in the small intestine. | [105] |
| Preterm infants | Plasma citrulline levels were measured during the first 48 h after necrotizing enterocolitis onset. | Plasma citrulline decreased in the first 48 h suggesting ongoing intestinal injury, thus plasma citrulline measurement may provide an indication for intestinal recovery rate during the first 24 h after NEC onset. | [106] |
| Male Wistar rats (n = 46; 230–250 g) | Varying citrulline levels were administered as 0.5,1, 2.5, 5 g/kg/d citrulline. | The jejunum weight was significantly positively correlated with plasma citrulline, suggesting a dose-dependent intestinal adaptation in gut resected rats. | [107] |
| In vitro analysis using IPEC-J2 cells | Citrulline (2 mM) and Lactobacillus helveticus ASCC 511 were co-treated to IPEC-J2 cells. | Lactobacillus helveticus and citrulline exhibited synergistic effects against adhesion of pathogenic bacteria, Escherichia coli; stimulated nitric oxide; improved transepithelial electrical resistance; and stimulated tight junction proteins expression, thus, promoting intestinal health. | [98] |
| Female C57BL/6J mice | Mice were induced non-alcoholic steatohepatitis using fat-, fructose-, and cholesterol-rich diet followed by +/− 2.5 g l -citrulline/kg body weight. | l-citrulline alleviated non-alcoholic fatty liver disease progression via attenuation of bacterial endotoxin translocation and the loss of tight junction proteins in small intestinal tissue. | [108] |
| Human model | Randomized, double-blind crossover study, 10 men cycled for 60 min at 70% of their maximum workload after l-citrulline (10 g) or placebo (l-alanine) intake. | Pre-exercise l-citrulline intake prevented splanchnic hypoperfusion-induced intestinal compromise by preserving splanchnic perfusion and attenuated intestinal injury during exercise probably by enhancing arginine availability. | [109] |
| Mice model | Mice undergoing intestinal obstruction were divided into three groups: sham, intestinal obstruction, and citrulline group receiving a diet containing 0.6% citrulline. | Citrulline pretreatment preserved barrier integrity and modulated immune response via decreasing intestinal permeability and bacterial translocation, whereas it preserved the ileum mucosa and increased secretory IgA concentration. | [110] |
| Male Wistar rats (n = 15) | Ulcerative colitis was established in rats and citrulline was administered intragastrically for 7 d. | Citrulline provided protective effects by lowering the peripheral blood monocytes, the infiltration of CD68-positive monocytes, and the concentrations of MCP-1, IL-6, and IL-17A in the colon tissues of effects in ulcerative colitis rats. | [111] |
| Adult male Sprague–Dawley rats (180–220 g) | l-citrulline (300, 600, and 900 mg/kg body weight) was administered to rats having ethanol-induced gastric ulcer in rats. | l-citrulline elicited gastro-protective effects by attenuating gastric lesions, prevented oxidative damage, decreased nitric oxide content and increased the myeloperoxidase activity | [19] |
| Male Wistar rats (n = 24, 220–230 g) | Rats were assigned to either citrulline, arginine, control, or sham groups. The sham group underwent transection and other groups had an 80% resection of the small intestine. | Citrulline increased arginine levels and improved nitrogen balance after massive intestinal resection. | [112] |
| Adult male Sprague–Dawley rats (200–240 g) | l-citrulline (300, 600, and 900 mg/kg) was pretreated to ischemia/reperfused rats. | l-citrulline reduced the gastric mucosal lesion, prevented the production of lipid peroxidation, and inhibited the increase in myeloperoxidase activity. | [113] |
| Male C57/Bl6 mice (n = 65; 26–28.5 g) | Mice received intravenous infusion of endotoxin (LPS, 0.4 µg/g bodyweight per h) combined with either l-citrulline (6.25 mg/h), l-arginine (6.25 mg/h), or l-alanine (12.5 mg/h). | During endotoxemia, l-citrulline supplementation reduced intestinal microcirculatory dysfunction and increased intracellular NO production via increasing plasma and tissue concentrations of arginine and citrulline, and restored intracellular NO production in the intestine. Jejunal tissues in the l-citrulline group showed an increase in degree of phosphorylation of eNOS phosphorylation and decreased iNOS protein level. | [45] |
