Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans
Abstract
1. Introduction
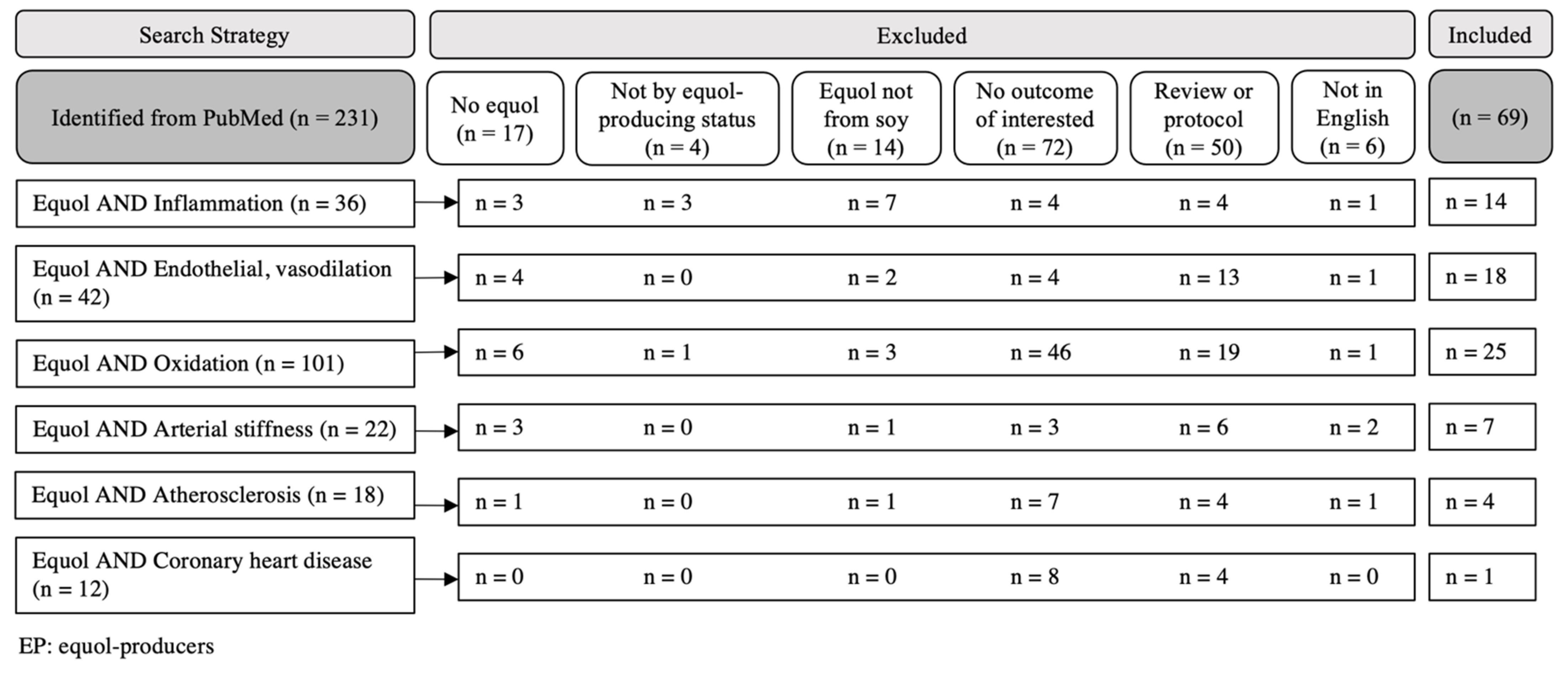
2. Materials and Methods
3. Results
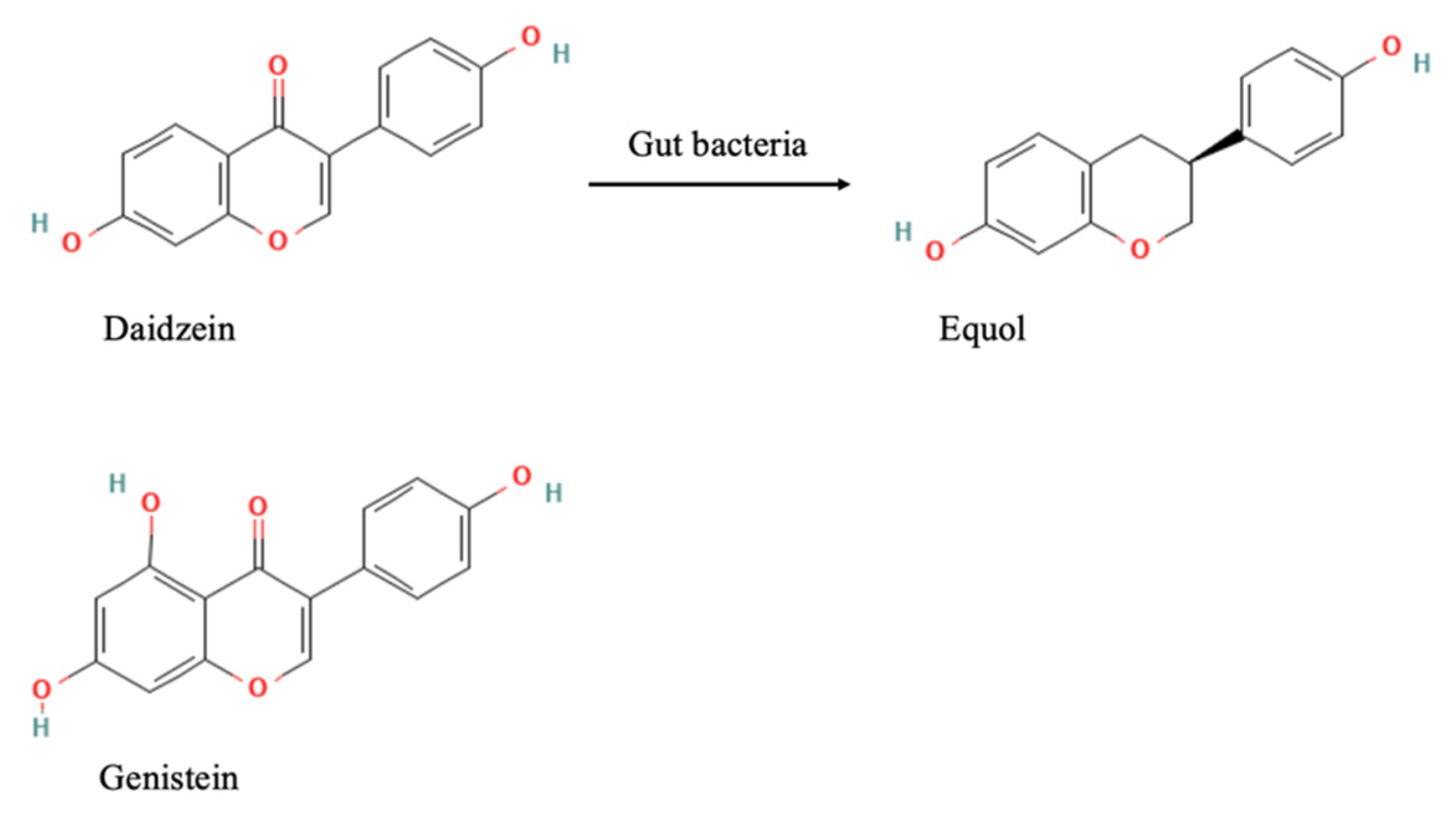
3.1. Characterization of Equol
3.2. ERβ
3.3. Potential Cardioprotective Properties of Equol
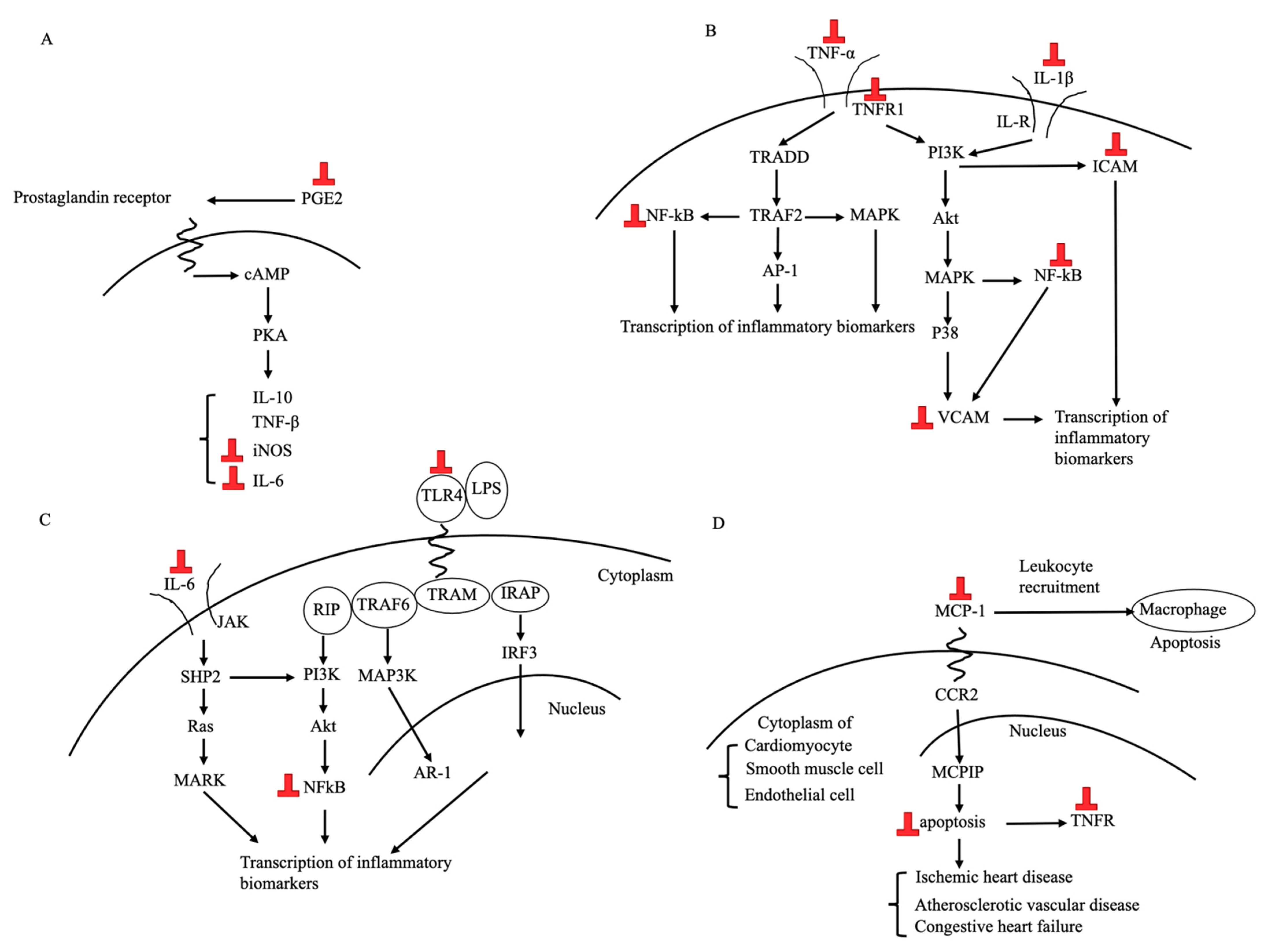
3.3.1. Anti-Inflammatory Effects
| # | Authors | Type | Findings | Has Effect |
|---|---|---|---|---|
| 1 | Blay [61] | In vitro | Equol (10 μM) significantly inhibited the overproduction of NO and PGE2 induced by LPS plus INF-γ when a pre-treatment was performed or when administered during activation. Moreover, equol regulated the gene transcription of cytokines and inflammatory markers. Genistein (20 μM) exerted similar anti-inflammatory effects, but daidzein did not. | Yes |
| 2 | Johnson [62] | In vitro | Equol exhibited protective effects against pro-inflammatory cytokines (IL-6 and TNF-α) and NO production in murine microglia cells. Equol also showed greater permeability through artificial gut and blood-brain barriers compared to daidzein. | Yes |
| 3 | Obiorah [63] | In vitro | Equol and ISFs induced endoplasmic reticulum stress and inflammatory response stress-related genes in a comparable manner to estrogens. Equol and ISFs induced proliferation of estrogenized breast cancer cells (simulating a perimenopausal state) but induced apoptosis of estrogen-deprived cells (simulating a postmenopausal state). | Yes |
| 4 | Nagarajan [64] | In vitro | In an in vitro LPS-induced inflammation model, equol dose-dependently inhibited LPS-induced MCP-1 secretion by macrophages. | Yes |
| 5 | Subedi [65] | In vitro | In microglial cells, equol inhibited TLR4 activation, MAPK activation, NF-kB-mediated transcription of inflammatory mediators, production of NO, release of PGE-2, and secretion of TNF-α and IL-6 in LPS-activated murine microglia cells. | Yes |
| 6 | Moriyama [66] | In vitro | Equol attenuated LPS-induced NO production with a concomitant decrease in the expression of iNOS. Equol did not affect the LPS-induced increase in intracellular ROS production. Increased NO production is a well-known inflammatory change in astrocytes stimulated by LPS. Attenuation of NO production by equol may mitigate LPS-induced neuroinflammation in astrocytes. | Yes |
| 7 | Lin [67] | In vivo | Equol-administered collagen-induced arthritis mice had a lower severity of arthritis symptoms. Equol administration suppressed the expression of IL-6 and its receptor in the inflamed area of collagen-induced arthritis mice. | Yes |
| 8 | Yokosuka [34] | In vivo | In ovariectomized mice induced to have intracranial aneurysms, equol protected against aneurysm formation; the disruption of the intestinal microbial conversion of daidzein to equol abolished daidzein’s protective effect against aneurysm formation. Moreover, mice treated with equol had lower inflammatory cytokines in their cerebral arteries. | Yes |
| 9 | van der Velpen [35] | Human | In the adipose tissue of postmenopausal women, the expression of inflammation-related genes was upregulated in equol producers but downregulated in non-producers. | Yes |
| 10 | Törmälä [68] | Human | ISFs caused a decrease in the VCAM-1 and platelet-selectin. The fall in platelet-selectin was more marked in equol producers. No changes appeared in SHBG, CRP, or ICAM-1. | Yes |
| 11 | Reverri [69] | Human | Consuming soy improved arterial stiffness as was assessed by the augmentation index, but did not improve the inflammatory biomarkers (CRP, TNF-α, IL-6, IL-18, and IL-10). The addition of equol-producing status as a covariate did not significantly change these results. | No |
| 12 | Nicastro [70] | Human | Equol, while not associated with a decrease in CRP level, was associated with decreased geometric mean WBC counts, comparing the highest quartile to the lowest. | Yes |
| 13 | Greany [71] | Human | An RCT of 34 postmenopausal women on 44 mg/day of ISFs showed that the ISFs did not influence the concentrations of Hcy, CRP, sE-selectin, sVCAM-1, and sICAM-1. Equol-producing status did not modify the associations. | No |
| 14 | Mangano [72] | Human | In women who received the ISFs intervention, there was no significant differences in percent change in the serum inflammatory markers between equol producers and non-producers. | No |
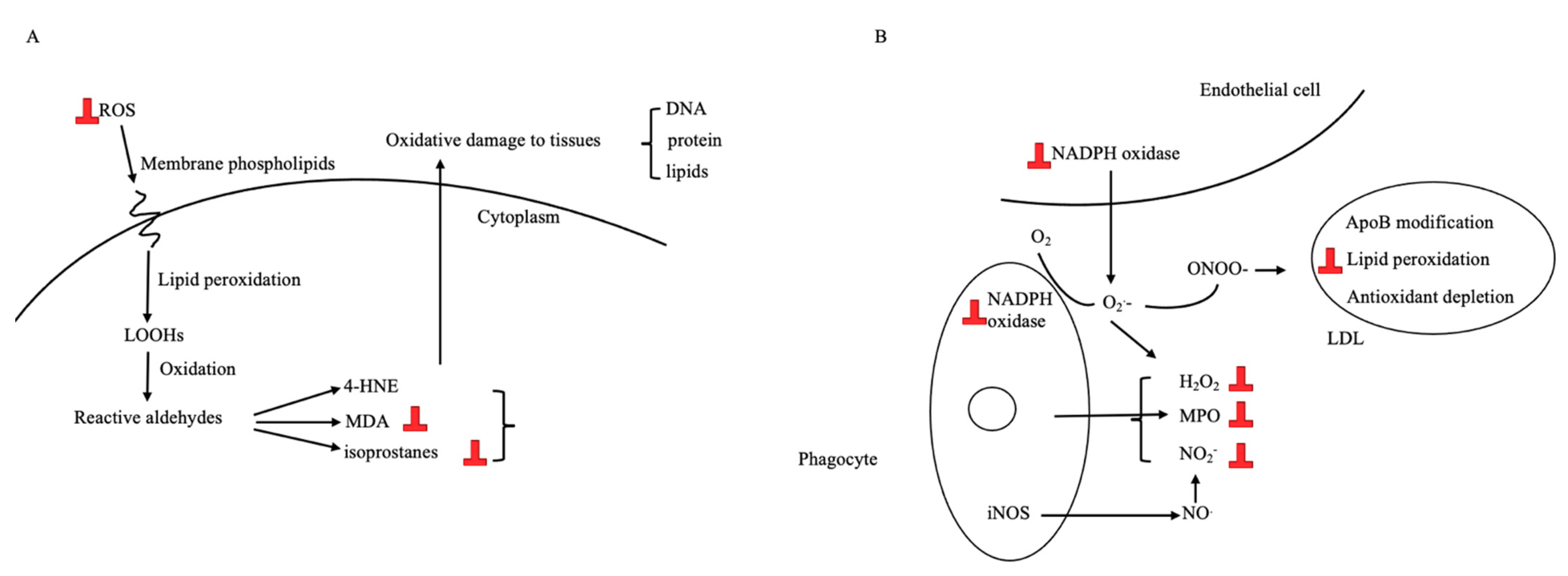
3.3.2. Antioxidative Effect
| # | Authors | Type | Findings | Has Effect |
|---|---|---|---|---|
| 1 | Lin [93] | In vitro | Equol was shown to protect chicken intestinal epithelial cells from oxidative damage by promoting the expression of antioxidant genes, increasing the activities of antioxidant enzymes, and enhancing antioxidant capacity. Equol significantly enhanced total SOD activity and the Nrf2 transcript. | Yes |
| 2 | Pereboom [92] | In vitro | Equol decreased the intracellular production of the superoxide anion and hydrogen peroxide content of phagocytic cells. | Yes |
| 3 | Hwang [96] | In vitro | Equol and ascorbic acid interacted synergistically in preventing LDL oxidation. All phases of LDL oxidation were affected by these compounds, which is atypical of the behavior of antioxidants that are consumed during the early phases. Equol was more potent than daidzein and genistein because of its absence of a carbonyl group, C2–C3 double bond and flanking hydroxyl groups in the pyran ring. | Yes |
| 4 | Pažoureková [91] | In vitro | Upon activation by ROS, neutrophils treated by equol produced less p40 phox (a component of NADPH oxidase, responsible for the assembly of functional oxidase in intracellular membranes) both extra- and intracellularly to the control. | Yes |
| 5 | Choi [97] | In vitro | Equol pretreatment significantly decreased levels of oxidative stress biomarkers such as thiobarbituric acid-reactive substances, carbonyl content and serum 8-hydroxy-2-deoxyguanosine. Moreover, equol increased the activity of CAT, superoxide dismutase, GPx, and glutathione reductase. In addition, equol possessed anticancer activity through acting as an antioxidant and therefore reduced apoptosis. | Yes |
| 6 | Wei [98] | In vitro | Low doses of equol could prevent skeletal muscle cell damage induced by hydrogen peroxide. Equol increased cell viability, the concentration of MDA content, and LDH activity. | Yes |
| 7 | Kamiyama [99] | In vitro | Equol might contribute to a reduced level of oxLDL-stimulated apoptosis linked to the reduced generation of intracellular ROS in human umbilical vein endothelial cells. | Yes |
| 8 | Sierens [100] | In vitro | Equol was able to function as an antioxidant, scavenging potentially harmful free radicals. Equol protected against oxidative-induced DNA damage. Pretreatment of a physiological range of equol offered protection against the hydrogen peroxide-mediated DNA damage in human lymphocytes cells. This protection was greater than that offered by the addition of antioxidant vitamins ascorbic acid and alpha-tocopherol, or the compounds 17β-estradiol and tamoxifen, which have similar structures to ISFs and are known to have moderate antioxidant activity. | Yes |
| 9 | Rüfer [101] | In vitro | Equol exhibited higher antioxidant activity than daidzein and about the same antioxidant capacity as the oxidative metabolites of daidzein and genistein despite the lack of the 2,3-double bond with the 4-oxo group and a 5,7-dihydroxyl structure. The antioxidative effect was tested by an ORAC assay which determined the ability of compounds to scavenge peroxyl radicals. | Yes |
| 10 | Hwang [51] | In vitro | Equol inhibited LDL oxidation in vitro and LDL oxidative modification by monocyte/macrophages. The antioxidant effect of equol was found to be mediated by the inhibition of superoxide radical production and manifested through enhanced levels of free NO. Equol had a greater antioxidant activity than genistein and daidzein. | Yes |
| 11 | Sierens [102] | In vitro | Pretreatment with equol significantly protected sperm DNA against oxidative damage. Compared with ascorbic acid and alpha-tocopherol, being added at physiological concentrations, genistein was the most potent antioxidant, followed by equol, ascorbic acid, and alpha-tocopherol. Equol might have a role to play in antioxidant protection against male infertility. | Yes |
| 12 | Arora [103] | In vitro | Compared to genistein and daidzein with their glycosylated and methoxylated derivatives, equol and its 4-hydroxy and 5-hydroxy derivatives were more potent antioxidants, suggesting that the absence of the 2, 3-double bond and the 4-oxo group on the ISF nucleus enhanced antioxidant activity. | Yes |
| 13 | Turner [104] | In vitro | Equol inhibited the oxidation of LDL 2.65-fold more than its parent compound daidzein. | Yes |
| 14 | Choi [94] | In vitro | Equol acted as an antioxidant in the brains of rats. The ratio of GSH/GSSG in primary cortical neuron cells exposed to equol for 24 and 72 h significantly decreased in a time- and dose-dependent manner. Moreover, equol treatment significantly increased the LDH release in a time-and dose-dependent manner. | Yes |
| 15 | Gou [90] | In vitro | Equol protected chicken macrophages from oxidative stress induced by lipopolysaccharide through reducing lipid peroxidation products such as MDA and enhancing the contents of antioxidants such as glutathione and the activities of relevant antioxidase enzymes such as total SOD; effects were also seen in gene expression related to the immune response and increased contents of cytokines. | Yes |