| Swiss male mice (6 weeks old) | Mice received supplemented citrulline or alanine in the drinking water for 10 d (1 g/kg/d) and on the seventh day, the animals were injected intraperitoneally with a single dose of phosphate-buffered saline (PBS) or 5-fluorouracil (200 mg/kg) for the induction of mucositis. | Citrulline administration contributed to a partial recovery of the mucosal architecture in mucositis-induced mice. There was an intermediate reduction in the histopathologic score, and functional intestinal permeability was partially rescued by citrulline treatment. Citrulline attenuated mucosal damage by reducing the size of the injured areas and decreased intestinal permeability in mucositis mice. | [114] |
| Subject | Design | Main Findings | References |
|---|---|---|---|
| The LDL receptor-deficient mouse C57BL/6 mice (90 days old; (24.76 ± 0.37 g) | Mice were randomly assigned to either the quercetin treatment (100µg/d; n = 12) or the control group (n = 12) and fed regular chow diet for 4 weeks, followed by a high-fat diet until 12 weeks. | Quercetin treatment to high-fat-diet-fed mice attenuated atherosclerotic lesions, elicited protective effects against immune/inflammatory responses and oxidative stress, and decreased intestinal lipid levels. Additionally, quercetin altered the gut microbiota composition by decreasing the abundance of Verrocomicrobia but increased microbiome diversity and the abundances of Actinobacteria, Cyanobacteria, and Firmicutes. Quercetin reduced the lipid level, areas of atherosclerotic lesions and sizes of plaques. | [124] |
| C57BL/6 mice | Dietary quercetin (30 mg/kg) was supplemented to a Citrobacter rodentium-induced colitis mouse model for 2 weeks. | Quercetin alleviated Citrobacter rodentium-induced colitis by suppressing pro-inflammatory cytokines production and modified the gut microbiota by increasing Bacteroides, Bifidobacterium, Lactobacillus, and Clostridia populations but reduced Fusobacterium and Enterococcus spp. | [125] |
| Broiler chickens (n = 240) | Chickens were randomized into four groups: saline-challenged; LPS-challenged; and LPS-treated broiler chickens, fed either 200 or 500 mg/kg of quercetin. | Quercetin alleviated LPS-induced oxidative stress via the MAPK/Nrf2 signaling in the intestines of chickens. Quercetin alleviated LPS-induced decrease in duodenal, jejunal, and illeal villus height and increased the crypt depth of these regions. Further, quercetin inhibited LPS-induced jejunal oxidative stress and relieved jejunal mitochondria damage. | [126] |
| Finishing pigs ((Large White × Landrace); n = 170; initial body weight of 72 ± 4 kg) | Pigs were randomly assigned to either a control group fed basal diet or treatment group consuming the same diet supplemented with 25 mg/kg feed quercetin, and after a 4-week period, pigs were transported for 5 h. | Quercetin-supplementation improved intestinal health and alleviated intestinal injury during transport through decreased serum endotoxin levels, lowered intestinal ROS and MDA, and lowered jejunal inflammatory cytokines expression, but increased jejunum villi height and upregulated the mRNA expression of occludin and zonula occudens-1 in the jejunum. | [127] |
| Male Wistar rats (8 weeks old; 250 ± 20 g) | Post-inflammatory irritable bowel syndrome (PI-IBS) model rats were administered quercetin by gavage at doses of 5, 10, and 20 mg/kg for 14 d. | Quercetin elicited an analgesic effect on PI-IBS and decreased the visceral pain threshold of PI-IBS rats, and the abdominal motor response to colon distension was markedly increased. Quercetin also reduced the colonic expression of genes responsible for enteroendocrine cell differentiation. | [128] |
| Rats | Rats were grouped as osteoarthritis-induced model, quercetin-treated, and control groups. Quercetin group received daily intragastric administration (100 mg/kg/d, i.g.) from day 1 to day 28. | Quercetin partially abrogated intestinal flora disorder and reversed fecal metabolite abnormalities. Diversity in the gut microbiota was decreased after quercetin treatment and at the genus level, Lactobacillus was increased whereas, unidentified Ruminococcaceae was decreased. | [129] |