| 16 | Liu [105] | In vitro | Equol elevated brain antioxidant activity by increasing SOD, CAT, and GPx levels. MDA levels and AChE activity were decreased in hypertensive and vascular dementia rats. Equol further improved the long- and short-term memory of the rats. | Yes |
| 17 | Vedavanam [106] | In vivo | The order of the half-maximal inhibitory concentration values, the indication of the potency of inhibiting glucose-induced LDL lipid peroxidation observed for the compounds, was equol > genistein > daidzein. | Yes |
| 18 | Choi [89] | In vivo | Equol might act as an antioxidant through an inhibition of oxidative stress and the stimulation of CAT and SOD, but could also cause pro-oxidant effects, such as the reduction of the GSH/GSSG ratio, depending on the treatment period. A study in mice showed that equol administration significantly inhibited biomarkers of oxidative stress (thiobarbituric acid-reactive substances value, carbonyl content, and serum 8-hydroxydeoxyguanosine). Moreover, the CAT and total SOD activities and their transcripts were significantly increased by equol. Although equol increased the glutathione peroxidase activity in mice treated with equol for 1-week, long-term administration of equol (7 weeks) caused a decrease in the ratio of GSH/GSSG and the activities of GPx and glutathione reductase. | Yes |
| 19 | Ma [107] | In vivo | A study in male and ovariectomized female rats with transient middle cerebral artery occlusion revealed that the pretreatment of equol significantly reduced infarct size in both sexes. This neuroprotection was accompanied by a decrease in the NADPH oxidase activity and superoxide levels in the brain. In addition, equol reduced plasma thiobarbituric acid reactive substances and neurological deficits up to 7 days after injury. | Yes |
| 20 | Horiuchi [108] | In vitro | The study demonstrated that equol had suppressive effects against oxidative stress in pancreatic β-cells in a dose-dependent manner and presumably through activating PKA signaling. | Yes |
| 21 | Jackman [33] | In vivo | Equol exerted weak antioxidant effects in cerebral arteries, whereas the effects of daidzein were insignificant. Antioxidant activity was assessed as the reduction in NADPH-induced superoxide levels. | Yes |
| 22 | Widyarini [109] | In vivo | In addition to the activation of estrogenic signaling pathways for photoprotection, equol also provided UV-protective antioxidant effects that depend partially on HO-1 induction. Equol dose-dependently inhibited the oxidative stress measured as UVA-induced lipid peroxidation on mouse skin. A component of the equol lipid protection capacity is attributed to endogenous cutaneous antioxidant enzymes, including the inducible stress protein HO-1. | Yes |
| 23 | Nhan [110] | Human | Urinary equol was not associated with the secretion of urinary F2 isoprostane, a measure of cellular lipid peroxidation, after ISF treatment in postmenopausal women. However, the observations on the effect of equol were limited because only two of the eight subjects were equol producers, one of whom experienced a large increase in the biomarker excretion, whereas the other experienced small decreases. | No |
| 24 | Hidayat [36] | Human | The level of MDA, an oxidative stress marker, was lower in equol producers than non-producers. This RCT was conducted with 190 postmenopausal women aged 47–60 who received 100 mg ISFs for 6 months. The random allocation of ISFs intervention was carried out separately by equol-producing status. | Yes |
| 25 | Richardson [95] | In vitro | Equol might have a beneficial effect in delaying the onset and decreasing the severity of symptoms in Friedreich’s ataxia patients by an antioxidant mechanism, such as reducing the ROS-induced modification of proteins and lipids and impaired mitochondrial function. These effects were independent of the ERβ. | Yes |
3.3.3. Endothelial Function and Vasodilation
| # | Authors | Type | Findings | Has Effect |
|---|---|---|---|---|
| 1 | Joy [117] | In vitro | Nutritionally relevant plasma concentrations of equol rapidly stimulated phosphorylation of ERK1/2 and PI3K/Akt, leading to the activation of NOS and increased NO production at resting cytosolic Ca2+ levels. | Yes |
| 2 | Rowlands [119] | In vitro | Equol-stimulated mitochondrial ROS modulated endothelial redox signaling and NO release through transactivation of epidermal growth factor receptor kinase and reorganization of the F-actin cytoskeleton. | Yes |
| 3 | Cheng [115] | In vitro | Equol prevented oxidative damage to vascular function in pulmonary cells via downregulating eNOS and oxidative stress. | Yes |
| 4 | Zhang [114] | In vitro | In HUVEC, equol increased Nrf2 mRNA as well as the mRNA of the gene products of HO-1 and NQO1. Pretreatment of cells with specific endoplasmic reticulum inhibitors or PI3K/Akt increased Nrf2, HO-1, and NQO1 protein. | Yes |
| 5 | Chung [121] | In vitro | Equol had a significant antioxidant effect on the bAECs that were exposed to hydrogen peroxide. Equol pretreatment effectively inhibited the hydrogen peroxide-induced cell death by the reduction in intracellular ROS production, probably through increasing phospho-p38 MAPK. | Yes |
| 6 | Zhang [123] | In vitro | The improvement of atherosclerosis by equol through attenuation of endoplasmic reticulum stress is mediated by activating the Nrf2 signaling pathway. Equol treatment inhibited cell apoptosis and attenuated upregulation of endoplasmic reticulum stress markers in HUVECs. In an oxidative stress environment, equol treatment dose-dependently activated the Nrf2 signaling pathway. | Yes |
| 7 | Somjen [122] | In vitro | Equol, but not daidzein and genistein, had a monophasic stimulatory effect on thymidine incorporation, which boosts DNA synthesis. In human endothelial cells, equol, daidzein, and genistein stimulated DNA synthesis in a dose-dependent manner. The administration of equol, daidzein, and genistein to immature and ovariectomized female rats resulted in increased creatine phosphokinase in the aorta and in the left ventricle of the heart. | Yes |
| 8 | Kim [124] | In vitro | Equol had a vasodilatory effect on human uterine arteries vascular smooth muscle, which was mediated through antagonistic action for a receptor-dependent Ca2+ channel. | Yes |
| 9 | Johnson [62] | In vitro | Equol exhibited protective effects against NO production in murine microglial cells. Equol also showed greater permeability through artificial gut and blood-brain barriers compared to daidzein. | Yes |
| 10 | Chin-Dusting [125] | In vivo | Equol had a dose-dependent inhibition of the contractile responses to noradrenaline in rat isolated aortic rings. Equol independently increased the release of a vasoconstrictor prostanoid, such as thromboxane. | Yes |
| 11 | Jackman [33] | In vivo | In normotensive rats, equol displayed vasorelaxant activity similar to daidzein. The relaxant effect of equol was independent of intact endothelium, NOS activity, K+ channels, and gender. In the basilar artery, where superoxide levels are higher, equol exerted weak antioxidant effects, whereas the effects of daidzein were insignificant. During hypertension, equol-induced vasorelaxation was preserved, whereas relaxant responses to daidzein were impaired. | Yes |
| 12 | Matsumoto [126] | In vivo | Contractions induced by a selective 5-HT receptor agonist increased with insulin treatment, but less so with equol + insulin. In the endothelium-denuded preparations, 5-HT-induced contractions were augmented with insulin treatment but less so by equol + insulin treatment. These differences in 5-HT-induced contractions were eliminated by a large-conductance of Ca2+-activated K+ channel inhibitor. | Yes |
| 13 | Yu [116] | In vivo | Equol significantly increased regional cerebral blood flow in rats and produced an endothelium-independent relaxation in rat cerebral basilar arteries. Selective Ca2+-activated K+ channel blockers significantly inhibited equol-induced vasodilation in cerebral arteries. | Yes |
| 14 | Ohkura [120] | In vivo | Ovariectomized rats were assigned to (1) an ISF-deficient but equol-sufficient group, (2) an ISFs-deficient and equol-deficient group. In the thoracic artery, endothelium-dependent relaxation, cyclic guanosine monophosphate levels in the tissue, and eNOS synthase expression and phosphorylation were significantly higher in the first group compared to the second group. | Yes |
| 15 | Törmälä [73] | Human | Before ISF intervention, women with a 4-fold elevation in equol levels had a lower endothelial function index compared to women without this capacity. Soy supplementation had no effect on arterial stiffness or endothelial function in either group. | Yes |
| 16 | Kreijkamp-Kaspers [37] | Human | This RCT did not support the hypothesis that ISFs have beneficial effects on endothelial function in older postmenopausal women. However, in the soy-only group, systolic and diastolic blood pressure decreased, and endothelial function improved in the equol producers, whereas blood pressure increased, and endothelial function deteriorated in the non-producers. | Yes |
| 17 | Hidayat [36] | Human | ISFs did not improve endothelial functions in both the equol producers and the non-producers. The VCAM-1 and NO did not differ by equol-producing status. | No |
| 18 | Clerici [127] | Human | After ISFs treatment, the brachial artery flow-mediated vasodilatation was improved more obviously in the equol producers. | Yes |
3.3.4. Arterial Stiffness
3.3.5. Atherosclerosis and CHD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. W.H.O. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Available online: https://www.who.int/healthinfo/global_burden_disease/estimates/en/ (accessed on 24 August 2021).