| Ross 308 chicks (n = 128 chicks; 41 gm/chick) | Quercetin was fed to groups of broiler chickens at concentrations of 200, 400, and 800 ppm, and a control group was supplemented with a basal diet. | Dietary quercetin improved the gut microbiota environment by decreasing total coliforms and Clostridium perfringens population but increased the Lactobacillus counts. Further, the intestinal mRNA expression of intestinal Cu/Zn-superoxide dismutase, glutathione peroxidase, and nutritional transporters was upregulated in quercetin-supplemented groups. | [130] |
| C57BL/6J mice | Monosodium glutamate (MSG)-treated mice were randomly divided into two groups: MSG group and quercetin group (5 mg/kg quercetin) administrated by gavage at a dose of 100 µL/10 g/body weight (BW)/ d for 6 weeks. | Dietary quercetin attenuated MSG-induced gut microbiota dysbiosis and improved intestinal barrier function. Quercetin reversed MSG-induced elevation in Firmicutes abundance and decreased the Firmicutes/Bacteroidetes ratio. Further, Lachnospiraceae and Ruminicoccaceae abundance was reduced. Colon damage was recovered and Muc2 and ZO-1 expression was upregulated after quercetin treatment. | [131] |
| Wistar rats (n = 23) | Wistar rats were randomized into four groups fed a high-fat sucrose diet supplemented or not with trans-resveratrol (15 mg/kg body weight (BW)/d), quercetin (30 mg/kg BW/d), or a combination of both polyphenols. | Quercetin supplementation eliminated gut dysbiosis by attenuating Firmicutes/Bacteroidetes ratio and inhibited the growth of bacterial species associated to diet-induced obesity (Erysipelotrichaceae, Bacillus, Eubacterium cylindroides). | [28] |
| Male C57BL/6J mice (7 weeks old) | Mice were challenged with high-fat diet (HFD) supplemented or not with quercetin (0.05% (wt/wt) aglycone quercetin) for 16 weeks. | Quercetin alleviated obesity-associated NAFLD via its anti-inflammatory, antioxidant, and prebiotic integrative response. Quercetin reverted gut microbiota imbalance and related endotoxemia-mediated TLR-4 pathway induction, with subsequent inhibition of inflammasome response and reticulum stress pathway activation. | [123] |
| Kunming male mice (n = 36; 18–20 g) | Mice were administrated 0.5 mL/d antibiotics cocktail intragastrically for 7 d to induce gut dysbiosis. Quercetin-treated mice were fed AIN-93G diet containing 0.2% quercetin for 10 d. | Quercetin supplementation combated gut dysbiosis since it recovered intestinal barrier function and improved the diversity of the gut bacterial community in antibiotic-treated mice. Intestinal villi length and mucosal thickness were increased and butyrate production was enhanced in quercetin-treated mice. | [120] |
| Sprague–Dawley rat (6 weeks old; male; 160−200 g) | Quercetin (50 mg/kg/d) was dissolved in distilled water and administered daily by gavage at 10 mL/kg for 12 weeks to streptozotocin (STZ)-induced diabetic peripheral neuropathy (DPN) rats. | Quercetin exerted a neuroprotective effect and modulated gut microbiota associated with DPN phenotypes and ROS production in STZ-induced DPN rats. Quercetin rescued gut dysbiosis by decreasing four potential pathogenic species and enriching two prebiotic species associated with DPN phenotypes and ROS production. | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients 2021, 13, 3782. https://doi.org/10.3390/nu13113782
Uyanga VA, Amevor FK, Liu M, Cui Z, Zhao X, Lin H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients. 2021; 13(11):3782. https://doi.org/10.3390/nu13113782
Chicago/Turabian StyleUyanga, Victoria Anthony, Felix Kwame Amevor, Min Liu, Zhifu Cui, Xiaoling Zhao, and Hai Lin. 2021. "Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review" Nutrients 13, no. 11: 3782. https://doi.org/10.3390/nu13113782
APA StyleUyanga, V. A., Amevor, F. K., Liu, M., Cui, Z., Zhao, X., & Lin, H. (2021). Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients, 13(11), 3782. https://doi.org/10.3390/nu13113782