- Clarkson, T.B. Soy, soy phytoestrogens and cardiovascular disease. J. Nutr. 2002, 132, 566S–569S. [Google Scholar] [CrossRef]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Koehler, K.F.; Gustafsson, J.-Å. Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 2011, 10, 778–792. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R. Experimental determination of octanol–water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Verdrengh, M.; Jonsson, I.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Blum, P.S.; Berhow, M.A.; Baumann, H.; Kuo, S.-M. Dietary isoflavones suppress endotoxin-induced inflammatory reaction in liver and intestine. Cancer Lett. 2004, 215, 21–28. [Google Scholar] [CrossRef]
- Duan, W.; Kuo, I.C.; Selvarajan, S.; Chua, K.Y.; Bay, B.H.; Wong, W.F. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am. J. Respir. Crit. Care Med. 2003, 167, 185–192. [Google Scholar] [CrossRef]
- Ibrahim, W.H.; Habib, H.M.; Chow, C.K.; Bruckner, G.G. Isoflavone-rich soy isolate reduces lipid peroxidation in mouse liver. Int. J. Vitam. Nutr. Res. 2008, 78, 217–222. [Google Scholar] [CrossRef]
- Liu, J.; Chang, S.K.; Wiesenborn, D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J. Agric. Food Chem. 2005, 53, 2333–2340. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Lajolo, F.M.; Genovese, M.I. Effect of free or protein-associated soy isoflavones on the antioxidant status in rats. J. Sci. Food Agric. 2011, 91, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Golden, D.L.; Williams, J.K.; Franke, A.A.; Register, T.C.; Kaplan, J.R. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J. Nutr. 2005, 135, 2852–2856. [Google Scholar] [CrossRef]
- Kondo, K.; Suzuki, Y.; Ikeda, Y.; Umemura, K. Genistein, an isoflavone included in soy, inhibits thrombotic vessel occlusion in the mouse femoral artery and in vitro platelet aggregation. Eur. J. Pharmacol. 2002, 455, 53–57. [Google Scholar] [CrossRef]
- Clarkson, T.B.; Anthony, M.S.; Morgan, T.M. Inhibition of postmenopausal atherosclerosis progression: A comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J. Clin. Endocrinol. Metab. 2001, 86, 41–47. [Google Scholar] [CrossRef]
- Anthony, M.S.; Clarkson, T.B.; Bullock, B.C.; Wagner, J.D. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arter. Thromb. Vasc. Biol. 1997, 17, 2524–2531. [Google Scholar] [CrossRef]
- Marventano, S.; Izquierdo Pulido, M.; Sanchez-Gonzalez, C.; Godos, J.; Speciani, A.; Galvano, F.; Grosso, G. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M.; American Heart Association Nutrition, C. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, Soy Isoflavones, and Protein Intake in Relation to Mortality from All Causes, Cancers, and Cardiovascular Diseases: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. J. Acad. Nutr. Diet. 2019, 119, 1483–1500.e17. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Dong, H.; Wang, D.; Gong, J.; Huang, W.; Lu, F. Soy isoflavones and glucose metabolism in menopausal women: A systematic review and meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2016, 60, 1602–1614. [Google Scholar] [CrossRef]
- Khodarahmi, M.; Jafarabadi, M.A.; Moludi, J.; Farhangi, M.A. A systematic review and meta-analysis of the effects of soy on serum hs-CRP. Clin. Nutr. 2019, 38, 996–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Chen, J.; Sun, K.; Wang, X.; Wang, X.; Hui, R. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef]
- Birru, R.L.; Ahuja, V.; Vishnu, A.; Evans, R.W.; Miyamoto, Y.; Miura, K.; Usui, T.; Sekikawa, A. The impact of equol-producing status in modifying the effect of soya isoflavones on risk factors for CHD: A systematic review of randomised controlled trials. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- Mayo, B.; Vazquez, L.; Florez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.-T.; Yang, G.; Li, H.; Cai, Q.; Xiang, Y.-B.; Ji, B.-T.; Franke, A.A.; Zheng, W.; Shu, X.-O. Urinary isoflavonoids and risk of coronary heart disease. Int. J. Epidemiol. 2012, 41, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Penalvo, J.L.; Gil, J.I.; Medina, S.; Horcajada, M.N.; Lafay, S.; Silberberg, M.; Llorach, R.; Zafrilla, P.; Garcia-Mora, P. Soy isoflavones and cardiovascular disease epidemiological, clinical and-omics perspectives. Curr. Pharm. Biotechnol. 2012, 13, 624–631. [Google Scholar] [CrossRef]
- Cano, A.; García-Pérez, M.Á.; Tarín, J.J. Isoflavones and cardiovascular disease. Maturitas 2010, 67, 219–226. [Google Scholar] [CrossRef]
- Jackman, K.A.; Woodman, O.L.; Chrissobolis, S.; Sobey, C.G. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007, 1141, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, K.; Rutledge, C.; Kamio, Y.; Kuwabara, A.; Sato, H.; Rahmani, R.; Purcell, J.; Eguchi, S.; Baranoski, J.F.; Margaryan, T. Roles of Phytoestrogen in the Pathophysiology of Intracranial Aneurysm. Stroke 2020, 52, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Van der Velpen, V.; Geelen, A.; Hollman, P.C.; Schouten, E.G.; van’t Veer, P.; Afman, L.A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: A double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 1269–1277. [Google Scholar] [CrossRef]
- Hidayat, A. Effect of soy isoflavone supplementation on endothelial dysfunction and oxidative stress in equol-producing postmenopausal women. Endocr. Metab. Immune Disord. -Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2015, 15, 71–79. [Google Scholar]
- Kreijkamp-Kaspers, S.; Kok, L.; Bots, M.L.; Grobbee, D.E.; Lampe, J.W.; van der Schouw, Y.T. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am. J. Clin. Nutr. 2005, 81, 189–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Setchell, K.D.; Zhao, X.; Shoaf, S.E.; Ragland, K. The pharmacokinetics of S-(-) equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Atkinson, C.; Berman, S.; Humbert, O.; Lampe, J.W. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J. Nutr. 2004, 134, 596–599. [Google Scholar] [CrossRef]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef]
- Jin, J.-S.; Kitahara, M.; Sakamoto, M.; Hattori, M.; Benno, Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int. J. Syst. Evol. Microbiol. 2010, 60, 1721–1724. [Google Scholar] [CrossRef]
- Matthies, A.; Clavel, T.; Gutschow, M.; Engst, W.; Haller, D.; Blaut, M.; Braune, A. Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Appl. Environ. Microbiol. 2008, 74, 4847–4852. [Google Scholar] [CrossRef]
- Shimada, Y.; Yasuda, S.; Takahashi, M.; Hayashi, T.; Miyazawa, N.; Sato, I.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Cloning and expression of a novel NADP (H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20–92. Appl. Environ. Microbiol. 2010, 76, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Ohtani, T.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of two novel reductases involved in equol biosynthesis in Lactococcus strain 20–92. J. Mol. Microbiol. Biotechnol. 2011, 21, 160–172. [Google Scholar] [CrossRef]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of a novel dihydrodaidzein racemase essential for biosynthesis of equol from daidzein in Lactococcus sp. strain 20–92. Appl. Environ. Microbiol. 2012, 78, 4902–4907. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Gonzalez, A.; Jokela, T.; Wähälä, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br. J. Nutr. 2005, 94, 873–876. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280961, Genistein. In PubChem; 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Genistein (accessed on 24 August 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281708, Daidzein. In PubChem; 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Daidzein (accessed on 24 August 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 91469, Equol. In PubChem; 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Equol (accessed on 24 August 2021).
- Hwang, J.; Wang, J.; Morazzoni, P.; Hodis, H.N.; Sevanian, A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: An antioxidant mechanism for cell-mediated LDL modification. Free Radic. Biol. Med. 2003, 34, 1271–1282. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.G. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Pelzer, T.; Loza, P.-A.A.; Hu, K.; Bayer, B.; Dienesch, C.; Calvillo, L.; Couse, J.F.; Korach, K.S.; Neyses, L.; Ertl, G. Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction. Circulation 2005, 111, 1492–1498. [Google Scholar] [CrossRef]
- Gabel, S.A.; Walker, V.R.; London, R.E.; Steenbergen, C.; Korach, K.S.; Murphy, E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2005, 38, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, I.; Liu, D.; Bell, J.A.; Collins, J.; Steenbergen, C.; Murphy, E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J. Mol. Cell. Cardiol. 2007, 42, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ventura, J.L.; Duran, M.C.; Blanco-Colio, L.M.; Meilhac, O.; Leclercq, A.; Michel, J.-B.; Jensen, O.N.; Hernandez-Merida, S.; Tuñón, J.; Vivanco, F. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation 2004, 110, 2216–2219. [Google Scholar] [CrossRef]
- Rayner, K.; Sun, J.; Chen, Y.-X.; McNulty, M.; Simard, T.; Zhao, X.; Wells, D.J.; de Belleroche, J.; O’Brien, E.R. Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: Role of selective estrogen receptor beta modulation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.; Delgado, M.; Baiges, I.; Blade, C.; Arola, L.; Salvado, J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol microbial metabolites exhibit gut and blood–brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef]
- Obiorah, I.E.; Fan, P.; Jordan, V.C. Breast cancer cell apoptosis with phytoestrogens is dependent on an estrogen-deprived state. Cancer Prev. Res. 2014, 7, 939–949. [Google Scholar] [CrossRef]
- Nagarajan, S.; Burris, R.L.; Stewart, B.W.; Wilkerson, J.E.; Badger, T.M. Dietary soy protein isolate ameliorates atherosclerotic lesions in apolipoprotein E-deficient mice potentially by inhibiting monocyte chemoattractant protein-1 expression. J. Nutr. 2008, 138, 332–337. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a dietary daidzein gut metabolite attenuates microglial activation and potentiates neuroprotection in vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef]
- Moriyama, M.; Hashimoto, A.; Satoh, H.; Kawabe, K.; Ogawa, M.; Takano, K.; Nakamura, Y. Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflamm. 2018, 2018, 8496973. [Google Scholar] [CrossRef]
- Lin, I.-C.; Yamashita, S.; Murata, M.; Kumazoe, M.; Tachibana, H. Equol suppresses inflammatory response and bone erosion due to rheumatoid arthritis in mice. J. Nutr. Biochem. 2016, 32, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Törmälä, R.; Appt, S.; Clarkson, T.B.; Mueck, A.O.; Seeger, H.; Mikkola, T.S.; Ylikorkala, O. Impact of soy supplementation on sex steroids and vascular inflammation markers in postmenopausal women using tibolone: Role of equol production capability. Climacteric 2008, 11, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Reverri, E.J.; LaSalle, C.D.; Franke, A.A.; Steinberg, F.M. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol. Nutr. Food Res. 2015, 59, 323–333. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Mondul, A.M.; Rohrmann, S.; Platz, E.A. Associations between urinary soy isoflavonoids and two inflammatory markers in adults in the United States in 2005–2008. Cancer Causes Control 2013, 24, 1185–1196. [Google Scholar] [CrossRef]
- Greany, K.; Nettleton, J.; Wangen, K.; Thomas, W.; Kurzer, M.S. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur. J. Clin. Nutr. 2008, 62, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Hutchins-Wiese, H.L.; Kenny, A.M.; Walsh, S.J.; Abourizk, R.H.; Bruno, R.S.; Lipcius, R.; Fall, P.; Kleppinger, A.; Kenyon-Pesce, L. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: A randomized controlled trial. Nutr. Res. 2013, 33, 1026–1033. [Google Scholar] [CrossRef]
- Törmälä, R.; Appt, S.; Clarkson, T.B.; Groop, P.-H.; Rönnback, M.; Ylikorkala, O.; Mikkola, T.S. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis 2008, 198, 174–178. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Polidori, A.; Arhami, M.; Gillen, D.L.; Kleinman, M.T.; Vaziri, N.D.; Longhurst, J.; Zaldivar, F. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ. Health Perspect. 2008, 116, 898–906. [Google Scholar] [CrossRef] [PubMed]
- van der Velpen, V.; Geelen, A.; Schouten, E.G.; Hollman, P.C.; Afman, L.A.; van’t Veer, P. Estrogen receptor–mediated effects of isoflavone supplementation were not observed in whole-genome gene expression profiles of peripheral blood mononuclear cells in postmenopausal, equol-producing women. J. Nutr. 2013, 143, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and oxidative stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pignatelli, P. Clinical application of NOX activity and other oxidative biomarkers in cardiovascular disease: A critical review. Antioxid. Redox Signal. 2015, 23, 514–532. [Google Scholar] [CrossRef]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Davidson, B.P.; Yue, Q.; Belcik, T.; Xie, A.; Inaba, Y.; McCarty, O.J.; Tormoen, G.W.; Zhao, Y.; Ruggeri, Z.M. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ. Cardiovasc. Imaging 2013, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Quesada, I.M.; Lucero, A.; Amaya, C.; Meijles, D.; Cifuentes, M.E.; Pagano, P.; Castro, C. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 2015, 242, 469–475. [Google Scholar] [CrossRef]
- Cipollone, F.; Ciabattoni, G.; Patrignani, P.; Pasquale, M.; Di Gregorio, D.; Bucciarelli, T.; Davı, G.; Cuccurullo, F.; Patrono, C. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation 2000, 102, 1007–1013. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hyun, Y.J.; Jang, Y.; Lee, B.K.; Chae, J.S.; Kim, S.E.; Yeo, H.Y.; Jeong, T.-S.; Jeon, D.W.; Lee, J.H. Lipoprotein-associated phospholipase A2 activity is associated with coronary artery disease and markers of oxidative stress: A case-control study. Am. J. Clin. Nutr. 2008, 88, 630–637. [Google Scholar] [CrossRef]
- Wang, B.; Pan, J.; Wang, L.; Zhu, H.; Yu, R.; Zou, Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis 2006, 184, 425–430. [Google Scholar] [CrossRef]
- Vassalle, C.; Petrozzi, L.; Botto, N.; Andreassi, M.; Zucchelli, G. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. J. Intern. Med. 2004, 256, 308–315. [Google Scholar] [CrossRef]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; Van de Werf, F.; Collen, D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Kutter, D.; Devaquet, P.; Vanderstocken, G.; Paulus, J.-M.; Marchal, V.; Gothot, A. Consequences of total and subtotal myeloperoxidase deficiency: Risk or benefit? Acta Haematol. 2000, 104, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, E. Evaluation of Equol Function on Anti-or Prooxidant Status in vivo. J. Food Sci. 2009, 74, H65–H71. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Jiang, S.; Zheng, C.; Tian, Z.; Lin, X. Equol inhibits LPS-induced oxidative stress and enhances the immune response in chicken HD11 macrophages. Cell. Physiol. Biochem. 2015, 36, 611–621. [Google Scholar] [CrossRef]
- Pažoureková, S.; Lucová, M.; Nosál, R.; Drábiková, K.; Harmatha, J.; Šmidrkal, J.; Jančinová, V. Equol effectively inhibits toxic activity of human neutrophils without influencing their viability. Pharmacology 2016, 97, 138–145. [Google Scholar] [CrossRef]
- Pereboom, D.; Gilaberte, Y.; Sinues, B.; Escanero, J.; Alda, J.O. Antioxidant intracellular activity of genistein and equol. J. Med. Food 1999, 2, 253–256. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, S.; Jiang, Z.; Zheng, C.; Gou, Z. Effects of equol on H2O2-induced oxidative stress in primary chicken intestinal epithelial cells. Poult. Sci. 2016, 95, 1380–1386. [Google Scholar] [CrossRef]
- Choi, E.J. The prooxidant, rather than antioxidant, acts of daidzein in vivo and in vitro: Daidzein suppresses glutathione metabolism. Eur. J. Pharmacol. 2006, 542, 162–169. [Google Scholar] [CrossRef]
- Richardson, T.E.; Simpkins, J.W. R-and S-Equol have equivalent cytoprotective effects in Friedreich’s Ataxia. BMC Pharmacol. Toxicol. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Hwang, J.; Sevanian, A.; Hodis, H.N.; Ursini, F. Synergistic inhibition of LDL oxidation by phytoestrogens and ascorbic acid. Free Radic. Biol. Med. 2000, 29, 79–89. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.-H. Anticancer mechanism of equol in 7, 12-dimethylbenz (a) anthracene-treated animals. Int. J. Oncol. 2011, 39, 747–754. [Google Scholar]
- Wei, X.-J.; Wu, J.; Ni, Y.-D.; Lu, L.-Z.; Zhao, R.-Q. Antioxidant effect of a phytoestrogen equol on cultured muscle cells of embryonic broilers. In Vitro Cell. Dev. Biol. -Anim. 2011, 47, 735–741. [Google Scholar] [CrossRef]
- Kamiyama, M.; Kishimoto, Y.; Tani, M.; Utsunomiya, K.; Kondo, K. Effects of equol on oxidized low-density lipoprotein-induced apoptosis in endothelial cells. J. Atheroscler. Thromb. 2009, 16, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Sierens, J.; Hartley, J.A.; Campbell, M.J.; Leathem, A.J.; Woodside, J.V. Effect of phytoestrogen and antioxidant supplementation on oxidative DNA damage assessed using the comet assay. Mutat. Res./DNA Repair 2001, 485, 169–176. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Kulling, S.E. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Sierens, J.; Hartley, J.; Campbell, M.; Leathem, A.; Woodside, J. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog. Carcinog. Mutagenesis 2002, 22, 227–234. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Baron, T.; Wolffram, S.; Minihane, A.M.; Cassidy, A.; Rimbach, G.; Weinberg, P.D. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic. Res. 2004, 38, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-H.; Tsai, T.-Y. Effects of equol on deoxycorticosterone acetate salt-induced hypertension and associated vascular dementia in rats. Food Funct. 2016, 7, 3444–3457. [Google Scholar] [CrossRef] [PubMed]
- Vedavanam, K.; Srijayanta, S.; O’Reilly, J.; Raman, A.; Wiseman, H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 601–608. [Google Scholar] [CrossRef]
- Ma, Y.; Sullivan, J.C.; Schreihofer, D.A. Dietary genistein and equol (4′, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2010, 299, R871–R877. [Google Scholar] [CrossRef]
- Horiuchi, H.; Harada, N.; Adachi, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-equol enantioselectively activates cAMP-protein kinase A signaling and reduces alloxan-induced cell death in INS-1 pancreatic β-cells. J. Nutr. Sci. Vitaminol. 2014, 60, 291–296. [Google Scholar] [CrossRef]
- Widyarini, S.; Domanski, D.; Painter, N.; Reeve, V.E. Photoimmune protective effect of the phytoestrogenic isoflavonoid equol is partially due to its antioxidant activities. Photochem. Photobiol. Sci. 2012, 11, 1186–1192. [Google Scholar] [CrossRef]
- Nhan, S.; Anderson, K.E.; Nagamani, M.; Grady, J.J.; Lu, L.-J.W. Effect of a soymilk supplement containing isoflavones on urinary F2 isoprostane levels in premenopausal women. Nutr. Cancer 2005, 53, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 43. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar]
- Zhang, T.; Liang, X.; Shi, L.; Wang, L.; Chen, J.; Kang, C.; Zhu, J.; Mi, M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, X.; Weakley, S.M.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J. Nutr. 2010, 140, 12–17. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Song, Z.; Zhao, L.-M.; Li, G.-R.; Deng, X.-L. Equol increases cerebral blood flow in rats via activation of large-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Pharmacol. Res. 2016, 107, 186–194. [Google Scholar] [CrossRef]
- Joy, S.; Siow, R.C.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cell. J. Biol. Chem. 2006, 281, 27335–27345. [Google Scholar] [CrossRef]
- Priestley, J.R.; Kautenburg, K.E.; Casati, M.C.; Endres, B.T.; Geurts, A.M.; Lombard, J.H. The NRF2 knockout rat: A new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am. J. Physiol. -Heart Circ. Physiol. 2016, 310, H478–H487. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.J.; Chapple, S.; Siow, R.C.; Mann, G.E. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: Roles for F-actin and GPR30. Hypertension 2011, 57, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, Y.; Obayashi, S.; Yamada, K.; Yamada, M.; Kubota, T. S-equol partially restored endothelial nitric oxide production in isoflavone-deficient ovariectomized rats. J. Cardiovasc. Pharmacol. 2015, 65, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-E.; Kim, S.Y.; Jo, H.H.; Hwang, S.J.; Chae, B.; Kwon, D.J.; Lew, Y.O.; Lim, Y.-T.; Kim, J.H.; Kim, E.J. Antioxidant effects of equol on bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 2008, 375, 420–424. [Google Scholar] [CrossRef]
- Somjen, D.; Knoll, E.; Kohen, F.; Stern, N. Effects of phytoestrogens on DNA synthesis and creatine kinase activity in vascular cells. Am. J. Hypertens. 2001, 14, 1256–1262. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Hu, Q.; Shi, L.; Qin, L.; Zhang, Q.; Mi, M. Equol attenuates atherosclerosis in apolipoprotein E-deficient mice by inhibiting endoplasmic reticulum stress via activation of Nrf2 in endothelial cells. PLoS ONE 2016, 11, e0167020. [Google Scholar]
- Kim, J.Y.; Lee, M.Y.; Park, H.M. The effect of eqoul, a metabolite of isoflavone, on endothelial cell-independent vasodilatation of human uterine artery in vitro. J. Bone Metab. 2015, 22, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chin-Dusting, J.P.; Fisher, L.J.; Lewis, T.V.; Piekarska, A.; Nestel, P.J.; Husband, A. The vascular activity of some isoflavone metabolites: Implications for a cardioprotective role. Br. J. Pharmacol. 2001, 133, 595–605. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takayanagi, K.; Kobayashi, S.; Kojima, M.; Taguchi, K.; Kobayashi, T. Effect of equol on vasocontractions in rat carotid arteries treated with high insulin. Biol. Pharm. Bull. 2019, 42, 1048–1053. [Google Scholar] [CrossRef]
- Clerici, C.; Setchell, K.D.; Battezzati, P.M.; Pirro, M.; Giuliano, V.; Asciutti, S.; Castellani, D.; Nardi, E.; Sabatino, G.; Orlandi, S. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J. Nutr. 2007, 137, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446–454. [Google Scholar] [CrossRef]
- Man, B.; Cui, C.; Zhang, X.; Sugiyama, D.; Barinas-Mitchell, E.; Sekikawa, A. The effect of soy isoflavones on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 603–614. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Curtis, P.J.; Potter, J.; Kroon, P.A.; Wilson, P.; Dhatariya, K.; Sampson, M.; Cassidy, A. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: A double-blind randomized controlled trial. Am. Clin. Nutr. 2013, 97, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of equol supplement on bone and cardiovascular parameters in middle-aged Japanese women: A prospective observational study. J. Altern. Complement. Med. 2018, 24, 701–708. [Google Scholar] [CrossRef]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.-M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Yoshikata, R.; Myint, K.Z.; Ohta, H. Relationship between equol producer status and metabolic parameters in 743 Japanese women: Equol producer status is associated with antiatherosclerotic conditions in women around menopause and early postmenopause. Menopause 2017, 24, 216–224. [Google Scholar] [CrossRef]
- Uemura, H.; Katsuura-Kamano, S.; Nakamoto, M.; Yamaguchi, M.; Fujioka, M.; Iwasaki, Y.; Arisawa, K. Inverse association between soy food consumption, especially fermented soy products intake and soy isoflavone, and arterial stiffness in Japanese men. Sci. Rep. 2018, 8, 9667. [Google Scholar] [CrossRef]
- Eyster, K.; Appt, S.; Chalpe, A.; Register, T.; Clarkson, T. Effects of equol on gene expression in female cynomolgus monkey iliac arteries. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 423–427. [Google Scholar] [CrossRef]
- Ahuja, V.; Miura, K.; Vishnu, A.; Fujiyoshi, A.; Evans, R.; Zaid, M.; Miyagawa, N.; Hisamatsu, T.; Kadota, A.; Okamura, T. Significant inverse association of equol-producer status with coronary artery calcification but not dietary isoflavones in healthy Japanese men. Br. J. Nutr. 2017, 117, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.-S.-Y.; Tang, X.-Y.; Xiong, F.; Liu, Y.-P.; Liu, M.; Ling, C.-W.; Sun, T.-Y.; Ling, W.; Zhang, Z.-Q.; Chen, Y.-M. Isoflavone biomarkers are inversely associated with atherosclerosis progression in adults: A prospective study. Am. J. Clin. Nutr. 2021, 114, 203–213. [Google Scholar] [CrossRef]
- Lee, I.-M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef]
- Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef]
- Albert, C.M.; Cook, N.R.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: A randomized trial. JAMA 2008, 299, 2027–2036. [Google Scholar] [CrossRef]
- Ebbing, M.; Bleie, Ø.; Ueland, P.M.; Nordrehaug, J.E.; Nilsen, D.W.; Vollset, S.E.; Refsum, H.; Pedersen, E.K.R.; Nygård, O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: A randomized controlled trial. JAMA 2008, 300, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. Bmj 2010, 341, c6273. [Google Scholar] [CrossRef]
- Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, J.M. Multivitamins in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2012, 308, 1751–1760. [Google Scholar] [CrossRef]
- Myung, S.-K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.-J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. Bmj 2013, 346, f10. [Google Scholar] [CrossRef] [PubMed]
- Arad, Y.; Spadaro, L.A.; Roth, M.; Newstein, D.; Guerci, A.D. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: The St. Francis Heart Study randomized clinical trial. J. Am. Coll. Cardiol. 2005, 46, 166–172. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.B. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 2007, 14, 373–384. [Google Scholar] [CrossRef]
- Dubey, R.K.; Imthurn, B.; Barton, M.; Jackson, E.K. Vascular consequences of menopause and hormone therapy: Importance of timing of treatment and type of estrogen. Cardiovasc. Res. 2005, 66, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A.; Debi, A. Phytoestrogens: The “natural” selective estrogen receptor modulators? Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 47–51. [Google Scholar] [CrossRef]
| # | Authors | Type | Findings | Has Effect |
|---|---|---|---|---|
| 1 | Usui [135] | Human | Compared with the placebo group, intervention with natural equol led to a significant decrease in the HbA1c, serum LDL-C levels and CAVI score. Furthermore, the effect was more prominent in a subgroup of female equol non-producers. | Yes |
| 2 | Curtis [136] | Human | Overall, the ISF intervention did not significantly change the common carotid artery or augmentation index, but the pulse pressure variability improved. Equol producers had larger reductions in mean arterial pressure and PWV compared with non–producers. | Yes |
| 3 | Hazim [138] | Human | In an RCT, acute ISF treatment (24 h) improved cfPWV in equol producers but had no effect on endothelial function and NO in non-producers. | Yes |
| 4 | Yoshikata [137] | Human | Reduction in arterial stiffness was observed after 12 months of equol supplementation. Significant reductions in respective parameters were observed in women with moderate to high risk for arteriosclerosis, hypertriglyceridemia, bone resorption risk, and bone fracture risk. | Yes |
| 5 | Yoshikata [139] | Human | Equol-producing women in their 50 s showed significantly lower PWV. In a multivariate logistic regression, for women in their 50 s, equol production was significantly associated with lower arterial stiffness. | Yes |
| 6 | Reverri [69] | Human | Consuming soy nuts improved arterial stiffness as assessed by the augmentation index using peripheral arterial tonometry. Addition of equol-producing status as a covariate did not significantly change the result. | No |
| 7 | Törmälä [73] | Human | Soy supplementation had no effect on arterial stiffness in either equol producers or non-producers. At baseline (before ISF treatment), women with a 4-fold elevation in equol level had a lower augmentation index compared to women without this capacity. | Yes |
| # | Authors | Type | Findings | Has Effect |
|---|---|---|---|---|
| 1 | Eyster [141] | In vivo | Equol did not impact atherosclerotic lesions. Similar responses of genes to both equol and estradiol might reflect that equol served as a natural selective estrogen receptor modulator in the arteries. Equol modulated the expression of 10 genes in the atherosclerosis model that estradiol did not. | No |
| 2 | Zhang [123] | In vivo | Equol intervention reduced atherosclerotic lesions in the aorta in high-fat-diet treated apolipoprotein E-deficient mice. Plasma lipid analysis showed that equol intervention reduced triglycerides, TC, and LDL-C and increased HDL-C. | Yes |
| 3 | Ahuja [142] | Human | In multivariable models, the odds ratio for the presence of CAC in equol producers compared with the equol in non-producers was 0.10 (95 % confidence interval: 0.01, 0.90, p < 0.04). However, serum ISFs were not related to CAC. | Yes |
| 4 | Zuo [143] | Human | An 8.8-year prospective study including 2572 subjects (40 to 75 years old) found that ISFs and equol were associated with reduced progression of carotid intima-media thickness. Path analyses indicated that the association of serum equol with atherosclerosis was mediated by increased SHBG and decreased blood pressure but not lipids. | Yes |
| 5 | Zhang [30] | Human | Urinary levels of ISFs and other metabolites of ISFs were not associated with incident CHD, while urinary equol was significantly associated with CHD. The adjusted odds ratios (95% confidence intervals) for CHD across increasing quartiles of equol levels in women were 1 (reference), 0.61 (0.32, 1.15), 0.51 (0.26, 0.98), and 0.46 (0.24, 0.89) (p = 0.02 for trend). | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13, 3739. https://doi.org/10.3390/nu13113739
Zhang X, Veliky CV, Birru RL, Barinas-Mitchell E, Magnani JW, Sekikawa A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans. Nutrients. 2021; 13(11):3739. https://doi.org/10.3390/nu13113739
Chicago/Turabian StyleZhang, Xiao, Cole V. Veliky, Rahel L. Birru, Emma Barinas-Mitchell, Jared W. Magnani, and Akira Sekikawa. 2021. "Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans" Nutrients 13, no. 11: 3739. https://doi.org/10.3390/nu13113739
APA StyleZhang, X., Veliky, C. V., Birru, R. L., Barinas-Mitchell, E., Magnani, J. W., & Sekikawa, A. (2021). Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans. Nutrients, 13(11), 3739. https://doi.org/10.3390/nu13113739







